Abstract
Introduction: long non-coding RNA ANRIL (lncRNA ANRIL) has been demonstrated to play a crucial role in cancer progression. However, its effects in hepatocellular carcinoma (HCC) have not been explored. The aim of this study was to investigate the clinical significance of lncRNA NRIL in human HCC. Methods: In this study, we determined for the first time the expression of lncRNA ANRIL in human HCC by quantitative Real-time-PCR analysis. Kaplan-Meier curves and multivariate Cox proportional models were used to study the impact on clinical outcome. Small interfering RNA (siRNA) was used to silence lncRNA ANRIL and to explore the effects of reduced lncRNA ANRIL expression on cell growth and metastasis. Results: lncRNA ANRIL expression in HCC tissues was significantly higher than in the adjacent non-tumor tissues (P < 0.05). The expression of lncRNA ANRIL was remarkably associated with the histologic grade and TNM stage of HCC patients (P < 0.05). In addition, HCC patients with higher lncRNA ANRIL expression had significantly poorer overall survival (P < 0.05). Multivariate analysis suggested that high lncRNA ANRIL expression was an independent predictor of poor prognosis (P < 0.05). Moreover, in vitro assays revealed that the decreased expression of lncRNA ANRIL could suppress the cell proliferation, migration and invasion HCC cells. Conclusions: Our results suggest that lncRNA ANRIL may serve as an efficient clinical biomarker and a therapeutic target for HCC patients.
Keywords: Hepatocellular carcinoma, lncRNA ANRIL, overall survival, proliferation, migration, invasion
Introduction
Hepatocellular carcinoma (HCC) is one of the most frequent human malignancy worldwide. Especially in China, it has become a major cause of cancer-related death [1]. The established risk factors of HCC are viral hepatitis, alcohol abuse, and non-alcoholic fatty liver disease [2]. The main features of HCC are fast infiltrating growth, early metastasis, high-grade malignancy, and poor therapeutic efficacy [3]. In most cases, surgical resection and liver transplantation remain the only curative treatment options. Frequent tumor metastasis and recurrence after surgical intervention lead to the dismal outcome of patients with HCC [4]. Therefore, identification of effective prognostic markers by better understanding the molecular mechanisms involved in HCC carcinogenesis is of crucial significance in the development of novel targeted therapeutic strategies for this disease.
Non-coding RNAs (ncRNAs), once regarded as “transcriptional noise”, have recently been demonstrated to be functional molecules [5]. These protein-non-coding sequences account for the majority of human genome while protein-coding genes only about 2% [6]. The ncRNAs include not only well characterized microRNAs and other non-coding transcripts less than 200 nucleotides (nt) but also a large class of long (> 200 nt) ncRNAs (lncRNAs), which have emerged as a new layer of cell biology [7]. A growing volume of literature has indicated the vital roles of lncRNAs in cancer biology. Alterations in lncRNAs have been shown to exhibit pro-oncogenic or tumor-suppressive activities [8,9]. Therefore, identification of cancer-associated lncRNAs and investigation of their molecular and biological functions are important in understanding the molecular biology of HCC development and progression.
A recently identified long non-coding RNA, named CDKN2B antisense RNA 1 (ANRIL), is transcribed as a 3.8 kb lncRNA in the opposite direction from the INK4B-ARF-INK4A gene cluster [10]. It suggests that higher levels of lncRNA ANRIL expression were seen in prostate cancer and involved in repressing of the p15/CDKN2B-p16/CDKN2Ap14/ARF gene cluster in Cis by directly binding to the Polycomb Repressor Complex (PRC) [10]. Bochenek et al indicated that ANRIL is the best replicated genetic risk locus of coronary artery disease (CAD) and periodontitis (PD) [11]. Zhang et al found that lncRNA ANRIL was up-regulated in gastric cancer tissues and associated with tumor size, TNM stage and poor prognosis of gastric cancer. Further experiments suggested that ANRIL knockdown significantly repressed the proliferation both in vitro and in vivo [12]. These results indicate that the dysregulation of ANRIL could participate in diverse human disease progression. However, the prognostic role and underlying mechanism of ANRIL in HCC is still unknown.
In the present study, we explored ANRIL expression pattern and its correlation with clinicopathological features in HCC. Then, its prognostic significance was assessed. Then oncogenic role of ANRIL was investigated in HCC cells.
Materials and methods
Patients and tissue specimens
A total of 130 patients with primary HCC who underwent a curative liver resection at the Department of General Surgery, Huaihe Clinical College of HeNan University, were included in this retrospective study between June 2006 and July 2012. All these tissues were immediately stored in liquid nitrogen and kept freshly frozen at -80°C. Written informed consent was accomplished from the patients for publication of this study and any accompanying images. Study protocol was approved by the Ethics Committee of Provincial Hospital affiliated to Huaihe Clinical College of HeNan University. Before surgical therapy, none of the patients had received neoadjuvant chemotherapy, radiation therapy, or immunotherapy. Related important clinical information was collected from each patient’s medical records. Clinical staging was performed according to American Joint Committee on Cancer/International Union Against Cancer TNM staging system [13]. Patient characteristics were shown in Table 1.
Table 1.
Clinicopathological features and the expression of lncRNA ANRIL in HCC patients
| Parameters | Group | Total | lncRNA ANRIL expression | P value | |
|---|---|---|---|---|---|
|
| |||||
| High | Low | ||||
| Gender | Male | 53 | 28 | 25 | 0.527 |
| Female | 39 | 18 | 21 | ||
| Age (years) | < 60 | 58 | 31 | 27 | 0.388 |
| ≥ 60 | 34 | 15 | 19 | ||
| Tumor size (cm) | < 5 cm | 41 | 19 | 22 | 0.529 |
| ≥ 5 cm | 51 | 27 | 24 | ||
| Tumor number | Solitary | 44 | 24 | 20 | 0.404 |
| Multiple | 48 | 22 | 26 | ||
| AFP | < 20 | 37 | 19 | 18 | 0.832 |
| > 20 | 55 | 27 | 28 | ||
| Hepatitis B | Negative | 22 | 12 | 10 | 0.625 |
| Positive | 70 | 34 | 36 | ||
| Histologic grade | High | 29 | 9 | 20 | 0.014 |
| Low | 63 | 37 | 26 | ||
| TNM stage | I-II | 39 | 8 | 31 | 0.000 |
| III-IV | 53 | 38 | 15 | ||
Cell culture and transfection
The human hepatoma cell line HepG2 was purchased from American Type Culture Collection. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco), containing 10% foetal bovine serum (FBS, Gibco) and antibiotics (penicillin and streptomycin) and were incubated in a 5% CO2 humidified incubator at 37°C.
HepG2 were transfected with siRNA oligonucleotides using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. The nucleotide sequences of siRNA for ANRIL were GGUCAUCUCAUUGCUCUAU [14], Negative control siRNA (si-NC) were purchased from Invitrogen. After transfection, cells were harvested for qRT-PCR analyses.
Cell proliferation assays
Cell viability of human HCC cells transfected with si-ANRIL or si-NC was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, 48 h after transfection, 20 μL MTT solution (Sigma) was added into the culture medium for 4 h incubation. Then, 150 μL DMSO (Sigma) was added into each well to dissolve the crystals. The absorbance of each sample was recorded at 490 nm. All experiments were performed in quadruplicate. For each treatment group, wells were assessed in triplicate.
Cell migration and invasion assay
For the migration assays, 48 h after transfection, 5×104 cells in serum-free media were placed into the upper chamber of an insert (8.0 μm, Millipore). For the invasion assays, the upper chamber was pre-coated with Matrigel (Sigma) and 1×105 cells were seeded. The chambers were then incubated for 24 h in culture medium with 10% FBS in the bottom chambers before examination. The cells on the upper surface were scraped and washed away, whereas the cells on the lower surface were fixed and stained with 0.05% crystal violet for 2 h. Finally, cells were counted under a microscope and the relative number was calculated. Experiments were independently repeated in triplicate.
RNA extraction and qRT-PCR analyses
Total RNA from tissues and cells was extracted using Trizol reagent (Invitrogen). RNA was reversed transcribed into cDNAs using the Primer-Script one step RT-PCR kit (TaKaRa). The cDNA template was amplified by real-time PCR using the SYBR Premix Dimmer Eraser kit (TaKaRa). GAPDH was used as an internal control, and lncRNA ANRIL values were normalized to GAPDH. Real-time PCR reactions were performed by the ABI7900 system (Applied Biosystems). The relative expression fold change of mRNAs was calculated by the 2-ΔΔCt method. The primer sequences were as follows: GADPH: 5’-GTCAACGGATTTGGTCTGTATT-3’ (forward), 5’-AGTCTTCTGGGTGGCAGTGAT-3’ (reverse); lncRNA ANRIL: 5’-TGCTCTATCCGCCAATCAGG-3’ (forward), 5’-GGGCCTCAGTGGCACATACC-3’ (reverse).
Statistical analysis
All statistical analyses were performed using SPSS 18.0 software (IBM). The significance of differences between groups was estimated by Student’s t-test, χ2 test or Wilcoxon test, as appropriate. Overall survival rates were calculated by the Kaplan-Meier method with the log-rank test applied for comparison. Survival data were evaluated using univariate and multivariate Cox proportional hazards model. Variables with a value of P < 0.05 in univariate analysis were used in subsequent multivariate analysis on the basis of Cox regression analyses. All tests were two tailed and results with P < 0.05 were considered statistically significant.
Results
Expression level of lncRNA ANRIL in HCC tissues and its relationship with clinicopathological features
The expression level of lncRNA ANRIL in 92 pairs of HCC tissues and adjacent non-tumor tissues was examined by qRT-PCR, ANRIL was significantly increased in HCC tissues compared with adjacent non-tumor tissues (Figure 1A, P < 0.05), suggesting that ANRIL may play an oncogenic role in HCC. To assess the association between ANRIL expression and clinicopathologic features of HCC patients, according to the relative ANRIL expression in tumor tissues, the 92 HCC patients were classified into two groups according to the median of ANRIL expression level: relative high group: ANRIL expression ratio ≥ median ratio and relative low group: ANRIL expression ratio < median ratio. Our data revealed that high ANRIL expression was associated with histologic grade and TNM stage. However, ANRIL expression was not correlated with age, gender, tumor size, tumor number, AFP, and hepatitis B infection.
Figure 1.
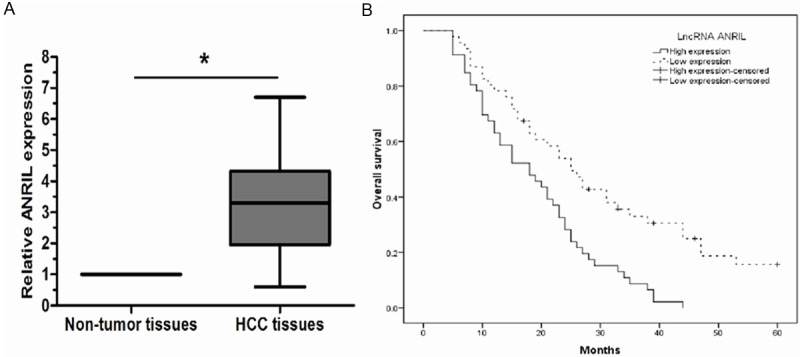
LncRNA ANRIL expression in HCC tissues and its clinical significance. A: Relative expression of lncRNA ANRIL in HCC tissues (n=42) compared with adjacent non-tumor normal tissues (n=42). LncRNA ANRIL expression was examined by qRT- PCR and normalized to GAPDH expression. B: Patients with higher levels of lncRNA ANRIL expression showed shorter overall survival times compared to patients with lower levels of lncRNA ANRIL expression (log-rank P < 0.05). * P < 0.05.
Prognostic value of ANRIL expression in HCC patients
The prognostic value of lncRNA ANRIL expression was further investigated using the Kaplan-Meier method and log-rank test. As shown in Figure 1B, there was a significant correlation between lncRNA ANRIL expression and overall survival of HCC patients (P < 0.05, log-rank test). The 5-year overall survival rate of HCC patients with high ANRIL expression was significantly lower than that of those patients with low ANRIL expression. Univariate Cox proportional hazards regressions model analysis revealed that histologic grade, TNM stage, and ANRIL expression were statistically significant risk factors affecting the overall survival of patients with HCC (Table 2). No significant associations were found for age, gender, tumor size, tumor number, AFP, and hepatitis B infection, and patient outcome. Multivariate analysis using the Cox proportional hazard model for all variables that were significant in the univariate analysis confirmed that histologic grade, TNM stage, and the level of lncRNA ANRIL expression (P < 0.05) were independent prognostic factors for patients with HCC (Table 2). These findings suggested that lncRNA ANRIL may play an important role in the progression of HCC.
Table 2.
Univariate and multivariate analyses for overall survival (Cox proportional hazards regression model)
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Gender | 0.894 | 0.472-1.503 | 0.364 | |||
| Male vs. Female | ||||||
| Age (years) | 1.213 | 0.724-1.791 | 0.287 | |||
| < 60 vs. ≥ 60 | ||||||
| Tumor size | 1.822 | 0.561-3.275 | 0.108 | |||
| < 5 cm vs. ≥ 5 cm | ||||||
| Tumor number | 2.165 | 0.601-4.281 | 0.229 | |||
| Solitary vs. Multiple | ||||||
| AFP (ng/ml) | 2.588 | 0.854-5.812 | 0.118 | |||
| < 25 vs. > 25 | ||||||
| Hepatitis B | 1.852 | 0.804-3.411 | 0.219 | |||
| Postive vs. Negative | ||||||
| Histologic grade | 2.718 | 1.336-5.257 | 0.007 | 2.407 | 1.262-4.832 | 0.012 |
| Low vs. High | ||||||
| TNM stage | 3.214 | 1.508-6.917 | 0.004 | 2.947 | 1.405-6.581 | 0.007 |
| I-II vs. III-IV | ||||||
| LncRNA ANRIL | 2.907 | 1.774-7.618 | <0.001 | 2.684 | 1.534-6.992 | 0.003 |
| High vs. Low | ||||||
Konckdown of lncRNA ANRIL inhibits proliferation, migration and invasion of HCC cells in vitro
To investigate the role of lncRNA ANRIL in HCC progression, we used HepG2 cells as our experimental model. We modulated the expression level of lncRNA ANRIL through RNA interference experiments. Our results showed the expression levels of lncRNA ANRIL in HepG2 cells transfected with si-ANRIL were significantly lower than in cells transfected with si-NC cells (Figure 2A; P < 0.05). The proliferation assay suggested that lncRNA ANRIL down-regulation significantly inhibited the proliferative activity of HepG2 cells (Figure 2B; P < 0.05). Furthermore, the effects of lncRNA ANRIL on cell migration and invasion of human HCC cells were evaluated by the migration and invasion assay in vitro. Our data indicated that down-regulated expression of lncRNA ANRIL led to a significant decrease in cell migration and invasion of human HCC cells (Figure 2C, 2D; P < 0.05).
Figure 2.
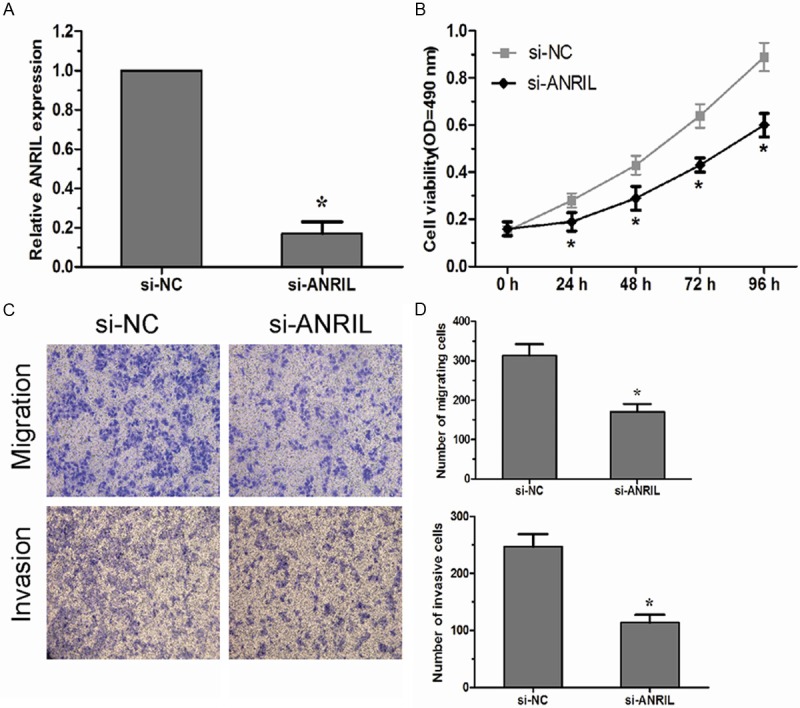
Knockdown of LncRNA ANRIL inhibits HCC cells proliferation migration and invasion in vitro. A: The lncRNA ANRIL expression in HepG2 cells transfected with si-ANRIL or si-NC was analyzed by qRT-PCR. B: MTT assay was performed to determine the proliferation of si-ANRIL transfected HepG2 cells. C, D: Transwell assays were performed to investigate the migratory and invasive ability of si-ANRIL transfected HepG2 cells. Data represent the mean±SD from three independent experiments. *P < 0.05.
Discussion
Although a growing number of novel treatment strategies have been developed for HCC, such as molecular targeted therapy and gene therapy, to our disappointment, satisfactory therapeutic outcomes have not been achieved [15,16]. Considering that the survival rate of HCC is still low, it is necessary to identify novel therapeutic targets to improve the clinical outcome of HCC patients.
Long non-coding RNAs (lncRNAs) represent a new class of non-protein-coding RNAs which are longer than 200 bases, and do not function as templates for protein synthesis [7]. Previous studies have proved that lncRNAs play a critical role in the development and progression of cancer [8]. Additionally, multiple studies have indicated that the expression levels of lncRNAs are dysregulated in different kinds of tumors, including HCC. For example, Endo et al found the expression of HOTAIR was significantly higher in gastric cancer tissues, the High-HOTAIR group was associated with venous invasion, lymph node metastases and lower overall survival rate compared to the Low-HOTAIR group [17]. Zheng et al suggested that expression of lncRNA MALAT1 was increased in colorectal cancer compared to that in the adjacent normal tissues, patients with higher expression of MALAT1 showed a significantly worse overall survival and disease free survival than that patients with lower expression of MALAT1 [18]. Shi et al showed GAS5 expression was down-regulated in non-small cell lung cancer tissues and was highly related to tumor size and TNM stage. Furthermore, they indicated that GAS5 overexpression increased tumor cell growth arrest and induced apoptosis in vitro and in vivo [19]. Ma et al revealed that down-regulation of lncRNA-LET was observed in gallbladder cancer and patients with low expression of lncRNA-LET have significantly poorer prognosis than those with high expression. Moreover, they demonstrated that up-regulated expression of lncRNA-LET could inhibit the invasion of gallbladder cancer cells in vitro [20]. However, to our knowledge, the roles of lncRNA ANRIL in the carcinogenesis of HCC are still unclear.
In the present study, our results showed that the expression of lncRNA ANRIL was increased in HCC tissues compared with non-tumor tissues. Importantly, high expression of lncRNA ANRIL was found to associate with low histologic grade and advanced TNM stage. Moreover, the overall survival time of patients with higher lncRNA ANRIL expression levels was shorter than that of patients with lower lncRNA ANRIL expression levels. These findings indicate that lncRNA ANRIL plays a direct role in the modulation of HCC progression and may be considered as a novel prognostic marker for HCC. To further investigate the underlying mechanism of lncRNA ANRIL in HCC progression, we explored the effects of loss of function of lncRNA ANRIL on HCC cells. Our studies showed that lncRNA ANRIL knockdown significantly repressed the proliferation, migration and invasion of HCC cells in vitro.
According to the results of our study, lncRNA ANRIL could serve as a potential therapeutic target for the following reasons. First, lncRNA ANRIL expression is significant increased in HCC tissues and strongly correlated with advanced clinical features. The second reason is lncRNA ANRIL could predict the prognosis of patients with HCC. Third, Down-regulated expression of lncRNA ANRIL could inhibit HCC cells proliferation, migration and invasion.
In summary, our findings demonstrated that lncRNA ANRIL plays a vital role in the development and progression of HCC. The development of ANRIL-based therapeutic strategies for the downregulation of such oncogenic lncRNAs may provide a novel and promising alternative therapeutic approach for future HCC treatment.
Disclosure of conflict of interest
None.
References
- 1.Shi J, Zhu L, Liu S, Xie WF. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005;92:607–612. doi: 10.1038/sj.bjc.6602333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300–4308. doi: 10.3748/wjg.14.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 4.Nagao T, Inoue S, Goto S, Mizuta T, Omori Y, Kawano N, Morioka Y. Hepatic resection for hepatocellular carcinoma. Clinical features and long-term prognosis. Ann Surg. 1987;205:33–40. doi: 10.1097/00000658-198701000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;1:R17–29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 6.Huttenhofer A, Schattner P, Polacek N. Non-coding RNAs: hope or hype? Trends Genet. 2005;21:289–297. doi: 10.1016/j.tig.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 8.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Chen Z, Wang X, Huang Z, He Z, Chen Y. Long non-coding RNA: a new player in cancer. J Hematol Oncol. 2013;6:37. doi: 10.1186/1756-8722-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yap KL, Li S, Munoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou MM. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bochenek G, Hasler R, El Mokhtari NE, Konig IR, Loos BG, Jepsen S, Rosenstiel P, Schreiber S, Schaefer AS. The large non-coding RNA ANRIL, which is associated with atherosclerosis, periodontitis and several forms of cancer, regulates ADIPOR1, VAMP3 and C11ORF10. Hum Mol Genet. 2013;22:4516–4527. doi: 10.1093/hmg/ddt299. [DOI] [PubMed] [Google Scholar]
- 12.Zhang EB, Kong R, Yin DD, You LH, Sun M, Han L, Xu TP, Xia R, Yang JS, De W, Chen J. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget. 2014;5:2276–2292. doi: 10.18632/oncotarget.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002;87:13–15. [PubMed] [Google Scholar]
- 14.Kotake Y, Nakagawa T, Kitagawa K, Suzuki S, Liu N, Kitagawa M, Xiong Y. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene. 2011;30:1956–1962. doi: 10.1038/onc.2010.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 16.Johnson PJ. Hepatocellular carcinoma: is current therapy really altering outcome? Gut. 2002;51:459–462. doi: 10.1136/gut.51.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endo H, Shiroki T, Nakagawa T, Yokoyama M, Tamai K, Yamanami H, Fujiya T, Sato I, Yamaguchi K, Tanaka N, Iijima K, Shimosegawa T, Sugamura K, Satoh K. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One. 2013;8:e77070. doi: 10.1371/journal.pone.0077070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng HT, Shi DB, Wang YW, Li XX, Xu Y, Tripathi P, Gu WL, Cai GX, Cai SJ. High expression of lncRNA MALAT1 suggests a biomarker of poor prognosis in colorectal cancer. Int J Clin Exp Pathol. 2014;7:3174–3181. [PMC free article] [PubMed] [Google Scholar]
- 19.Shi X, Sun M, Liu H, Yao Y, Kong R, Chen F, Song Y. A critical role for the long non-coding RNA GAS5 in proliferation and apoptosis in non-small-cell lung cancer. Mol Carcinog. 2013 doi: 10.1002/mc.22120. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Ma MZ, Kong X, Weng MZ, Zhang MD, Qin YY, Gong W, Zhang WJ, Quan ZW. Long noncoding RNALET is a positive prognostic factor and exhibits tumor suppressive activity in gallbladder cancer. Mol Carcinog. 2014 doi: 10.1002/mc.22215. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


