Abstract
Aquaporin-4 (AQP4) is the most popular water channel protein expressed in brain tissue and plays a very important role in regulating the water balance in and outside of brain parenchyma. To investigate the expression of aquaporin-4 in the rat brain tissue after dexamethasone therapy of meningitis induced by Streptococcus pneumonia, total 40 of 3-week old Sprague-Dawley rats were divided into infection group (n=30) and normal control group (n=10). The meningitis groups were infected with 1×107 cfu/ml of Streptococcus pneumoniae and then randomized into no treatment (untreated group, n=10), treatment with ceftriaxone (CTRX group, n=10) and treatment with dexamethasone combined ceftriaxone (CTRX + DEXA group, n=10). The normal control group was established by using saline. The rats were euthanized when they reached terminal illness or five days after infection, followed by detection of AQP4 through using immunohistochemistry and Western blot methods. Data has showed that expression of AQP4 in model group remained higher than the control and treatment group (P<0.05). AQP4 expression in CTRX + DEXA group was lower than that in CTRX group (P<0.05). There was no statistical difference between CTRX + DEXA group and the control group (P>0.05). These data suggested that Dexamethasone could down-regulate the expression of AQP4 in the brain tissue of rats with meningitis and provides evidence for the mechanism of protective effect of Dexamethasone on central neurosystem.
Keywords: Dexamethasone, bacterial meningitis, aquaporin-4
Introduction
Meningitis is a very important acute infectious disease of the central nervous system and remains a major challenge globally, especially for neonatal and children patients [1]. Bacterial infection is the major cause of meningitis in children younger than 5 years old. Among the major pathogens, Gram-positive bacteria Streptococcus pneumoniase is a very common and aggressive pathogen and culprit for about 6% of all cases [2]. Despite all the advances of the antimicrobial therapy, bacterial meningitis patients’ long-term sequel, including blindness, deafness, cerebral palsy, seizures, hydrocephalus impairments are still high after recover and present in approximately 25-50% of survivor [3-5]. A major complication associated with poor outcome in bacterial meningitis is brain edema, which could cause the intracranial pressure to rise and leading to brain herniation, ischemia and even death [6]. The molecular mechanisms responsible for the brain edema with bacterial meningitis are poorly understood. Meta-analysis has shown that adjunctive administration of dexamethasone in the treatment of children with acute bacterial meningitis could significantly reduce the fever days, hearing impairment and reactive arthritis and without increase the risks of serious adverse events [7]. At the same time there is also some controversy about the application of dexamethasone [8,9]. It is known that dexamethasone has the ability to decrease pro-inflammatory cytokines and inhibits the reactive oxygen spices produced by leukocytes thus could do a favorable effect during the treatment as adjunctive therapy [10], while the underlying mechanism is not fully understand yet.
Aquaporin-4 (AQP4) is one of the most popular water channel protein expressed in brain tissue and play a significant important role in keeping water balance and regulation of water distribution of the brain [11]. It is well recognized that hippocampus along with cortex are the two brain structures which were prominently affected in bacterial meningitis. Nau’s group observed the apoptosis of granular cells in the dentate gyrus frequently occurs in the autopsy cases [12]. We want to explore if AQP4 also plays a role in the hippocampus during the bacterial meningitis by detecting the expression changes in the rat bacterial meningitis model to better understanding the mechanism of dexamethasone on brain tissue under the bacterial meningitis context.
Material and methods
Reagents and bacterial strain
streptococcus pneunoniae serotype III was purchased from CMCC (stain number 31003). All other reagents were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA) or specifically marked.
Experimental animals
Total 40 mixed gender of 3 weeks old Sprague-Dawley rats were used, weighting approximately 60-80 g. Animals were randomly assigned to one of these four groups (n=10 each): 1) saline control group; 2) untreated group; 3) ceftriaxone (CTRX) group; 4) CTRX + dexamethasone (DEXA) group. All groups were introduced with bacteria injection into the cisterna magna except for saline control group, which only got injected with saline. After 24 hours of injection of bacteria, animals were treated differently according to the group assign. Control group use 2ml of saline injected intraperitoneally. Dose of the subcutaneous injection of ceftriaxone and intraperitoneally administration of dexamethasone are 100 mg/kg/d and 3 mg/kg/d, respectively. Medical treatment continued for three days and animals were sacrificed at day five.
Bacterial meningitis model
Animal model were established based on method published previously [13]. Briefly speaking, rats were anesthetized with 10% chloral hydrate (200 mg/kg) injected intraperitoneally. The head was then secured with a stereotactic frame and after the injection needle reached cisterna magna, move away 50 microliter of cerebrospinal fluid and inject 50 ul (1×107/ml CFU) of streptococcus pneumoniae. After 24 h of injection, cisternal cerebrospinal fluid was drawn and leukocytes numbers were counted and neurological examinations were performed every 12 hours based on Longza et al literature [14]. Specifically, the neurologic findings were scored on this five-point scale: score 0 represents no neurologic deficit; a score of 1 (failure to extend left forepaw fully) represents a mild focal neurological deficit; a score of 2 (circling to the left) means a moderate focal neurologic deficit and a score of 3 (falling to the left) represents for a severe focal deficit. Rats with a score of 4 could not walk spontaneously and has a depressed level of consciousness. Rats with score above 1 are considered neurologic deficit.
Immunohistochemistry assay of APQ4
Rats were sacrificed by decapitation and the left hemisphere was conventional formaldehyde-fixed (10%) for 24 h and then went through steps of paraffin embedding. The right side samples were frozen in -80°C for western blot assay. Specifically, deparaffinated and rehydrated brain tissue were serially sectioned into 5 μm thickness slices through hippocampus at the coronal direction. All sections were incubated with 3% HO 2 in Tris-buffered saline (PBS) for 10 min at room temperature to quench endogenous peroxidase. After that, the sections were heated with microwave in citrate buffer (0.016 M citric acid and 0.084 M sodium citrate) adjustment of the buffer between each heating session. The slides were incubated with 5% bovine serum albumin (BSA) dissolved in PBS for 10 min at room temperature to block non-specific binding. Then the sections were incubated with the anti-aquaporin monoclonal antibodies (1:200 dilution. Bostar) overnight at 4°C. Following completely rinses, the samples were incubated for 1 hour with horseradish peroxidase-conjugated goat anti-rabbit I gG (1:500 dilution. Boster). After washing, slides were incubated in 3, 3-diaminobenzidine (DAB) (0.5 mg/ml in PBS) for 5 minutes at room temperature. In some case we have used the Boster Kits. Finally the sections were washed in distilled water, dehydrated with ethanol, cleaned in xylene and mounted permanently. Quantification of the staining intensity by acquiring 10 fields under high magnification on each slide with Olympus microscope (CX31-LV320) and assessing the IOD (integrated optical density) with Image-pro Plus software (version 6.0. PA, USA).
Western blotting analysis
The infrared imager (Li-Cor Odyssey, NA, USA) was used to detect and quantify the AQP4 protein expression in brain. Specifically, the right-side frozen hemisphere of hippocampus was dissected out and mechanically homogenized. Protein concentration was determined using the Pierce BCA protein assay kit (Thermo Scientific Inc. USA). For Western blots, 80 microgram of protein extracts was loaded into Nupage Bis-Tris gel (4%-12%. Invitrogen, USA) and run under 80 volts for 2 hours. Then protein was transferred onto PVDF Immobilon Membrane (Millipore, USA) at 4°C overnight. To identify AQP4, rabbit anti-rat AQP4 polyclonal primary antibody (1:400, Bostar, China) was used. Membrane was then incubated with infrared dye conjugated goat anti-rabbit (1:20000, IRDye 800). Data was acquired by using the Odyssey Infrared Imaging System, where signal intensities were determined using Li-Cor imaging software and exported to Microsoft Excel.
Data analysis
Data were expressed as mean ± standard deviation. Comparisons of signal intensity among different groups were performed with ANOVA (analysis of variance) followed by Newman-Keuls test. P value less than 0.05 is considered as significant difference.
Results
Rat bacterial meningitis model
After rat were infected with streptococcus pneumonia, animal started to show neurological disorder symptoms from mild to severe extent, including weariness, reduced feeding and spontaneous activity, drowsiness, fast, paralysis, ataxia, seizures, coma and ever death. Among all these rats, four were found dead in untreated group and one found dead in CTRX group, while the other two groups didn’t record any mortality. The saline control group didn’t show any visible neurological symptoms and thus the score was 0. All others groups score were indicated in Table 1. The average score for 24 hours after infection were similar among different groups. Then after intervention, score of CTRX + DEXA group drops the most, from 2.80±0.72 to 0.90±1.0. The other two groups’ score also reduced accordingly.
Table 1.
Neurological scores of different groups after infection for 24 hours and 5 days
| Group | 24 h after infection | 5 days after infection |
|---|---|---|
| Untreated | 2.70±0.67 | 3.33±0.81 |
| Ceftriaxone treatment | 2.90±0.74 | 1.44±1.42* |
| Ceftriaxone + Dexamethasone treatment | 2.80±0.72 | 0.90±1.10* |
compared to untreated group, P value is less than 0.05.
AQP-4 protein expression in hippocampus
AQP4 protein was observed in all these tested hippocampus tissue with different expression intensity. Compare to CTRX treatment and untreated group, AQP4 expression in CTRX + DEXA group has been significantly reduced (P<0.05), while it didn’t have significant difference with saline control group (Table 2, Figure 1).
Table 2.
AQP4 protein expression in different groups
| Group | N number | AQP4 IOD value |
|---|---|---|
| Saline control | 10 | 43.52±5.71 |
| Untreated | 6 | 119.45±13.13* |
| Ceftriaxone treatment | 9 | 82.15±11.27 |
| Ceftriaxone + Dexamethasone treatment | 10 | 74.45±9.26* |
compared to CTRX group, P value is less than 0.05.
Figure 1.
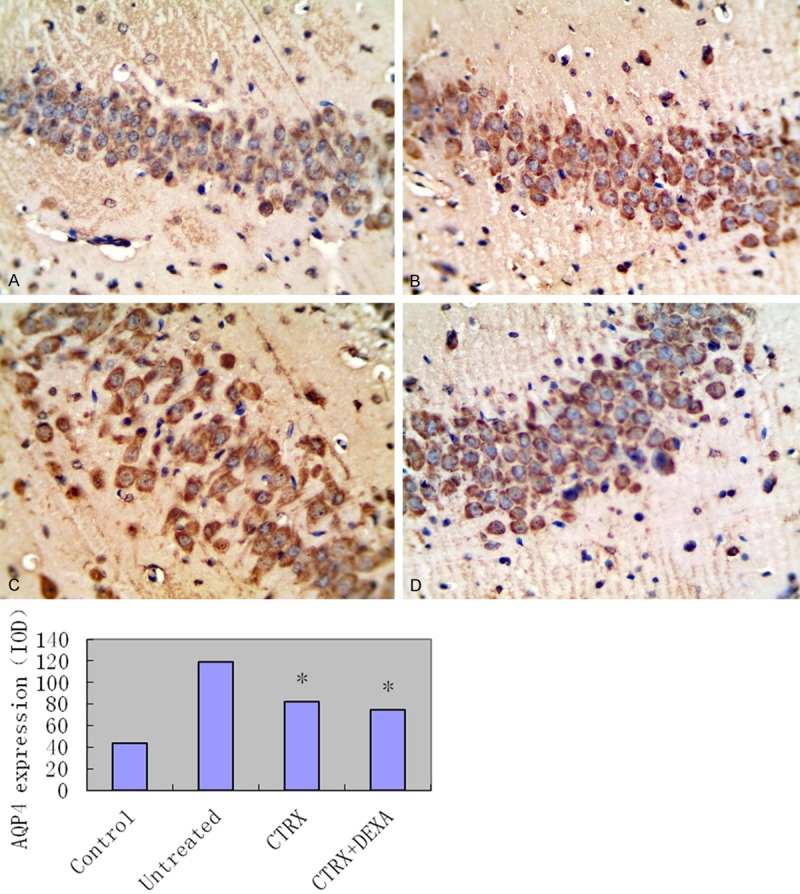
Upper panel: representative picture of the Immunohistochemistry stain for AQP4 expression of hippocampus tissue in different treatment groups. A: Hippocampus tissue of saline control group; B: Hippocampus tissue of untreated group; C: Hippocampus tissue of CTRX treatment group; D: Hippocampus tissue of CTRX + DEXA treatment group. All slides were using DAB as the chromogenic reagent and counterstained with hematoxylin. All pictures were taken under high magnification of 400X. Lower panel: Integrated optical density of immunohistochemistry of Aquaporin-4 (AQP4) expressions in four groups: 1 saline control group; 2 untreated group; 3 Ceftriaxone treatment group (CTRX group); 4 Ceftriaxone plus Dexamethasone group (CTRX + DEXA group). Compared CTRX group with both untreated and CTRX + DEXA group, P value is less than 0.05. Compared CTRX + DEXA group with saline group, P value is more than 0.05.
Western blot
Tissue level of AQP-4 expression showed CTRX and untreated groups have higher expression of AQP4 than saline control and CTRX + DEXA group and the difference are statistically significant (Figure 2).
Figure 2.
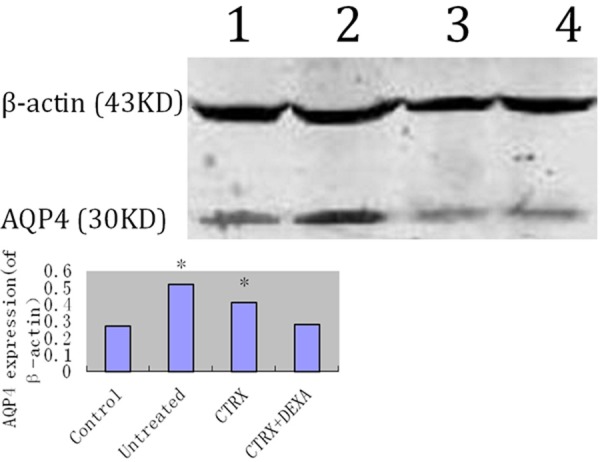
Upper panel: representative western blotting for AQP4 expression. Lane 1: saline control group; Lane 2 untreated group; Lane 3: ceftriaxone treatment group (CTRX group); Lane 4: ceftriaxone plus dexamethasone group (CTRX + DEXA group). Lower panel: integrated intensity ratio of AQP4 protein in different groups normalized to internal control. Compared CTRX group with both untreated and CTRX + DEXA group, P value is less than 0.05. Compared CTRX + DEXA group with saline control group, P value is more than 0.05.
Discussion
Bacterial meningitis is a very common and severe infectious disease in pediatric patients. Mortality could exceed 5% and approximately 10% patients suffer permanent central nerve system sequelae [15]. In this study, we injected streptococcus pneumonia into the rat intracisternal ventricle to induce meningitis. 24 hours after injection, all of the rats have shown different extend of neurological symptoms and the neurological score are similar, except for the saline control group, which indicated that the model was introduced successfully. After the bacterial infected brain tissue, brain edema could be a major complication associated with the unfavorable outcome of bacterial meningitis. Cytotoxic and vasogenic edema are two main types of brain edema and both are thought to co-exist in meningitis [16].
The presence of water channel, aquaporins, within the neurovascular unit has let to intensive research in understanding the underlying roles of AQPs under normal condition and in different diseases. Corticosteroids are drugs that can reduce the inflammation caused by infection. Although researches on the use of corticosteroid in addition to antibiotics had conflicting results [9], there is a distinguished body of literature have shown that using Dexamethasone as a conjunctive treatment could reduce the neurological sequelae and hearing loss in children patients [5,7,17]. In recent years, experimental studies with animal models have shown that including dexamethasone, generic targeting and/or pharmacological blockage of related signal pathways hold major promises for the adjunctive treatment of acute bacterial meningitis [8]. Yang et al [18] found that over-expression of AQP4 in mice brain glial cell could result in accelerated progression of cytotoxic brain swelling and thus AQP4 expression is rate-limiting for brain water accumulation, which indicate that altered AQP4 expression can be functionally significant. Not only in normal brain tissue, Saadoun et al [19] also found that in high-grade astrocytomas and adenocarcinomas patient’s brain tissue, there is increased expression of AQP4 and this increase has a significant correlation between blood-brain barrier openings. Review paper [20] has stated that AQPs, and in particular AQP4, have important role in the resolution of edema after brain injury.
In our study, after the brain tissue was infected with S. pneumonia for 24 hours, CTRX + DEXA group decreased the expression of AQP4 in hippocampus and had significantly difference compared with either CTRX or untreated groups, while no significant difference was observed between this group and the saline control group. This data indicate that ceftriaxone plus dexamethasone treatment might reduce brain edema by decrease the expression of AQP4. While the underlying mechanism of this protective effects of dexamethasone on brain edema through the adjusted expression of AQP4 still remain unclear. It has shown that the activation of protein-kinase C (PKC) could relieve brain edema by reducing AQP4 in both in vivo and in vitro studies [20-24]. And PKC was found to be one of the short-term regulators of AQP4 via phosphorylation on a consensus PKC phosphorylation site and the effect is likely mediated by gating of the water channel [25,26]. Previous studies demonstrated that Dexamethasone treatment could decrease osmotic changes after brain injury by reducing inflammatory cytokines [27]. Thus on the basis of our results, we speculate that due to the powerful antiinflammation effects of dexamethasone, it could reduce the releasing of inflammatory cytokines and activate the phosphorylation of PKC on AQP4 protein channel, down-regulate the mRNA expression of AQP4, thus reduce the water channel function of AQP4, which resulted in reduced edema of brain. The results support the idea of applying Dexamethasone in the early treatment stage of bacterial meningitis with less brain edema occurrence, better survival rate and less severe sequelae.
Acknowledgements
This work was supported by the Science and Technology Research Key Project Grand from Education Department of Henan Province.
Disclosure of conflict of interest
None.
References
- 1.Grandgirard D, Leib SL. Meningitis in neonates: bench to bedside. Clin Perinatol. 2010;373:655–676. doi: 10.1016/j.clp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Heath PT, Nik Yusoff NK, Baker CJ. Neonatal meningitis. Arch Dis Child Fetal Neonatal Ed. 2003;883:F173–8. doi: 10.1136/fn.88.3.F173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Louvois J, Halket S, Harvey D. Neonatal meningitis in England and Wales: sequelae at 5 years of age. Eur J Pediatr. 2005;16412:730–734. doi: 10.1007/s00431-005-1747-3. [DOI] [PubMed] [Google Scholar]
- 4.Lepage P, Dan B. Infantile and childhood bacterial meningitis. Handb Clin Neurol. 2013;112:1115–1125. doi: 10.1016/B978-0-444-52910-7.00031-3. [DOI] [PubMed] [Google Scholar]
- 5.van de Beek D, Brouwer MC, Thwaites GE, Tunkel AR. Advances in treatment of bacterial meningitis. Lancet. 2012;3809854:1693–1702. doi: 10.1016/S0140-6736(12)61186-6. [DOI] [PubMed] [Google Scholar]
- 6.Saez-Llorens X, McCracken GH Jr. Bacterial meningitis in children. Lancet. 2003;3619375:2139–2148. doi: 10.1016/S0140-6736(03)13693-8. [DOI] [PubMed] [Google Scholar]
- 7.van de Beek D, Farrar JJ, de Gans J, Mai NT, Molyneux EM, Peltola H, Peto TE, Roine I, Scarborough M, Schultsz C, Thwaites GE, Tuan PQ, Zwinderman AH. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol. 2010;93:254–263. doi: 10.1016/S1474-4422(10)70023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koedel U, Scheld WM, Pfister HW. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis. 2002;212:721–736. doi: 10.1016/s1473-3099(02)00450-4. [DOI] [PubMed] [Google Scholar]
- 9.Peltola H, Roine I. Improving the outcomes in children with bacterial meningitis. Curr Opin Infect Dis. 2009;223:250–255. doi: 10.1097/QCO.0b013e328329c47a. [DOI] [PubMed] [Google Scholar]
- 10.Dandona P, Mohanty P, Hamouda W, Aljada A, Kumbkarni Y, Garg R. Effect of dexamethasone on reactive oxygen species generation by leukocytes and plasma interleukin-10 concentrations: a pharmacodynamic study. Clin Pharmacol Ther. 1999;661:58–65. doi: 10.1016/S0009-9236(99)70054-8. [DOI] [PubMed] [Google Scholar]
- 11.Zador Z, Bloch O, Yao X, Manley GT. Aquaporins: role in cerebral edema and brain water balance. Prog Brain Res. 2007;161:185–194. doi: 10.1016/S0079-6123(06)61012-1. [DOI] [PubMed] [Google Scholar]
- 12.Nau R, Soto A, Bruck W. Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J Neuropathol Exp Neurol. 1999;583:265–274. doi: 10.1097/00005072-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Papadopoulos MC, Verkman AS. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J Biol Chem. 2005;28014:13906–13912. doi: 10.1074/jbc.M413627200. [DOI] [PubMed] [Google Scholar]
- 14.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;201:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 15.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;399:1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 16.Klatzo I. Evolution of brain edema concepts. Acta Neurochir Suppl (Wien) 1994;60:3–6. doi: 10.1007/978-3-7091-9334-1_1. [DOI] [PubMed] [Google Scholar]
- 17.McIntyre PB, Berkey CS, King SM, Schaad UB, Kilpi T, Kanra GY, Perez CM. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA. 1997;27811:925–931. doi: 10.1001/jama.278.11.925. [DOI] [PubMed] [Google Scholar]
- 18.Yang B, Zador Z, Verkman AS. Glial cell aquaporin-4 overexpression in transgenic mice accelerates cytotoxic brain swelling. J Biol Chem. 2008;28322:15280–15286. doi: 10.1074/jbc.M801425200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002;722:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu SM, Xiong XX, Zheng YY, Pan CF. Propofol inhibits aquaporin 4 expression through a protein kinase C-dependent pathway in an astrocyte model of cerebral ischemia/reoxygenation. Anesth Analg. 2009;1095:1493–1499. doi: 10.1213/ANE.0b013e3181b893f3. [DOI] [PubMed] [Google Scholar]
- 21.Kleindienst A, Fazzina G, Amorini AM, Dunbar JG, Glisson R, Marmarou A. Modulation of AQP4 expression by the protein kinase C activator, phorbol myristate acetate, decreases ischemia-induced brain edema. Acta Neurochir Suppl. 2006;96:393–397. doi: 10.1007/3-211-30714-1_81. [DOI] [PubMed] [Google Scholar]
- 22.de Louvois J, Halket S, Harvey D. Neonatal meningitis in England and Wales: sequelae at 5 years of age. Eur J Pediatr. 2005;164:730–734. doi: 10.1007/s00431-005-1747-3. [DOI] [PubMed] [Google Scholar]
- 23.Nakahama K, Nagano M, Fujioka A, Shinoda K, Sasaki H. Effect of TPA on aquaporin 4 mRNA expression in cultured rat astrocytes. Glia. 1999;253:240–246. [PubMed] [Google Scholar]
- 24.Yamamoto N, Sobue K, Miyachi T, Inagaki M, Miura Y, Katsuya H, Asai K. Differential regulation of aquaporin expression in astrocytes by protein kinase C. Brain Res Mol Brain Res. 2001;95:110–116. doi: 10.1016/s0169-328x(01)00254-6. [DOI] [PubMed] [Google Scholar]
- 25.Han Z, Wax MB, Patil RV. Regulation of aquaporin-4 water channels by phorbol ester-dependent protein phosphorylation. J Biol Chem. 1998;27311:6001–6004. doi: 10.1074/jbc.273.11.6001. [DOI] [PubMed] [Google Scholar]
- 26.Zelenina M, Zelenin S, Bondar AA, Brismar H, Aperia A. Water permeability of aquaporin-4 is decreased by protein kinase C and dopamine. Am J Physiol Renal Physiol. 2002;2832:F309–18. doi: 10.1152/ajprenal.00260.2001. [DOI] [PubMed] [Google Scholar]
- 27.Sugimura Y, Murase T, Takefuji S, Hayasaka S, Takagishi Y, Oiso Y, Murata Y. Protective effect of dexamethasone on osmotic-induced demyelination in rats. Exp Neurol. 2005;1921:178–183. doi: 10.1016/j.expneurol.2004.10.018. [DOI] [PubMed] [Google Scholar]


