Abstract
Aims: This study was to clarify the regulated effects of TNF-α -308G/A polymorphism on TNF-α and investigate the relationship of -308G/A polymorphisms with diabetic nephropathy (DN) susceptibility. Methods: 86 DN patients and 94 healthy individuals were enrolled in our study. Polymerase chain reaction-sequence specific primer (PCR-SSP) detection technology was used to testify single nucleotide polymorphism (SNP) of TNF-α gene. Enzyme-linked immunosorbent assay (ELISA) was used to measure the content of TNF-α protein. Odds ratio (OR) with 95% CI were used to evaluate the association of TNF-α -308G/A polymorphism and DN susceptibility. Results: The level of TNF-α protein was much higher in DN patients compared to that of controls (P < 0.05). For TNF-α -308G/A, G/A genotype could increase the risk for DN (OR = 2.15, 95% CI = 1.08-4.30). Moreover, a allele frequency was found higher in cases compared with controls, which suggested that A allele served as an genetic-susceptibility factor for DN (OR = 1.89, 95% CI = 1.10-3.26). Further analysis indicated that level of TNF-α for individuals with mutant genotype (GA and AA) were higher than that of individuals with wild genotype (P < 0.05). However, AA genotype showed no effects on DN susceptibility (OR = 2.08, 95% CI = 0.56-7.33). Conclusion: TNF-α-308G/A polymorphism was associated with expression level of TNF-α and served as an genetic-susceptibility factor for DN.
Keywords: Tumor necrosis factor-α, diabetic nephropathy, protein expression
Introduction
Tumor necrosis factor-α (TNF-α) is a multifunctional pro-inflammatory cytokine, which is associated with some pathological processes, such as apoptosis, proliferation, inflammation and immunoreguation. Effects of single nucleotide polymorphisms (SNPs) within tumor necrosis factor-α (TNF-α) on inflammatory diseases have attracted extensive attention. Furthermore, some studies have confirmed that SNPs within TNF-αpromoter affected the expression of TNF-α, which were related to the occurrence and severity of some inflammatory diseases [1-5], however, some studies have not come to any conclusion or even come to opposite conclusion [6-8].
It was generally accepted that TNF-α -308G/A polymorphism might affect the transcription of TNF-α, of which A allele could strengthen the transcription of TNF-α and G allele is associated with low-level expression of TNF-α [9,10]. In addition, Navarro et al. found that expression level of TNF-α is upregulated in patients of diabetic nephropathy [11]. Nevertheless, there was no report about association of TNF-α -308G/A polymorphism and diabetic nephropathy (DN) based on Chinese population.
In the study, we analyzed the association of TNF-α -308G/A polymorphism and the expression level of TNF-α protein, and further explored the role of -308G/A loci in the pathogenesis of DN.
Materials and methods
Subjects
According to the diagnosis and classification criteria for diabetes mellitus of World Health Organization (WHO) in 1999, 86 patients with DN were enrolled. The control group was 94 healthy checkers in the hospital, who were required for no histories of high blood pressure and coronary heart disease. The subjects were unrelated Chinese, and the controls were matched to cases in age and gender.
Methods
DNA extraction
5 ml peripheral blood was obtained from each subject, and then processed with anticoagulation by 5% EDTA. Genome DNA was extracted from blood with the method of Resse et al [12].
PCR amplification
Primers were provided by American Lambda Biotec, co, LTD. Primer sequence was shown in Table 1. PCR reaction was performed under the following conditions: predenaturation 94°C for 5 min, followed by 30 cycles of 94°C for 30 s, 60°C for 50 s, 72°C for 40 s and final 72°C for 10 min. PCR products were detected with 2% agarose electrophoresis for 15-20 min.
Table 1.
Primers sequence of TNF-α-308
| Primers | Sequence |
|---|---|
| Normal primer | F: 5’-GATGGACTCACCAGGTGAG-3’ |
| R: 5’-CTCATGGTGTCCTTTCCAGG-3’ | |
| Primer of -308A | 5’-GTGGAGGCTGAPLCCCCGTCCT-3’ |
| Primer of -308G | 5’-GTGGAGGCTGAPLCCCCGTCCT-3’ |
ELISA assay
Serum TNF-α level of cases and controls were determined with ELISA kit (Beijing Tiantan Biological Products co., LTD).
Statistical treatment
Hardy Weinberg Equilibrium (HWE) was tested by χ2 test. T-test was adopted to calculate the comparison in TNF-α protein level between two groups or mutant and wild genotypes. χ2 test was used to evaluate the differences in genotype distribution between cases and controls. Odds ratio (OR) with 95% confidence interval (CI) were used to assess the relationship of TNF-α-308 and DN susceptibility. All the analysis were conducted in SPSS 18.0. If P < 0.05, there is statistical significance.
Results
Comparisons of genotype and allele frequencies of TNF-α-308 in two groups
Genotype frequencies of TNF-α-308 in case and control groups were consistent with HWE (P > 0.05), which suggested the population in the study was representative. As shown in Table 2, frequency of G/A genotype was found higher in case group than that of controls (32.6% vs. 19.1%), which indicated that G/A genotype could increase the risk for DN (OR = 2.15, 95% CI = 1.08-4.30). However, A/A genotype showed no significant association with DN risk (OR = 2.08, 95% CI = 0.56-7.33). Additionally, risk for DN of A allele carriers was 0.89 times than that of G allele (OR = 1.89, 95% CI = 1.10-3.26).
Table 2.
Genotype and allele frequencies of TNF-α-308 in case and control groups
| Genotype/Allele | DN cases n (%) | Controls n (%) | OR (95% CI) | χ2 | P value |
|---|---|---|---|---|---|
| Genotype | |||||
| -308G/A | |||||
| G/G | 52 (60.5) | 72 (76.6) | 1.00 | - | - |
| G/A | 28 (32.6) | 18 (19.1) | 2.154 (1.079-4.300) | 4.828 | 0.038 |
| A/A | 6 (6.9) | 4 (4.3) | 2.077 (0.558-7.332) | 1.230 | 0.328 |
| Allele | |||||
| G | 132 (76.7) | 162 (86.2) | 1.00 | - | - |
| A | 40 (23.3) | 26 (13.8) | 1.888 (1.095-3.255) | 5.330 | 0.029 |
Expression level of TNF-α
In the study, we measured the content of serum TNF-α in the case and control groups.
The result indicated that expression level of TNF-α was remarkably upregulated in DN patients (P < 0.05) (Figure 1).
Figure 1.

Comparison of expression level of TNF-α in two groups.
Association of TNF-α-308 polymorphism and TNF-α level
And then we investigated the association of -308 polymorphism and expression level of TNF-α. As shown in Figure 2, expression level of TNF-α for patients with mutant genotype (GA and AA) was much higher than that of patients with mutant genotype (P < 0.05).
Figure 2.
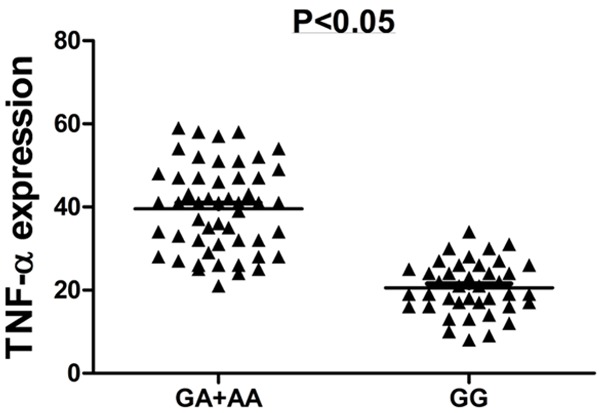
Comparison of expression level TNF-α between mutant and wild genotype.
Discussion
DN, the most common chronic microvascular complication of diabetes mellitus (DM), seriously affects living quality of the patients. Inflammation, cell hypertrophy and dedifferentiation contribute to the progression of DN [13]. The occurrence of DN is related to various factors including oxidative stress, high glucose, hemodynamics change, metabolic disorder and genetic factor [14,15]. And the role of genetic factor in the pathogenesis of DN attracted much more attention.
TNF-α gene located at 6p21.3, is a key member in the central part of major histocompatibility complex (MHC) [16]. And TNF-α is an important inflammatory mediator, produced by T cell and macrophages [17]. Recently, the studies have found that TNF-α is also produced by multiple cells in kidney, such as glomerular mesangial cell (GMC), proximal tubular cell and blood cell in kidney [18], among which GMC plays a crucial role in the progression of DN and the function change of GMC increases the risk of DN [19].
The study conducted by Baudl et al. with the method of Northern blot suggested that TNF-α mRNA expression level was low in the unactivated GMC [20], while it was upregulated with lipopolysaccharide (LPS) activation [21]. For GMC, not only endotoxin, but also fragment of complement, immune complex, platelet-derived growth factor, epidermal growth factor, platelet activating factor and interleukin could trigger the expression of TNF-α [22]. In addition, TNF-α content is found to associated with pathological changes of mesangial proliferative glomerulonephritis (MsPGN) [23-25], which indicated that TNF-α might one of important factor resulted in proliferation, sclerosis, disease progression of GMC.
In consideration of the role of TNF-α for GMC, the study focusing on the association of TNF-α and DN is valuable. In the study, we took -308 polymorphism into consideration, which is related to the expression level of TNF-α. And the result found that the expression level of TNF-α is much higher in DN patients than that of controls. Further analysis suggested that TNF-α protein level of individuals with mutant genotype was higher than that of individuals with wild genotype. Moreover, G/A genotype could increase the risk for DN. So we concluded that TNF-α -308 polymorphism was a genetic-susceptibility factor for DN, which would contribute to the prevention and early diagnosis of DN.
Disclosure of conflict of interest
None.
References
- 1.Sharma R, Agrawal S, Saxena A, Sharma RK. Association of IL-6, IL-10, and TNF-alpha gene polymorphism with malnutrition inflammation syndrome and survival among end stage renal disease patients. J Interferon Cytokine Res. 2013;33:384–391. doi: 10.1089/jir.2012.0109. [DOI] [PubMed] [Google Scholar]
- 2.Gallo E, Cabaleiro T, Roman M, Abad-Santos F, Dauden E. Study of genetic polymorphisms in the tumor necrosis factor alpha promoter region in Spanish patients with psoriasis. Actas Dermosifiliogr. 2012;103:301–307. doi: 10.1016/j.ad.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Sousa H, Oliveira S, Santos AM, Catarino R, Moutinho J, Medeiros R. Tumour necrosis factor alpha 308 G/A is a risk marker for the progression from high-grade lesions to invasive cervical cancer. Tumour Biol. 2014;35:2561–2564. doi: 10.1007/s13277-013-1337-3. [DOI] [PubMed] [Google Scholar]
- 4.Lu CC, Sheu BS, Chen TW, Yang HB, Hung KH, Kao AW, Chuang CH, Wu JJ. Host TNF-alpha-1031 and -863 promoter single nucleotide polymorphisms determine the risk of benign ulceration after H. pylori infection. Am J Gastroenterol. 2005;100:1274–1282. doi: 10.1111/j.1572-0241.2005.40852.x. [DOI] [PubMed] [Google Scholar]
- 5.Ghazouani L, Khalifa SB, Abboud N, Addad F, Khalfallah AB, Brahim N, Mediouni M, Almawi WY, Mahjoub T. -308G>A and -1031T>C tumor necrosis factor gene polymorphisms in Tunisian patients with coronary artery disease. Clin Chem Lab Med. 2009;47:1247–1251. doi: 10.1515/CCLM.2009.287. [DOI] [PubMed] [Google Scholar]
- 6.Bonyadi M, Abdolmohammadi R, Jahanafrooz Z, Somy MH, Khoshbaten M. TNF-alpha gene polymorphisms in Iranian Azari Turkish patients with inflammatory bowel diseases. Saudi J Gastroenterol. 2014;20:108–112. doi: 10.4103/1319-3767.129475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nossent JC, Sagen-Johnsen S, Bakland G. Tumor necrosis factor-alpha promoter -308/238 polymorphism association with less severe disease in ankylosing spondylitis is unrelated to serum TNF-alpha and does not predict TNF inhibitor response. J Rheumatol. 2014;41:1675–1682. doi: 10.3899/jrheum.131315. [DOI] [PubMed] [Google Scholar]
- 8.Kotsaki A, Raftogiannis M, Routsi C, Baziaka F, Kotanidou A, Antonopoulou A, Orfanos SE, Katsenos C, Koutoukas P, Plachouras D, Mandragos K, Giamarellos-Bourboulis EJ. Genetic polymorphisms within tumor necrosis factor gene promoter region: a role for susceptibility to ventilator-associated pneumonia. Cytokine. 2012;59:358–363. doi: 10.1016/j.cyto.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 9.Kroeger KM, Carville KS, Abraham LJ. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997;34:391–399. doi: 10.1016/s0161-5890(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 10.Navarro JF, Milena FJ, Mora C, Leon C, Claverie F, Flores C, Garcia J. Tumor necrosis factor-alpha gene expression in diabetic nephropathy: relationship with urinary albumin excretion and effect of angiotensin-converting enzyme inhibition. Kidney Int Suppl. 2005:S98–102. doi: 10.1111/j.1523-1755.2005.09918.x. [DOI] [PubMed] [Google Scholar]
- 11.Yoon SZ, Jang IJ, Choi YJ, Kang MH, Lim HJ, Lim YJ, Lee HW, Chang SH, Yoon SM. Association between tumor necrosis factor alpha 308G/A polymorphism and increased proinflammatory cytokine release after cardiac surgery with cardiopulmonary bypass in the Korean population. J Cardiothorac Vasc Anesth. 2009;23:646–650. doi: 10.1053/j.jvca.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Sisk TJ, Gourley T, Roys S, Chang CH. MHC class II transactivator inhibits IL-4 gene transcription by competing with NF-AT to bind the coactivator CREB binding protein (CBP)/p300. J Immunol. 2000;165:2511–2517. doi: 10.4049/jimmunol.165.5.2511. [DOI] [PubMed] [Google Scholar]
- 13.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124:2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng MC, Baum L, So WY, Lam VK, Wang Y, Poon E, Tomlinson B, Cheng S, Lindpaintner K, Chan JC. Association of lipoprotein lipase S447X, apolipoprotein E exon 4, and apoC3 -455T>C polymorphisms on the susceptibility to diabetic nephropathy. Clin Genet. 2006;70:20–28. doi: 10.1111/j.1399-0004.2006.00628.x. [DOI] [PubMed] [Google Scholar]
- 15.Collins AJ, Kasiske B, Herzog C, Chavers B, Foley R, Gilbertson D, Grimm R, Liu J, Louis T, Manning W, McBean M, Murray A, St Peter W, Xue J, Fan Q, Guo H, Li Q, Li S, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zhang R, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L. Excerpts from the United States Renal Data System 2006 Annual Data Report. Am J Kidney Dis. 2007;49:A6–7. S1–296. doi: 10.1053/j.ajkd.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 17.Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991;88:9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baud L, Fouqueray B, Bellocq A, Haymann JP, Peltier J. Inflammation, prelude to renal sclerosis: the importance of NF-kappa B. J Soc Biol. 2002;196:269–273. [PubMed] [Google Scholar]
- 19.Young BA, Johnson RJ, Alpers CE, Eng E, Gordon K, Floege J, Couser WG, Seidel K. Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int. 1995;47:935–944. doi: 10.1038/ki.1995.139. [DOI] [PubMed] [Google Scholar]
- 20.Caughey GE, Cleland LG, Gamble JR, James MJ. Up-regulation of endothelial cyclooxygenase-2 and prostanoid synthesis by platelets. Role of thromboxane A2. J Biol Chem. 2001;276:37839–37845. doi: 10.1074/jbc.M010606200. [DOI] [PubMed] [Google Scholar]
- 21.Affres H, Perez J, Hagege J, Fouqueray B, Kornprobst M, Ardaillou R, Baud L. Desferrioxamine regulates tumor necrosis factor release in mesangial cells. Kidney Int. 1991;39:822–830. doi: 10.1038/ki.1991.103. [DOI] [PubMed] [Google Scholar]
- 22.Stuber F. Effects of genomic polymorphisms on the course of sepsis: is there a concept for gene therapy? J Am Soc Nephrol. 2001;12(Suppl 17):S60–64. [PubMed] [Google Scholar]
- 23.Hirahashi J, Takayanagi A, Hishikawa K, Takase O, Chikaraishi A, Hayashi M, Shimizu N, Saruta T. Overexpression of truncated I kappa B alpha potentiates TNF-alpha-induced apoptosis in mesangial cells. Kidney Int. 2000;57:959–968. doi: 10.1046/j.1523-1755.2000.00924.x. [DOI] [PubMed] [Google Scholar]
- 24.Kacprzyk F, Chrzanowski W. Tumor necrosis factor (TNF) and interleukin-6 (IL-6) in patients with glomerulonephritis. Pol Arch Med Wewn. 1996;96:224–233. [PubMed] [Google Scholar]
- 25.Ozen S, Saatci U, Tinaztepe K, Bakkaloglu A, Barut A. Urinary tumor necrosis factor levels in primary glomerulopathies. Nephron. 1994;66:291–294. doi: 10.1159/000187825. [DOI] [PubMed] [Google Scholar]


