Abstract
We conducted a case-control study to investigate the role of ABCB1 C3435T and G2677T/A in the susceptibility and prognosis of colorectal cancer patients. A total of 316 patients with colorectal cancer and 316 controls were collected between January 2009 and January 2011. Genotyping of ABCB1 C3435T and G2677T/A was conducted by the methods of Polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP). Conditional logistic regression analysis showed that subjects carrying CT and CC genotypes of ABCB1 C3435T were more frequently observed in colorectal cancer patients when compared with controls, and the adjusted ORs were 1.62 (1.05-2.52) and 2.05 (1.25-3.36), respectively. By Cox regression analysis, we found that the TT genotype of ABCB1 C3435T was significantly associated with shorter PFS and OS in patients with colorectal cancer when compared with CC genotype, with adjusted HR (95% CI) of 2.57 (1.14-6.04) and 2.54 (1.05-6.61), respectively. We found that the ABCB1 C3435T polymorphism could affect the susceptibility and clinical outcome of colorectal cancer patients.
Keywords: ATP-binding cassette B1 gene, colorectal cancer, susceptibility, clinical outcome
Introduction
Colorectal cancer is the third most common cancer worldwide, and it is estimated that there were 663,000 cases in males and 571,000 cases in females [1]. In China, colorectal cancer is the fourth leading cause of cancer death [1]. The incidence and mortality of colorectal cancer has greatly increased since the rapid change of dietary and life styles and inherited genetic background [2]. Standard treatment of colorectal cancer involves neoadjuvant therapy before surgical resection, and followed by chemotherapy after operation [3]. Despite of this, about many colorectal cancer patients showed recurrence or metastasis during five years period.
Individualized chemotherapy through molecular biomarkers may improve individuals’ response to chemotherapy, and thus prolong the life of colorectal cancer patients. Thus, better understanding the role of molecular biomarkers on the chemotherapy could play an important role in guide patients to have an individualized chemotherapy, and colorectal cancer patients could benefit more from chemotherapy to prolong their survival time.
It is well known that gene polymorphisms could affect the drug absorption, distribution, metabolism and excretion if these gene polymorphisms can influence the clinical response to chemotherapeutics [4]. APT-binding cassette transporters, namely ABC proteins, are main types of transport suferfamilies and are responsible for mediate efflux of many endogenous substrates, xenobiothics and chemotherapeutic agents as well as drug resistance in many cancers [4-6]. P-glycoprotein (P-gp) is encoded by the ATP-binding cassette B1 (ABCB1) gene and is located on the luminal side of intestinal epithelial cells, and it is an efflux transporter and is responsible for protecting the gut from endogenous exogenous toxins [5,6]. It is reported that the ABCB1 C3435T and G2677T/A are the most frequent two gene polymorphisms of ABCB1 gene, and they are correlated with altered P-gp and lowered intestinal P-glycoprotein expression [7,8]. Recently, several studies have investigated that ABCB1 polymorphisms contribute to the risk of colorectal cancer in several kinds of populations, but the results are inconsistent [9-17]. Recently, few studies investigate the role of ABCB1 gene polymorphisms with the development and prognosis of colorectal cancer in a Chinese population.
Therefore, we conducted this case-control study to analyze the genetic polymorphisms in the drug transporter ABCB1, and investigate the role of ABCB1 C3435T and G2677T/A in the susceptibility and prognosis of colorectal cancer patients.
Materials and methods
Patient recruitment and data collection
A total of 316 patients with colorectal cancer were collected from the Affiliated Hospital of Inner Mongolia Medical University between January 2009 and January 2011. All the patients were histologically confirmed to be colorectal cancer. The pathologically diagnosed were conducted at the Pathology Department in our hospital. Patients who had secondary or recurrent tumors and a history of other malignant neoplasm were excluded from this study.
316 hospital-based subjects without malignancy were randomly selected from a health examination center as controls, and they were matched with colorectal cancer patients by sex and age. Controls that have a history of other malignant neoplasm and any digestive disease were excluded from our study.
The clinical characteristics of colorectal cancer patients and controls were gained through a self-designed questionnaires and medical records. The tumor differentiation or pathological grade of these colorectal cancer patients were conducted according to the World Health Organization criteria. All the patients received chemotherapy regimens, such as oxaliplatin-based chemotherapy, leucovorin plus 5-fluorouracil (FU), or FU alone.
Each patient signed an informed consent before participating into this study. This study was approved by the Ethics Committee of Affiliated Hospital of Inner Mongolia Medical University.
Our primary end point was overall survival (OS) calculated as the time from diagnosis until death from any cause or last known date alive. Progression-free survival (PFS) following treatment was calculated from the initiation of therapy to first recorded date of progression, death, or last follow-up evaluation. All the patients were followed up to death or the end of study (January 2014).
Genotyping
Each patient was asked to provide a sample of 5 mL venous blood, and was kept at -20°C. Genomic DNA was extracted from venous blood using the TIANamp blood DNA kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s instructions. Genotyping of ABCB1 C3435T and G2677T/A was conducted by the methods of Polymerase chain reaction restriction fragment length polymorphism, (PCR-RFLP). Primers of ABCB1 C3435T and G2677T/A which used for PCR-RFLP were designed using Sequenom Assay Design 3.1 software (Sequenom®). PCR was performed using 100 ng of genomic DNA, and conducted in a reaction volume of 20 μL, containing 10 ng of genomic DNA, 200 μM dNTP and 0.5 units of Taq DNA polymerase as well as 300 μM of primers. The PCR condition was conducted using the following steps: 94°C for 2 min, 35 cycles of 94°C for 30 sec, and with an annealing temperature reduced to 64°C for 30 sec and 72°C for 1 min. For ABCB1 C3435T, the PCR product was 244 bp, and digested with Mbo I. For G2677T/A, the PCR product was 224 bp, and digested with Ban I. Finally, DNA sequencing was performed to validate the results of genotyping.
Statistical analysis
All analyses were performed using the Statistical Package for the Social Sciences (SPSS) software 13.0 for windows. Correlation between ABCB1 C3435T and G2677T/A polymorphisms and risk of colorectal cancer was assessed using Odd Ratios (ORs) and 95% Confidence Intervals (CIs) from multivariate logistic regression analysis. Homozygote for the most frequent allele was regarded as the reference group. Moreover, the association between ABCB1 C3435T and G2677T/A polymorphisms and PFS and OS of colorectal cancer was assessed by Cox proportional hazards model with hazard ratios (HR) and their confidence intervals (CI). PFS and OS curves were plotted using the Kaplan-Meier method. The All P values were two-tailed, and difference was considered statistically significant when a value of P < 0.05.
Results
This study consisted of 316 colorectal cancer patients and 316 control subjects. The average age of cases and controls were 63.5 ± 10.9 and 62.8 ± 11.7 years, respectively. There were 122 males and 194 females in cases and controls, respectively (Table 1). Patients with colorectal cancer cases were more likely to be smokers (P < 0.05).
Table 1.
Basic characteristics of included colorectal cancer patients and control subjects
| Variables | Colorectal cancer patients N = 316 (%) | Control subjects N = 316 (%) | χ2 value | P value |
|---|---|---|---|---|
| Age, years | ||||
| Average | 63.5 ± 10.9 | 62.8 ± 11.7 | ||
| < 60 | 175 (55.38) | 179 (56.65) | ||
| ≥ 60 | 141 (44.62) | 137 (43.35) | 0.11 | 0.75 |
| Sex | ||||
| Male | 122 (38.61) | 122 (38.61) | ||
| Female | 194 (61.39) | 194 (61.39) | 0.00 | 1.00 |
| Smoking status | ||||
| Never | 204 (64.56) | 231 (73.10) | ||
| Ever | 112 (35.44) | 85 (26.90) | 5.38 | 0.02 |
| Drinking status | ||||
| Never | 174 (55.06) | 188 (59.49) | ||
| Ever | 142 (44.94) | 128 (40.51) | 1.27 | 0.26 |
| Tumor size | ||||
| < 4 cm | 77 (24.37) | |||
| ≥ 4 cm | 239 (75.63) | |||
| Tumor differentiation | ||||
| Grade 1 | 21 (6.65) | |||
| Grade 2 | 262 (82.91) | |||
| Grade 3 | 33 (10.44) | |||
| Lymph mode metastases | ||||
| No | 144 (45.57) | |||
| Yes | 172 (54.43) | |||
| Relapse | ||||
| No | 300 (94.94) | |||
| Yes | 16 (5.06) | |||
Of 316 colorectal cancer patients, 239 (75.63%) had tumor size ≥ 4 cm, 262 (82.91%) had tumor differentiation at Grade 2, 172 (54.43%) had lymph mode metastases, and 300 (94.94%) had no relapse.
The allele and genotype distributions of ABCB1 C3435T and G2677T/A were found to be in Hardy–Weinberg equilibrium in the control group. Conditional logistic regression analysis showed that subjects carrying CT and CC genotypes of ABCB1 C3435T were more frequently observed in colorectal cancer patients when compared with controls, and the adjusted ORs were 1.62 (1.05-2.52) and 2.05 (1.25-3.36), respectively (Table 2). Moreover, we found that patients carrying the C allele of ABCB1 C3435T had a significantly increased risk of colorectal cancer relative to the CC genotype, with adjusted OR (95% CI) of 1.38 (1.10-1.74). However, no association was found between variants of ABCB1 G2677T/A polymorphism and risk of colorectal cancer.
Table 2.
Distribution of ABCB1 C3435T and G2677T/A polymorphisms and associations with risk of colorectal cancer
| Genotypes | Colorectal cancer patients N = 316 (%) | Control subjects N = 316 (%) | Adjusted OR (95% CI)1 | P value |
|---|---|---|---|---|
| ABCB1 C3435T | ||||
| TT | 49 (15.51) | 77 (24.37) | 1.0 (Ref.) | - |
| CT | 168 (53.16) | 163 (51.58) | 1.62 (1.05-2.52) | 0.02 |
| CC | 99 (31.33) | 76 (24.05) | 2.05 (1.25-3.36) | 0.003 |
| Allele | ||||
| T | 266 (42.09) | 317 (50.16) | 1.0 (Ref.) | - |
| C | 366 (57.91) | 315 (49.84) | 1.38 (1.10-1.74) | 0.004 |
| G2677T/A | ||||
| GG | 39 (12.34) | 51 (16.14) | 1.0 (Ref.) | - |
| GT/GA | 153 (48.42) | 149 (47.15) | 1.37 (0.83-2.26) | 0.19 |
| TT/AA | 124 (39.24) | 116 (36.71) | 1.43 (0.85-2.39) | 0.15 |
| Allele | ||||
| G | 231 (73.10) | 251 (39.72) | 1.0 (Ref.) | - |
| T/A | 401 (126.90) | 381 (60.28) | 1.15 (0.91-1.46) | 0.22 |
Adjusted for sex, age, smoking and drinking status.
The association between ABCB1 C3435T and G2677T/A polymorphisms and PFS and OS of colorectal cancer patients are presented in Table 3. By Cox regression analysis, we found that the TT genotype of ABCB1 C3435T was significantly associated with shorter PFS and OS in patients with colorectal cancer when compared with CC genotype, with adjusted HR (95% CI) of 2.57 (1.14-6.04) and 2.54 (1.05-6.61), respectively, respectively. By Kaplan-Meier method, the TT genotype of ABCB1 C3435T showed a tendency towards shorter PFS and OS in colorectal cancer patients (Figures 1 and 2).
Table 3.
Cox regression analysis of ABCB1 C3435T and G2677T/A polymorphisms with the PFS and OS of colorectal cancer
| Genotype | PFS | HR (95% CI)1 | P value | OS | HR (95% CI)1 | P value | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Event (%) | Alive (%) | Death (%) | Alive (%) | |||||
|
|
|
|||||||
| N = 107 | N = 209 | N = 82 | N = 234 | |||||
| ABCB1 C3435T | ||||||||
| CC | 12 (11.21) | 37 (17.70) | 1.0 (Ref.) | - | 9 (10.98) | 40 (17.09) | 1.0 (Ref.) | - |
| CT | 50 (46.73) | 118 (56.46) | 1.57 (0.69-3.81) | 0.25 | 37 (45.12) | 131 (55.98) | 1.26 (0.54-3.21) | 0.58 |
| TT | 45 (42.06) | 54 (25.84) | 2.57 (1.14-6.04) | 0.01 | 36 (43.90) | 63 (26.92) | 2.54 (1.05-6.61) | 0.03 |
| Allele | ||||||||
| C | 74 (34.58) | 192 (45.93) | 1.0 (Ref.) | - | 55 (67.07) | 211 (90.17) | 1.0 (Ref.) | - |
| T | 140 (65.42) | 226 (54.07) | 1.61 (1.13-2.30) | 0.006 | 109 (132.93) | 257 (109.83) | 1.63 (1.11-2.41) | 0.009 |
| G2677T/A | ||||||||
| CC | 11 (10.28) | 28 (13.40) | 1.0 (Ref.) | - | 8 (9.76) | 31 (13.25) | 1.0 (Ref.) | - |
| CA | 50 (46.73) | 103 (49.28) | 1.24 (0.54-2.98) | 0.59 | 40 (48.78) | 113 (48.29) | 1.37 (0.56-3.74) | 0.47 |
| AA | 46 (42.99) | 78 (37.32) | 1.50 (0.65-3.66) | 0.31 | 33 (40.24) | 91 (38.89) | 1.41 (0.56-3.90) | 0.44 |
| Allele | ||||||||
| A | 72 (33.64) | 159 (38.04) | 1.0 (Ref.) | - | 56 (68.29) | 175 (74.79) | 1.0 (Ref.) | - |
| G | 142 (66.36) | 259 (61.96) | 1.21 (0.85-1.74) | 0.28 | 106 (129.27) | 295 (126.07) | 1.12 (0.76-1.67) | 0.54 |
Adjusted for tumor size, tumor differentiation, lymph mode metastases and relapse.
Figure 1.
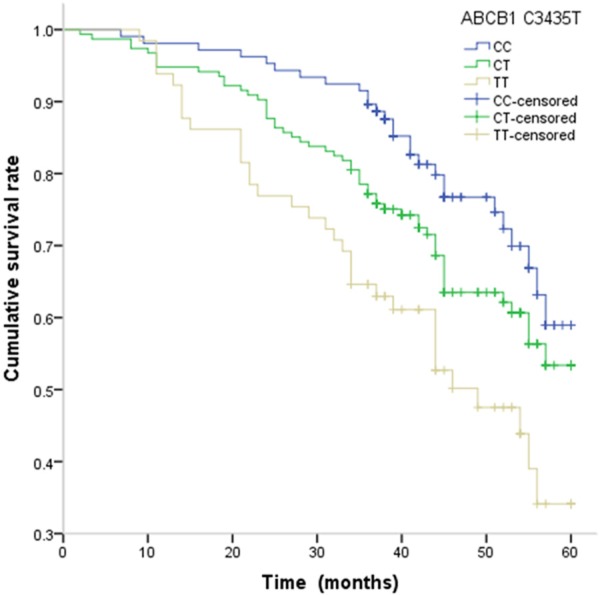
Kaplan-Meier estimates of PFS of colorectal cancer with ABCB1 C3435T.
Figure 2.
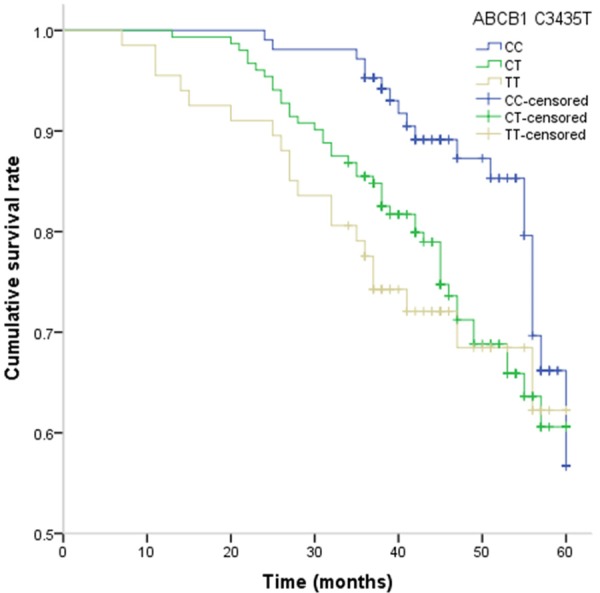
Kaplan-Meier estimates of OS of colorectal cancer with ABCB1 C3435T.
Discussion
ABCB1 (P-glycoprotein, multidrug resistance is reported to express in normal human tissues and multidrug-resistant cancer cells, which contributes to the absorption and distribution of xenobiotics, toxins and drugs. Previous studies reported that ABCB1 C3435T and G2677T/A gene polymorphisms are associated with altering expression or transporter activity of P-gp [7,8,18,19], and thus these two gene polymorphism can play an important role in susceptibility of developing cancer, but their biological significance is not fully understood yet. Moreover, ABCB1 is also a transmembrane protein, and it acts as an energy-dependent drug efflux pump for chemotherapeutic drugs [20]. This gene can elinate the parent drug through hepatobiliary and intestinal secretion, and be involved in proliferation and survival of epithelial cells and malignant cells during tumorigenesis [21]. ABCB1 variation may influence the efflux of chemotherapeutic agents from tumor cells or their elimination from the body, reduce the plasma concentrations, and thus influence their therapeutic efficacy.
This study assessed the most comprehensive pharmacogenetic SNPs of APT-binding cassette which involved in platinum, adriamycin, methotrexate, vincristine and cyclophosphamide pathways in the colorectal cancer patients receiving chemotherapy. Identification of genes involved in the genetic predisposition or progression of cancer has an important rolein clinical practice and basic medicine research. In this study, we investigated the associtions of ABCB1 C3435T and G2677T/A with develoment and prognosis of colorectal cancer patients in a Chinese population. Our multivariable logistic analysis showed that CC genotype of ABCB1 C3435T was associated with an increased risk of colorectal cancer, and this genotype was significantly associated with increased risk of death from colorectal cancer.
Several previous studies showed that CC genotype or CT genotype could influence the susceptibility and clinical outcome of colorectal cancer, which are in line with our results [8,22,23]. Andersen et al. conducted a prospective case-cohort study, and found that C allele of ABCB1 C3435T was significantly associated with risk of colorectal cancer, and has interaction with meat intake in relation to colorectal cancer risk [22]. Khedri et al. reported that significantly increased frequencies of the 3435T allele and the 3435T were observed in patients with colorectal cancer compared with controls [23]. However, Bae et al. reported conducted a case-control study with 111 patients with colorectal cancer and 93 cancer-free cases, and found that no significant association between ABCB1 C3435T and colorectal cancer risk [24]. De Iudicibus et al. reported that G2677T and C3435T polymorphisms are not associated with colorectal cancer risk and prognosis in an Italian population [25]. The inconsistent role of these results may be caused by different ethnicities, sample size, control subjects or by chance. Further studies are greatly needed to elucidate the potential role of ABCB1 C3435T polymorphism tumor biology.
This study has several major limitations. First, were several limitations in our study. First, cases were selected from one hospital, which may not be representative of the general population. There was still a certain risk of selection bias since they were not a random sample of the colorectal cancer population and may not well represent the real situation of these patients. Second, the relative small number of colorectal cancer cases could limit the statistical power to find the difference between groups. Third, other genes may affect the susceptibility and clinical outcome of colorectal cancer patients besides the ABCB1 gene. Therefore, further large sample and well designed studies are greatly needed to investigate the association between ABCB1 gene polymorphisms and clinical outcome of colorectal cancer.
In conclusion, we found that the ABCB1 C3435T polymorphism could affect the susceptibility and clinical outcome of colorectal cancer patients. Our study provides significant information on detective and prognostic value of ABCB1 C3435T, and ABCB1 C3435T polymorphism could be used as predictive markers for susceptibility and clinical outcome of colorectal cancer patients.
Disclosure of conflict of interest
None.
References
- 1.GLOBOCAN 2012. Colorectal Cancer. Estimated Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 2.Pourhoseingholi MA. Increased burden of colorectal cancer in Asia. World J Gastrointest Oncol. 2012;4:68–70. doi: 10.4251/wjgo.v4.i4.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sridharan M, Hubbard JM, Grothey A. Colorectal cancer: how emerging molecular understanding affects treatment decisions. Oncology (Williston Park) 2014;28:110–8. [PubMed] [Google Scholar]
- 4.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 5.Leonessa F, Clarke R. ATP binding cassette transporters and drug resistance in breast cancer. Endocr Relat Cancer. 2003;10:43–73. doi: 10.1677/erc.0.0100043. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson LR, De Flora S. Multiple drug resistance, antimutagenesis and anticarcinogenesis. Mutat Res. 2005;591:24–33. doi: 10.1016/j.mrfmmm.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S, Lan LB, Schuetz JD, Schuetz EG, Wilkinson GR. Identification of functionally variant MDR1 alleles among EuropeanAmericans and African Americans. Clin Pharmacol Ther. 2001;70:189–99. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 8.Samanian S, Mahjoubi F, Mahjoubi B, Mirzaee R, Azizi R. MDR1 gene polymorphisms: possible association with its expression and clinicopathology characteristics in colorectal cancer patients. Asian Pac J Cancer Prev. 2011;12:3141–5. [PubMed] [Google Scholar]
- 9.De Mattia E, Toffoli G, Polesel J, D’Andrea M, Corona G, Zagonel V, Buonadonna A, Dreussi E, Cecchin E. Pharmacogenetics of ABC and SLC transporters in metastatic colorectal cancer patients receiving first-line FOLFIRI treatment. Pharmacogenet Genomics. 2013;23:549–57. doi: 10.1097/FPC.0b013e328364b6cf. [DOI] [PubMed] [Google Scholar]
- 10.Andersen V, Vogel U, Godiksen S, Frenzel FB, Sæbø M, Hamfjord J, Kure E, Vogel LK. Low ABCB1 gene expression is an early event in colorectal carcinogenesis. PLoS One. 2013;8:e72119. doi: 10.1371/journal.pone.0072119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue AM, Xie ZB, Zhao HF, Guo SP, Shen YH, Wang HP. Associations of ABCB1 and XPC genetic polymorphisms with susceptibility to colorectal cancer and therapeutic prognosis in a Chinese population. Asian Pac J Cancer Prev. 2013;14:3085–91. doi: 10.7314/apjcp.2013.14.5.3085. [DOI] [PubMed] [Google Scholar]
- 12.Sun ZW, Wang XC, Gao J, Li J, Li YY, Dang YZ, Shen L. Correlation of MDR1 and ABCG2 genetic polymorphisms with the efficacy and adverse events of irinotecan chemotherapy in patients with colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2013;16:524–8. [PubMed] [Google Scholar]
- 13.Wu H, Kang H, Liu Y, Xiao Q, Zhang Y, Sun M, Liu D, Wang Z, Zhao H, Yao W, Jia T, Wang E, Zheng Z, Wei M. Association of ABCB1 genetic polymorphisms with susceptibility to colorectal cancer and therapeutic prognosis. Pharmacogenomics. 2013;14:897–911. doi: 10.2217/pgs.13.78. [DOI] [PubMed] [Google Scholar]
- 14.Jia Y, Tian W, Sun S, Han P, Xue W, Li M, Liu Y, Jiang S, Cui B. The influence of genetic polymorphisms in MDR1 gene on breast cancer risk factors in Chinese. Med Oncol. 2013;30:601. doi: 10.1007/s12032-013-0601-0. [DOI] [PubMed] [Google Scholar]
- 15.Cortejoso L, García MI, García-Alfonso P, González-Haba E, Escolar F, Sanjurjo M, López-Fernández LA. Differential toxicity biomarkers for irinotecan- and oxaliplatin-containing chemotherapy in colorectal cancer. Cancer Chemother Pharmacol. 2013;71:1463–72. doi: 10.1007/s00280-013-2145-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L, Li K, Li W, Yang Z. Association between the C3435T polymorphism of ABCB1/MDR1 gene (rs1045642) and colorectal cancer susceptibility : a meta-analysis based on 11,339 subjects. Tumour Biol. 2013;34:1949–57. doi: 10.1007/s13277-013-0740-0. [DOI] [PubMed] [Google Scholar]
- 17.He T, Mo A, Zhang K, Liu L. ABCB1/MDR1 gene polymorphism and colorectal cancer risk: a meta-analysis of case-control studies. Colorectal Dis. 2013;15:12–8. doi: 10.1111/j.1463-1318.2012.02919.x. [DOI] [PubMed] [Google Scholar]
- 18.Fromm MF. The influence of MDR1 polymorphisms on P-glycoprotein expression and function in humans. Adv Drug Deliv Rev. 2002;54:1295–310. doi: 10.1016/s0169-409x(02)00064-9. [DOI] [PubMed] [Google Scholar]
- 19.Leschziner GD, Andrew T, Leach JP, Chadwick D, Coffey AJ, Balding DJ, Bentley DR, Pirmohamed M, Johnson MR. Common ABCB1 polymorphisms are not associated with multidrug resistance in epilepsy using a gene-wide tagging approach. Pharmacogenet Genomics. 2007;17:217–20. doi: 10.1097/01.fpc.0000230408.23146.b1. [DOI] [PubMed] [Google Scholar]
- 20.Clarke R, Leonessa F, Trock B. Multidrug resistance/P-glycoprotein and breast cancer: review and meta-analysis. Semin Oncol. 2005;32(Suppl):S9–15. doi: 10.1053/j.seminoncol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Tahara T, Arisawa T, Shibata T, Hirata I, Nakano H. Multi-drug resistance 1 polymorphism is associated with reduced risk of gastric cancer in the Japanese population. J Gastroenterol Hepatol. 2007;22:1678–82. doi: 10.1111/j.1440-1746.2007.04848.x. [DOI] [PubMed] [Google Scholar]
- 22.Andersen V, Ostergaard M, Christensen J, Overvad K, Tjønneland A, Vogel U. Polymorphisms in the xenobiotic transporter multidrug resistance 1 (MDR1) and interaction with meat intake in relation to risk of colorectal cancer in a Danish prospective case-cohort study. BMC Cancer. 2009;9:407. doi: 10.1186/1471-2407-9-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khedri A, Nejat-Shokouhi A, Salek R, Esmaeili H, Mokhtarifar A, Entezari Heravi R, Tatari F, Behravan J, Miladpour B, Omidvar Tehrani S. Association of the colorectal cancer and MDR1 gene polymorphism in an Iranian population. Mol Biol Rep. 2011;38:2939–43. doi: 10.1007/s11033-010-9957-9. [DOI] [PubMed] [Google Scholar]
- 24.Bae SY, Choi SK, Kim KR, Park CS, Lee SK, Roh HK, Shin DW, Pie JE, Woo ZH, Kang JH. Effects of genetic polymorphisms of MDR1, FMO3 and CYP1A2 on susceptibility to colorectal cancer in Koreans. Cancer Sci. 2006;97:774–9. doi: 10.1111/j.1349-7006.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Iudicibus S, De Pellegrin A, Stocco G, Bartoli F, Bussani R, Decorti G. ABCB1 gene polymorphisms and expression of P-glycoprotein and long-term prognosis in colorectal cancer. Anticancer Res. 2008;28:3921–8. [PubMed] [Google Scholar]


