Abstract
Background: Microalbuminuria is the earliest clinical sign of diabetic nephropathy (DN). However, earlier markers as a diagnostic tool for DN was required for the invalid of microalbuminuria in some cases. Osteoinductive factor (OIF) was known to be an essential component of the normal vascular matrix. We aimed to research the relationship between DN and OIF, and discussed the availability of the serological markers for earlier stage of DN. Method: One hundred twenty Chinese subjects, who included patients with type 2 diabetes mellitus (T2DM), DN with microalbuminuria, and DN with macroalbuminuria, as well as healthy controls, were enrolled in this study. Serum OIF levels were examined by ELISA and other clinical biochemical parameters were tested based on standard methods. Results: Our results indicated that, serum OIF levels were significantly increased in DN subjects compared with healthy and T2DM subjects (P < 0.05 respectively). However, no significant changes in serum OIF levels were found between T2DM and healthy subjects. Furthermore, serum OIF had negative correlation with estimated glomerular filtration rate (eGFR) and positive correlation with blood urea nitrogen(BUN) and creatinine. ROC curve analysis showed that serum OIF level was a good sensitive and specificity marker for microalbuminuria and early renal damage with sensitivity of 86.7% and specificity of 95%, as well as for macroalbuminuria and damage progress with sensitivity of 90% and specificity of 95%. Conclusion: OIF may be an indicator of the earlier-stage DN in subjects with T2DM. Understanding the exact mechanism of up-regulated OIF in subjects with DN requires further study.
Keywords: Diabetic nephropathy, osteoinductive factor, early diagnosis, biomarker
Introduction
Diabetic nephropathy (DN) is a diabetic syndrome called chronic capillaries pathological change and has become the leading cause of end stage renal diseases in diabetic patients from Asia, Europe and United States [1,2]. According to the present diabetic nephropathy definition with the presence of proteinuria > 0.5 g/24 h, it was commonly so late that patients already has overt nephropathy, clinical nephropathy, proteinuria, or macroalbuminuria at this time [3]. Therefore, diagnosis earlier and reasonable treatments for patients at risk are of extremely concernment and in urgent need. To date, diabetic nephropathy has been categorized into microalbuminuria and macroalbuminuria stages based on the values of urinary albumin excretion [3]. Accumulating reports suggested that the risk of patients with type 2 diabetes and microalbuminuria developing to diabetic nephropathy was 10 to 20 times than that of patients with normoalbuminuria [4,5]. Although microalbuminuria has been globally considered as a risk factor for macroalbuminuria and widely used for diagnosing diabetic nephropathy, not all patients progress to this stage and some may regress to normoalbuminuria [6]. Thus, an earlier and more sensitive marker was demanded to predict the development of diabetic nephropathy and help us screen the patients with more advanced stages of diabetic nephropathy.
Osteoinductive factor (OIF) belongs to the small leucine-rich repeat proteoglycan (SLRP) family and is a secretory protein [7]. Because osteoinductive factor was initially isolated and found to induce ectopic bone formation, it was also named osteoglycin [8,9]. It has been reported that osteoinductive factor exerted this function through associating with TGFβ-like bone morphogenetic proteins [10]. In addition, osteoinductive factor was known to be an essential component of the normal vascular matrix. Catherine M. Shanahan demonstrated that osteoinductive factor was highly expressed in differentiated adult rat vascular smooth muscle cells (VSMCs) but downregulated in VSMCs that had undergone proliferation in vitro, which indicated that osteoinductive factor may be a new marker of differentiated VSMCs [11]. Except for the important roles in regulation of capillaries, osteoinductive factor also could regulate lipid metabolism, sugar metabolism and energy metabolism. However, whether osteoinductive factor levels could be attributed to the attack and development of DN from the early stages of diabetes is still unclear.
Therefore, we undertook studies to identify the osteoinductive factor in patients with type 2 diabetes with different stages of nephropathy in order to assess their significance as markers of early renal dysfunction.
Materials and methods
Subjects
This study included a total of 120 Chinese subjects contained those with type 2 diabetic mellitus (T2DM, n = 90), and their respective age and sex-matched controls (n = 30), and they were recruited from the Henan Provincial People’s Hospital of Zhengzhou University. T2DM was diagnosed according to American Diabetic Association criteria. All healthy subjects were selected based on the results of a physician’s questionnaire and laboratory tests. The study was approved by the local ethical committee of Henan Provincial People’s Hospital of Zhengzhou University and informed consent was obtained from every subject.
Clinical data and laboratory test
Clinical examination and assessment of body mass index (BMI) were performed. Blood pressure was measured 3 times, and the average value was considered for data analysis. Serum and early morning urine samples were collected, centrifuged, aliquoted and stored at -80°C until various routine laboratory test and quantification by ELISA.
Glycosylated haemoglobin (HbA1c) was quantitatively detected by ion exchange chromatography (Stanbio Laboratory). Fasting plasma glucose (FBG) was estimated using glucose oxidase enzymatic assay (Bio Merieux). Creatinine in urine and lipid profile (total cholesterol, triglycerides, HDL-C, and LDL-C) were calculated by enzymatic colorimetry using Olympus AU 400 auto analyzer (Olympus Diagnostics, GmbH, Germany). Albumin in urine was measured by immunoturbidimetric method using Boerhinger reagents (Germany). Albumin/creatinine ratio was calculated through comparing the albumin in the sample against its creatinine concentration.
ELISA
The concentration of OIF in serum was measured with an ELISA kit (Zymed Laboratories Inc, USA) using ab126749 (Abcom, China) as the OIF-specific antibody. The standard curve was created using the suppliers’ OIF. And the assay was performed according to the manufacturer’s specifications.
Statistical analysis
Data were described as mean ± standard deviation. Comparison between the two groups was performed using the Student’s unpaired t-test. A receiver operating characteristics (ROC) analysis was performed to calculate the area under the curve (AUC) to find the best cutoff values providing the highest diagnostic specificity followed by the best sensitivity. All analyses were performed with Statistical Package for Social Sciences version 13.0 (SPSS, Chicago, IL). In all statistical tests, differences with P-values < 0.05 were considered as significant.
Results
Characteristics of study subjects
Based on the albumin/creatinine ratio, diabetic mellitus group was classified to Normoalbuminuric (albumin/creatinine ratio < 30 µg/mg, n = 30), Microalbuminuric (male: albumin/creatinine ratio 30-300 µg/mg, female: 400 µg/mg, n = 30) and Macroalbuminuric (male: albumin/creatinine ratio > 300 µg/mg, female: > 400 µg/mg, n = 30). Characteristics and laboratory data of patients and controls were described in Table 1.
Table 1.
Characteristics of patients and controls
| Parameter | Control (n = 30) | Normoalbuminuric (n = 30) | Microalbuminuric (n = 30) | Macroalbuminuric (n = 30) |
|---|---|---|---|---|
| Age (years) | 50.1±2.1 | 49.8±2.0 | 50.3±1.9 | 51.2±2.1 |
| Gender, male (%) | 60% | 70% | 65% | 63% |
| BMI (kg/m2) | 22.9±2.7 | 23.6±3.3 | 24.1±3.5 | 23.7±2.9 |
| SBP (mmHg) | 120.5±3.8 | 123.4±4.2 | 138.1±5.1*,# | 142.3±5.3*,# |
| DBP (mmHg) | 83.2±2.7 | 85.4±3.3 | 90.1±3.5* | 94.3±3.6*,# |
| TC (mmol/L) | 4.9±0.32 | 5.3±0.35 | 5.7±0.41* | 5.9±0.46*,# |
| HDL-C (mmol/L) | 1.33±0.08 | 1.25±0.06 | 1.11±0.06* | 1.02±0.04*,# |
| LDL-C (mmol/L) | 2.55±0.12 | 2.59±0.18 | 2.63±0.17 | 2.91±0.19*,# |
| TGs (mmol/L) | 1.35±0.13 | 1.83±0.25* | 2.01±0.24* | 2.18±0.27* |
| FBG (mmol/L) | 5.03±0.11 | 9.57±0.54* | 10.31±0.71* | 10.78±0.85* |
| HbA1c (%) | 5.25±0.5 | 7.8±0.72* | 8.12±0.78* | 8.2±0.81* |
| BUN (mmol/L) | 4.18±0.39 | 6.14±1.2* | 6.47±1.98* | 10.58±4.1*,#,& |
| Creatinine (mg/dL) | 62.3±4.2 | 65.7±5.97 | 67.3±8.5 | 150.4±23.1*,#,& |
| eGFR | 112.4±4.7 | 138.3±5.9* | 134.6±6.7* | 75.7±5.3*,#,& |
Notes: Data are presented as mean ± SD.
P < 0.05 versus control;
P < 0.05 versus Normoalbuminuric;
P < 0.05 versus Microalbuminuric.
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TGs, triglycerides; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; BUN, blood urea nitrogen; eGFR, Estimated glomerular filtration rate.
Compared to the health control and Normoalbuminuric, the other two DN patients group had significantly higher systolic pressure and diastolic blood pressure. While, higher fasting blood glucose, HbA1c and blood urea nitrogen were observed among T2DM patients comparing to the healthy. For lipid profiles, plasma concentrations of total cholesterol, low density lipoprotein cholesterol and triglycerides were a little higher in T2DM patients than in health controls; whereas HDL cholesterol levels were clearly lower in DN patients than that in healthy (Table 1). Notably, creatinine in Macroalbuminuric group was exactly significantly higher than the other three groups, but with the eGFR the opposite was the case.
OIF levels are increased in patients with DN, but not in T2DM
ELISA was performed to identify the serum concentration of osteoinductive factor among all subjects, and we found that OIF concentration in Microalbuminuric patients and Macroalbuminuric patients were significantly increased than that in Normoalbuminuric patients and healthy subjects (Figure 1).
Figure 1.
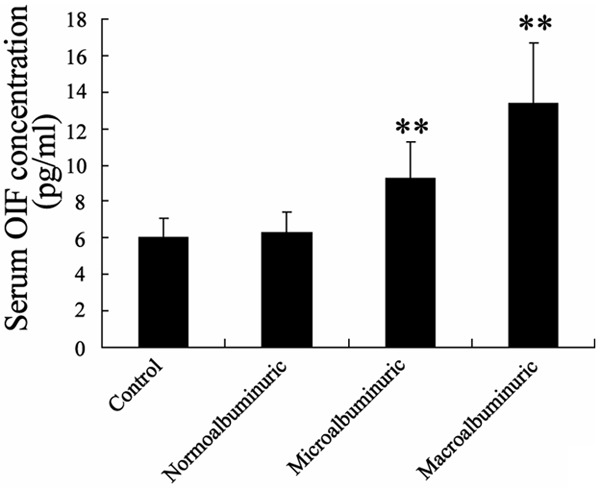
Serum concentration of osteoinductive factor (OIF) among Normoalbuminuric patients, Microalbuminuric patients, Macroalbuminuric patients and healthy subjects.
OIF levels were strongly associated with renal function in subjects with DN
Correlation studies revealed that OIF negative correlated with eGFP and here was positive correlation between OIF and creatinine, which were conventional biomarkers reflecting the decline of renal function in chronic kidney disease patients (Table 2).
Table 2.
Univariate correlations with serum OIF levels concentrations
| Parameter | r | P |
|---|---|---|
| Age | 0.15 | NS |
| Gender | -0.17 | NS |
| BMI | 0.11 | NS |
| SBP | 0.371 | 0.011 |
| DBP | 0.352 | 0.017 |
| TC | 0.291 | 0.032 |
| HDL-C | -0.307 | 0.03 |
| LDL-C | 0.267 | 0.041 |
| TGs | 0.205 | 0.09 |
| FBG | 0.2 | 0.092 |
| HbA1c | 0.231 | 0.076 |
| BUN | 0.524 | < 0.001 |
| Creatinine | 0.54 | < 0.001 |
| eGFR | -0.471 | < 0.005 |
OIF in T2DM patients may be a good predictor for DM
To investigate the diagnostic of microalbuminuria and macroalbuminuria in patients with diabetes, the ROC curve analysis of OIF levels in T2DM patients was performed. As showed in Figure 2A, the area under the curve (AUC) was 0.869 with a sensitivity of 86.7% and a specificity of 95% for microalbuminuria prediction. Meanwhile, there was an AUC of 0.955 with a sensitivity of 90% and a specificity of 95% for macroalbuminuria prediction (Figure 2B). Taken together, these data suggested that serum OIF levels of T2DM patients may be a good predictor for DN.
Figure 2.
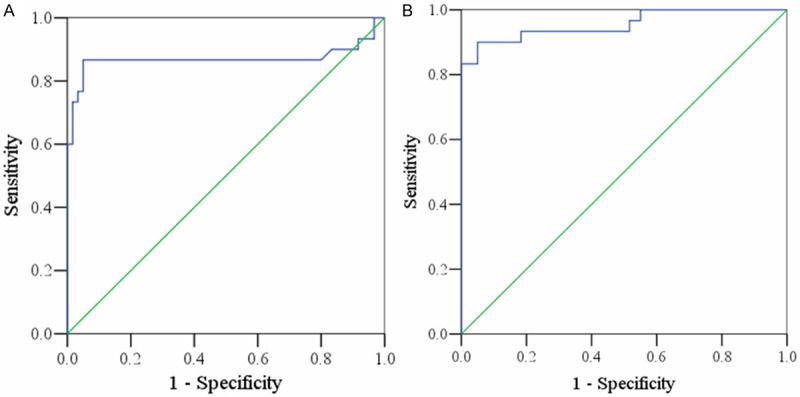
Receiver operating characteristic (ROC) curve analysis of OIF concentrations for prediction of microalbuminuria (A) and macroalbuminuria (B) in T2DM patients.
Discussion
To date, microalbuminuric has been an internationally admitted standard for DN and was excessively used on clinical [12]. Unfortunately, this standard easily interfered by excretion, sports, urinary tract infection, hypertension, heart function failure, urgent fever and so on [13]. Based on local community’s syndromes of diabetic patients, the former epidemiological investigation showed 44.3% of patients who got kidney functional failure (glomerular filtration rate < 60 ml/min/1.73 m2) were normoalbuminuric [6]. Thus, microalbuminuric still cannot completely demonstrate whether patients may get the risk of DN or not. It was strongly recommended for a more sensitive marker to predict DN in order to help us screen earlier stage DN.
Glomerular hypertrophy, increasing of extracellular matrix and glomerular sclerosis, were involved in the pathologically change of DN. It was usually displayed as high glomerular filtration, high injection state and changes in the glomerular filtration barrier. Many factors contributed to the occurring of DN, and the long term hyperglycemia caused by insulin metabolism disorder was a crucial reason [14]. Pathologically changes in kidney resulted from hyperglycemia rendering blood dynamics and abnormal glycometabolism. Moreover, the activation of several growth factors and cytokines was the direct account for the onset of DN. In addition, the functional abnormal of vascellum was an initiate reason for DN.
Sema Uslu et al reported that tubular involvement may be prior to glomerular involvement in DN patients, owing to several tubular proteins and enzymes were detectable even before the appearance of microalbuminuria or rising in serum creatinine [15]. In our study, it was showed that serum OIF levels were significantly increased in DN subjects compared with healthy and T2DM subjects (P < 0.05 respectively). However, no significant changes in serum OIF levels were found between T2DM and healthy subjects. Recent researches demonstrated that OIF may be one of fundamental compounds of capillary and plays an important role in regulation of capillaries. OIF mRNA levels was sufficiently high in lung, skeletal muscle, testis and lipid tissue [16-18]. Some other studies indicated that OIF have a still vague function which helped to rebuild capillaries. In this study, correlation studies revealed that OIF was positively correlated with BUM, creatinine and negatively correlated with eGFR. eGFR, creatinine, and BUN are conventional biomarkers reflecting changes in renal function in DN patients. In fact, GFR was the best parameter of overall kidney function, and BUN and creatinine were conventional biomarkers reflecting changes in renal function in CKD and DN patients [19-22]. These results suggested that OIF levels were strongly associated with renal function in subjects with DN. Through carrying out the nonparametric ROC plots, we found that serum OIF had a high sensitive and specificity for the prediction of microalbuminuria (86.7% and 95%, respectively) and macroalbuminuria (90% and 95%, respectively). The AUC of OIF for the prediction of microalbuminuria reached 0.869. Our results revealed the potential role of serum OIF levels for the onset and development of DN among DM subjects.
In conclusion, this study provided clinical evidence revealing that serum concentrations of OIF were increased in subjects with DN. OIF was a sensitive marker for early microalbuminuria. These data indicated that OIF may be a potential biomarker for diagnosing and evaluating the onset and development of DN among DM subjects. For there were seldom studies related to OIF all over the world, understanding the role of OIF in progression of DN will extend the application of OIF, which used as a serological labeling marker for diagnose earlier stage of DN. It also provided a new possibility target to cure early stage of DN. Ulteriorly, understanding the exact mechanism of up-regulated OIF in subjects with DN requires further study.
Disclosure of conflict of interest
None.
References
- 1.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 2.Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003;26:2392–2399. doi: 10.2337/diacare.26.8.2392. [DOI] [PubMed] [Google Scholar]
- 3.Gross JL, De Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 4.Ravid M, Savin H, Jutrin I, Bental T, Katz B, Lishner M. Long-term stabilizing effect of angiotensin-converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Ann Int Med. 1993;118:577–581. doi: 10.7326/0003-4819-118-8-199304150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Gæde P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353:617–622. doi: 10.1016/S0140-6736(98)07368-1. [DOI] [PubMed] [Google Scholar]
- 6.Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003;289:3273–3277. doi: 10.1001/jama.289.24.3273. [DOI] [PubMed] [Google Scholar]
- 7.Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17:1–19. doi: 10.1016/s0945-053x(98)90121-4. [DOI] [PubMed] [Google Scholar]
- 8.Fisher LW, Termine JD, Young MF. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989;264:4571–4576. [PubMed] [Google Scholar]
- 9.Bentz H, Nathan RM, Rosen DM, Armstrong RM, Thompson AY, Segarini PR, Mathews MC, Dasch JR, Piez KA, Seyedin SM. Purification and characterization of a unique osteoinductive factor from bovine bone. J Biol Chem. 1989;264:20805–20810. [PubMed] [Google Scholar]
- 10.Dasch JR, Pace DR, Avis PD, Bentz H, Chu S. Characterization of monoclonal antibodies recognizing bovine bone osteoglycin. Connect Tissue Res. 1993;30:11–21. doi: 10.3109/03008209309032927. [DOI] [PubMed] [Google Scholar]
- 11.Shanahan CM, Cary NR, Osbourn JK, Weissberg PL. Identification of osteoglycin as a component of the vascular matrix differential expression by vascular smooth muscle cells during neointima formation and in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1997;17:2437–2447. doi: 10.1161/01.atv.17.11.2437. [DOI] [PubMed] [Google Scholar]
- 12.Mogensen C. Microalbuminuria as a predictor of clinical diabetic nephropathy. Kidney Int. 1987;31:673–689. doi: 10.1038/ki.1987.50. [DOI] [PubMed] [Google Scholar]
- 13.Yang YH, Zhang S, Cui JF, Lu B, Dong XH, Song XY, Liu YK, Zhu XX, Hu RM. Diagnostic potential of serum protein pattern in Type 2 diabetic nephropathy. Diabet Med. 2007;24:1386–1392. doi: 10.1111/j.1464-5491.2007.02312.x. [DOI] [PubMed] [Google Scholar]
- 14.Hodgkinson AD, Bartlett T, Oates PJ, Millward BA, Demaine AG. The response of antioxidant genes to hyperglycemia is abnormal in patients with type 1 diabetes and diabetic nephropathy. Diabetes. 2003;52:846–851. doi: 10.2337/diabetes.52.3.846. [DOI] [PubMed] [Google Scholar]
- 15.Uslu S, Efe B, Alatas O, Kebapci N, Colak O, Demirustu C, Yoruk A. Serum cystatin C and urinary enzymes as screening markers of renal dysfunction in diabetic patients. J Nephrol. 2005;18:559. [PubMed] [Google Scholar]
- 16.Lee JY, Eom EM, Kim DS, Ha-Lee YM, Lee DH. Analysis of gene expression profiles of gastric normal and cancer tissues by SAGE. Genomics. 2003;82:78–85. doi: 10.1016/s0888-7543(03)00098-3. [DOI] [PubMed] [Google Scholar]
- 17.Pelissier P, Masquelet A, Bareille R, Pelissier SM, Amedee J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res. 2004;22:73–79. doi: 10.1016/S0736-0266(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka KI, Matsumoto E, Higashimaki Y, Katagiri T, Sugimoto T, Seino S, Kaji H. Role of osteoglycin in the linkage between muscle and bone. J Biol Chem. 2012;287:11616–11628. doi: 10.1074/jbc.M111.292193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004;27:195–200. doi: 10.2337/diacare.27.1.195. [DOI] [PubMed] [Google Scholar]
- 20.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients an indicator of more advanced glomerular lesions. Diabetes. 2003;52:1036–1040. doi: 10.2337/diabetes.52.4.1036. [DOI] [PubMed] [Google Scholar]
- 21.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 22.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]


