Abstract
We conducted a perspective study to assess the association between ERCC1 and XPF polymorphisms and response to chemotherapy and clinical outcome of NSCLC receiving chemotherapy. Between May 2009 and May 2011, a prospective study was conducted on 240 NSCLC cases. Genotypes of ERCC1 (rs11615, rs3212986 and rs2298881) and XPF (rs2276465 and rs6498486) were performed by Polymerase Chain Reaction Restriction Fragment Length Polymorphism (PCR-RFLP) assay. By conditional logistic regression analysis, patients carrying AA genotype of ERCC1 rs11615 showed more CR+PR to chemotherapy when compared with GG genotype, and the adjusted OR (95% CI) was 2.73 (1.21-6.18). By Cox regression analysis, AA genotype of ERCC1 rs11615 was associated with longer overall survival of NSCLC, and the adjusted HR (95% CI) was 0.38 (0.14-0.96). In conclusion, our study found that ERCC1 rs11615 polymorphism can influence the chemotherapy response and overall survival of NSCLC patients receiving cisplatin-based chemotherapy.
Keywords: ERCC1, XPF, polymorphism, clinical outcome, non-small cell lung cancer
Introduction
Lung cancer is one of the most common cancers worldwide for several decades, and it is the main cause of cancer-related mortality [1]. There are estimated to be 1.8 million new cases in 2012 (12.9% of the total), 58% of which occurred in the less developed regions [1]. It is estimated that about 80% of lung cancer patients are non-small cell lung cancer (NSCLC). Most of NSCLC patients present advanced status when they are diagnosed.
Despite the development of therapeutics for NSCLC and the improvement of diagnosis in recent years, the NSCLC patients showed a 19%-38% 5-years survival rate after diagnosis [2]. Previous study reported that the outcome of patients was significantly improved after receiving cisplatin-based chemotherapy, and the efficacy and toxicity of chemotherapy treatment are largely individual in patients. Recent studies showed that genetic factors can play an important role in different treatment effects of cisplatin-based chemotherapy [3,4]. Gene polymorphisms involved in altering the activity of enzymes and transporters can influence the efficacy of cisplatin-based chemotherapy [5].
Nucleotide excision repair (NER) is one of the important DNA repair pathways for repairing the chromosomal breakage or rearrangement from DNA damage, replication errors and recombination processes [6]. The excision repair cross-complimentary group 1 gene (ERCC1) encodes a subunit of the NER complex required for the incision step of NER, which forms a heterodimer with the xeroderma pigmentosum complementation group F (XPF) endonuclease to catalyze the 5’ incision during excision of the DNA lesion [7]. The ERCC1-XPF complex is a structure-specific endonuclease essential for the repair of DNA damage through the NER pathway, and it has an important role in several key cellular processes, such as DNA interstrand crosslink repair and DNA double-strand break repair [7].
Previous studies report that polymorphisms in ERCC1 and XPF are associated with response to chemotherapy and clinical outcome of several kinds of cancers, including gastric cancer, non-small cell lung cancer, head and neck cancer and ovarian cancer [8-12]. However, previous studies reported inconsistent results on the association between ERCC1 and XPF polymorphisms and the response to chemotherapy and clinical outcome of NSCLC [13-16]. Therefore, we conducted a perspective study to assess the association between ERCC1 and XPF polymorphisms and response to chemotherapy and clinical outcome of NSCLC receiving chemotherapy.
Patients and methods
Subjects
Between May 2009 and May 2011, a prospective study was conducted on 240 NSCLC cases, and they were diagnosed at stage III-IV, and the median age 61.5 years (34-78 years). All the patients were treated with cisplatin-based chemotherapy. All of the patients were histopathologically confirmed. All patients did not receive systemic anticancer chemotherapy previously. Patients who had serious concomitant systemic disorder unable to receive chemotherapy, concurrent chemo-radiotherapy, brain metastasis with symptoms, without comprehensive data, developing gastric ulcer and neutral system diseases which may affect the safety of patients or the evaluation of results were excluded from our study. Written informed consent was obtained from all patients for blood sample collection to establish the clinical significance of genetic polymorphisms in the cisplatin-based chemotherapy. This study was approved by ethics committee of the Fifth Affiliated Hospital of Zhengzhou University.
Chemotherapy regimens
All participants were treated with one of the following cisplatin-based combination chemotherapy regimen: cisplatin and gemcitabine (GP), cisplatin and vinorelbine (NP), cisplatin and paclitaxel (TP), or cisplatin and docetaxel (DP). The chemotherapy treatment was repeated every three weeks. The treatment was continued for a maximum of four cycles. The treatments were suspended until disease progression or unacceptable toxicity.
The patients were followed up until May 2014, with a median follow-up time of 21.6 months (range from 2 months to 60 months). All patients were followed up by telephone or attending clinics every one month until death or the end of follow-up.
All the patients completed two cycles of chemotherapy, and the treatment efficacy was evaluated according to Response Evaluation Criteria in Solid Tumors criteria (Duffaud and Therasse, 2000). NSCLC patients with complete remission (CR) and partial remission (PR) to chemotherapy were considered as responders, and patients with stable disease (SD) and progressive disease (PD) to chemotherapy were regarded as non-responders. Overall survival (OS) was defined as the period between the date of treatment and the data of death from any cause. Patients without an event or death at the time of the analysis were censored at the end of this study.
Blood samples and genotyping
5 ml peripheral blood was gained from each patient and control subject, and the blood sample was kept in -70°C until use. Genomic DNA was isolated from peripheral blood lymphocytes using Qiagen blood mini kit (Qiagen, Germany) by the manufacturer’s protocol. Genotypes of ERCC1 (rs11615, rs3212986 and rs2298881) and XPF (rs2276465 and rs6498486) were performed by Polymerase Chain Reaction Restriction Fragment Length Polymorphism (PCR-RFLP) assay. Primers and probes of ERCC1 (rs11615, rs3212986 and rs2298881) and XPF (rs2276465 and rs6498486) were designed using Sequenom Assay Design 3.1 software. Briefly PCR was carried out in a final volume of 25 μL containing 50 ng genomic DNA templates, 1×PCR buffer with 2 mM MgCl2, 0.5 μM of each primer, 50 μM dNTPs and 0.5 U DNA polymerase. For PCR amplification, the standard program was used as follows: one initial denaturation step at 94°C for 7 min, followed by 35 denaturation cycles of 1 min at 94°C, 1 min of annealing at 60°C, and 1 min of extension at 72°C, followed by a final elongation cycle at 72°C for 10 min.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were expressed as n (%) of study participants. The association between response to chemotherapy and ERCC1 (rs11615, rs3212986 and rs2298881) and XPF (rs2276465 and rs6498486) were described as odds ratio (ORs) and 95% confidence interval (CI) in conditioned logistic regression. The prognostic value of different SNPs for the PFS was estimated by multivariate analysis using the Cox regression analysis, describing as the hazard ratio (HR) and 95% confidence interval (CI). Survival curves were analyzed by the Kaplan-Meier method. Meanwhile, the demographic characteristics were adjusted in order to avoid potential confounding effects, including age, sex, tobacco smoking, histological types and TNM stage at entry. P values <0.05 with two-sided were considered statistical differences. Data were performed by the statistical software SPSS Statistics (version 16.0, SPSS Inc., Chicago, IL, USA).
Results
The demographic and clinical characteristics of the NSCLC cases are presented in Table 1. The mean age of the NSCLC subjects was 63.1±10.5 years old (range: 31 and 80 years old). Of 240 patients, 155 (64.58%) were males, 93 (38.75%) had a habit of tobacco smoking, 127 (52.92%) were at IV of TNM stage, and 132 (55.00%) were squamous cell carcinoma and 148 (61.67%) showed SD+PD to chemotherapy.
Table 1.
Demographic characteristics of patients
| Characteristics | Patients | % |
|---|---|---|
| Age, years | ||
| <60 | 103 | 42.92 |
| ≥60 | 137 | 57.08 |
| Gender | ||
| Male | 155 | 64.58 |
| Female | 85 | 35.42 |
| Tobacco smoking | ||
| Never | 147 | 61.25 |
| Ever | 93 | 38.75 |
| TNM stage | ||
| III | 113 | 47.08 |
| IV | 127 | 52.92 |
| Histology | ||
| Squamous cell carcinoma | 132 | 55.00 |
| Adenocarcinoma | 100 | 41.67 |
| Other | 8 | 3.33 |
| Response to chemotherapy | ||
| CR+PR | 92 | 38.33 |
| SD+PD | 148 | 61.67 |
At the end of the follow-up, 92 NSCLC patients showed good response to cisplatin-based chemotherapy, with a response rate of 38.33%. By conditional logistic regression analysis, patients carrying AA genotype of ERCC1 rs11615 showed more CR+PR to chemotherapy when compared with GG genotype, and the adjusted OR (95% CI) was 2.73 (1.21-6.18) (Table 2). However, we did not find significant association between ERCC1 rs3212986, ERCC1 rs2298881, XPF rs2276465 and XPF rs6498486 polymorphisms and response to chemotherapy in NSCLC.
Table 2.
Association between ERCC1 and XPF polymorphisms and response to chemotherapy in NSCLC patients
| SNPs | Total frequencies | % | CR+PR N=92 | % | SD+PD N=148 | % | OR (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|
| ERCC1 rs11615 | ||||||||
| GG | 112 | 46.67 | 36 | 39.13 | 76 | 51.12 | Ref. | - |
| AG | 89 | 37.08 | 34 | 36.96 | 55 | 33.84 | 1.31 (0.70-2.44) | 0.37 |
| AA | 39 | 16.25 | 22 | 23.91 | 17 | 15.04 | 2.73 (1.21-6.18) | 0.007 |
| ERCC1 rs3212986 | ||||||||
| CC | 99 | 41.25 | 37 | 40.22 | 62 | 41.72 | Ref. | - |
| AC | 102 | 42.50 | 38 | 41.30 | 64 | 40.88 | 0.99 (0.54-1.83) | 0.99 |
| AA | 39 | 16.25 | 17 | 18.48 | 22 | 17.40 | 1.29 (0.57-2.93) | 0.5 |
| ERCC1 rs2298881 | ||||||||
| AA | 147 | 61.25 | 53 | 57.61 | 94 | 61.65 | Ref. | - |
| AC | 60 | 25.00 | 24 | 26.09 | 36 | 24.32 | 1.18 (0.61-2.28) | 0.59 |
| CC | 33 | 13.75 | 15 | 16.30 | 18 | 14.03 | 1.48 (0.64-3.39) | 0.31 |
| XPF rs2276465 | ||||||||
| CC | 142 | 59.17 | 51 | 55.43 | 91 | 61.51 | Ref. | - |
| CG | 58 | 24.17 | 23 | 25.00 | 35 | 22.22 | 1.17 (0.59-2.29) | 0.62 |
| GG | 40 | 16.67 | 18 | 19.57 | 22 | 16.27 | 1.46 (0.67-3.15) | 0.3 |
| XPF rs6498486 | ||||||||
| AA | 157 | 65.42 | 57 | 61.96 | 100 | 67.35 | Ref. | - |
| AC | 50 | 20.83 | 20 | 21.74 | 30 | 19.61 | 1.17 (0.57-2.35) | 0.63 |
| CC | 33 | 13.75 | 15 | 16.30 | 18 | 13.04 | 1.46 (0.63-3.33) | 0.32 |
Adjusted for sex, age, tobacco smoking, TNM stage and histology.
At the end of May 2014, 157 patients died from all causes, and the five-year survival rate is 34.58%. By Cox regression analysis, AA genotype of ERCC1 rs11615 was associated with longer overall survival of NSCLC, and a decreased risk of death from NSCLC. The adjusted HR (95% CI) was 0.38 (0.14-0.96) for AA genotype compared to GG genotype (Table 3, Figure 1).
Table 3.
Association between ERCC1 and XPF polymorphisms and overall survival of NSCLC patients
| SNPs | Death N=157 | % | Alive N=83 | % | HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| ERCC1 rs11615 | ||||||
| GG | 85 | 54.14 | 27 | 32.53 | Ref. | - |
| AG | 57 | 36.31 | 32 | 38.55 | 0.76 (0.41-1.41) | 0.35 |
| AA | 15 | 9.55 | 24 | 28.92 | 0.38 (0.14-0.96) | 0.03 |
| ERCC1 rs3212986 | ||||||
| CC | 66 | 42.04 | 33 | 39.76 | Ref. | - |
| AC | 63 | 40.13 | 39 | 46.99 | 0.87 (0.47-1.64) | 0.65 |
| AA | 28 | 17.83 | 11 | 13.25 | 1.14 (0.49-2.62) | 0.73 |
| ERCC1 rs2298881 | ||||||
| AA | 100 | 63.69 | 47 | 56.63 | Ref. | - |
| AC | 38 | 24.20 | 22 | 26.51 | 0.89 (0.44-1.74) | 0.71 |
| CC | 19 | 12.10 | 14 | 16.87 | 0.77 (0.30-1.84) | 0.53 |
| XPF rs2276465 | ||||||
| CC | 95 | 60.51 | 47 | 56.63 | Ref. | - |
| CG | 38 | 24.20 | 20 | 24.10 | 0.97 (0.48-1.92) | 0.92 |
| GG | 24 | 15.29 | 16 | 19.28 | 0.89 (0.38-1.97) | 0.75 |
| XPF rs6498486 | ||||||
| AA | 106 | 67.52 | 51 | 61.45 | Ref. | - |
| AC | 32 | 20.38 | 18 | 21.69 | 0.93 (0.44-1.90) | 0.83 |
| CC | 19 | 12.10 | 14 | 16.87 | 0.78 (0.31-1.86) | 0.56 |
Adjusted for sex, age, tobacco smoking, TNM stage and histology.
Figure 1.
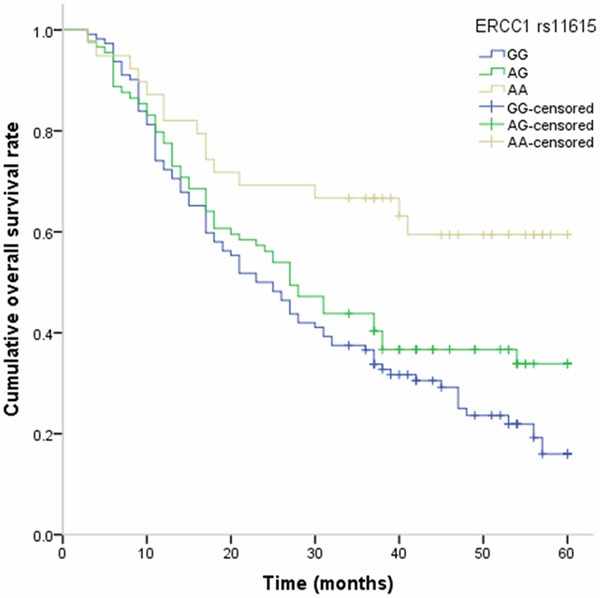
Kaplan-Meier analysis on the association between ERCC1 rs11615 polymorphism and overall survival of NSCLC.
Discussion
It is well known that traditional selection of chemotherapy treatment could significantly improve chemotherapy efficacy and clinical outcome of cancer patients. However, individualized chemotherapy through molecular biomarkers could greatly improve the treatment efficacy of cancer patients. In this prospective study, we investigated the role of ERCC1 and XPF polymorphisms in response to chemotherapy and clinical outcome of NSCLC receiving chemotherapy. Our results found that patients carrying AA genotype of ERCC1 rs11615 showed more CR+PR to chemotherapy when compared with GG genotype, and was associated with longer overall survival of NSCLC patients.
ERCC1 is one important protein of the NER pathway, and it is involved in repairing interstrand and intrastrand cross-links caused by cisplatin-based chemotherapy in several kinds of cancer [17-20]. For the association between ERCC1 polymorphisms and treatment efficacy of NSCLC, several previous studies reported their association in different kinds of populations [9,13-16]. Zhao et al. reported that ERCC1 rs11615 and rs3212986 polymorphisms may be helpful for designing individualized cancer treatment for NSCLC patients [13]. Lv et al. found that ERCC1 polymorphism are correlated with response to platinum-based chemotherapy in NSCLC [15]. Huang et al. investigated the association between ERCC1 and XPF polymorphisms and clinical outcome of advanced NSCLC, and reported that patients carrying the ERCC1 rs3212986 polymorphism were significantly associated with increased risk of death from NSCLC [9]. However, some studies reported that ERCC1 could not influence the treatment efficacy of NSCLC patients treated with cisplatin-based chemotherapy. Sullivan et al. investigated the role of DNA repair genes in the treatment effectiveness of platinum-based chemotherapy in NSCLC, but it did not find significant association between ERCC1 polymorphisms and clinical outcome of NSCLC [21]. A recent meta-analysis with 9615 cases and 5542 controls assessed the role of ERCC1 gene polymorphisms (C118T and C8092A) in this clinical outcome of NSCLC, and suggested that ERCC1 rs11615 polymorphism may serve as a biomarker for lung cancer risk and have prognostic value in patients with advanced NSCLC undergoing platinum-based treatment [22]. The results of our study are in line with previous, which suggest that ERCC1 rs11615 polymorphism can influence the clinical outcome of NSCLC.
XPF is also another important protein in the NER pathway, and this protein plays an important role in recombination repair, mismatch repair, and possibly, immunoglobulin class switching, owing to its function in identifying damage sites [23]. The polymorphisms of XPF can alter the function of XPF, and thus influence its role in the treatment efficacy of chemotherapy. Only three previous studies reported the role of XPF polymorphism in the clinical outcome of cancers [9,23,24]. However, the three studies did not find significant association between XPF polymorphisms and clinical outcome of cancers. In our study, we also did not find that XPF polymorphisms can influence the response to chemotherapy and clinical outcome of NSCLC. Therefore, further studies are greatly needed to confirm our findings.
In conclusion, our study found that ERCC1 rs11615 polymorphism can influence the chemotherapy response and overall survival of NSCLC patients receiving cisplatin-based chemotherapy. ERCC1 rs11615 may substantially contribute to the future design of individualized cancer treatment in NSCLC patients. Therefore, further large sample studies are greatly required to confirm our results.
Disclosure of conflict of interest
None.
References
- 1.International Agency for Research on Cancer. Lung Cancer. Estimated Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed in 2014-1-1.
- 2.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:985. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 3.Alberts DS, Garcia D, Mason-Liddil N. Cisplatin in advanced cancer of the cervix: an update. Semin Oncol. 1991;18:11–24. [PubMed] [Google Scholar]
- 4.Zhou F, Yu Z, Jiang T, Lv H, Yao R, Liang J. Genetic polymorphisms of GSTP1 and XRCC1: prediction of clinical outcome of platinum-based chemotherapy in advanced non-small cell lung cancer (NSCLC) patients. Swiss Med Wkly. 2011;141:w13275. doi: 10.4414/smw.2011.13275. [DOI] [PubMed] [Google Scholar]
- 5.Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487–491. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- 6.Tuteja N, Tuteja R. Unraveling DNA repair in human: molecular mechanisms and consequences of repair defect. Crit Rev Biochem Mol Biol. 2001;36:261–90. doi: 10.1080/20014091074192. [DOI] [PubMed] [Google Scholar]
- 7.Wang AT, Sengerová B, Cattell E, Inagawa T, Hartley JM, Kiakos K, Burgess-Brown NA, Swift LP, Enzlin JH, Schofield CJ, Gileadi O, Hartley JA, McHugh PJ. Human SNM1A and XPF-ERCC1 collaborate to initiate DNA interstrand cross-link repair. Genes Dev. 2011;25:1859–70. doi: 10.1101/gad.15699211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen ZH, Wang L, Luo LP. Association of DNA repair gene polymorphisms with response to chemotherapy and prognosis of gastric cancer. Genet Mol Res. 2014;13:7484–91. doi: 10.4238/2014.September.12.15. [DOI] [PubMed] [Google Scholar]
- 9.Huang SJ, Wang YF, Jin ZY, Sun JY, Guo ZL. Role of ERCC1 variants in response to chemotherapy and clinical outcome of advanced non-small cell lung cancer. Tumour Biol. 2014;35:4023–9. doi: 10.1007/s13277-013-1526-0. [DOI] [PubMed] [Google Scholar]
- 10.Bauman JE, Austin MC, Schmidt R, Kurland BF, Vaezi A, Hayes DN, Mendez E, Parvathaneni U, Chai X, Sampath S, Martins RG. ERCC1 is a prognostic biomarker in locally advanced head and neck cancer: results from a randomised, phase II trial. Br J Cancer. 2013;109:2096–105. doi: 10.1038/bjc.2013.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNeil EM, Melton DW. DNA repair endonuclease ERCC1-XPF as a novel therapeutic target to overcome chemoresistance in cancer therapy. Nucleic Acids Res. 2012;40:9990–10004. doi: 10.1093/nar/gks818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming ND, Agadjanian H, Nassanian H, Miller CW, Orsulic S, Karlan BY, Walsh CS. Xeroderma pigmentosum complementation group C single-nucleotide polymorphisms in the nucleotide excision repair pathway correlate with prolonged progression-free survival in advanced ovarian cancer. Cancer. 2012;118:689–97. doi: 10.1002/cncr.26329. [DOI] [PubMed] [Google Scholar]
- 13.Zhao X, Zhang Z, Yuan Y, Yuan X. Polymorphisms in ERCC1 gene could predict clinical outcome of platinum-based chemotherapy for non-small cell lung cancer patients. Tumour Biol. 2014;35:8335–41. doi: 10.1007/s13277-014-2033-7. [DOI] [PubMed] [Google Scholar]
- 14.Xie F, Sun Q, Wu S, Xie X, Liu Z. Nucleotide excision repair gene ERCC1 19007T>C polymorphism contributes to lung cancer susceptibility: a meta-analysis. Genet Test Mol Biomarkers. 2014;18:591–5. doi: 10.1089/gtmb.2013.0329. [DOI] [PubMed] [Google Scholar]
- 15.Lv H, Han T, Shi X, Yao Y, Yao Y, Qiu W, Yue L, Liang J. Genetic polymorphism of GSTP1 and ERCC1 correlated with response to platinum-based chemotherapy in non-small cell lung cancer. Med Oncol. 2014;31:86. doi: 10.1007/s12032-014-0086-5. [DOI] [PubMed] [Google Scholar]
- 16.Mlak R, Krawczyk P, Ramlau R, Kalinka-Warzocha E, Wasylecka-Morawiec M, Wojas-Krawczyk K, Kucharczyk T, Homa I, Kozioł P, Ciesielka M, Chudziak D, Milanowski J. Predictive value of ERCC1 and RRM1 gene single-nucleotide polymorphisms for first-line platinum- and gemcitabine-based chemotherapy in non-small cell lung cancer patients. Oncol Rep. 2013;30:2385–98. doi: 10.3892/or.2013.2696. [DOI] [PubMed] [Google Scholar]
- 17.Rumiato E, Cavallin F, Boldrin E, Cagol M, Alfieri R, Basso D, Castoro C, Ancona E, Amadori A, Ruol A, Saggioro D. ERCC1 C8092A (rs3212986) polymorphism as a predictive marker in esophageal cancer patients treated with cisplatin/5-FU-based neoadjuvant therapy. Pharmacogenet Genomics. 2013;23:597–604. doi: 10.1097/FPC.0b013e3283653afc. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Wang F, Wang Z, Li C, Luo H, Liang Y, An X, Shao J, Li Y. Polymorphisms in ERCC1 C8092A predict progression-free survival in metastatic/recurrent nasopharyngeal carcinoma treated with cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2013;72:315–22. doi: 10.1007/s00280-013-2196-8. [DOI] [PubMed] [Google Scholar]
- 19.Hao T, Feng W, Zhang J, Sun YJ, Wang G. Association of four ERCC1 and ERCC2 SNPs with survival of bone tumour patients. Asian Pac J Cancer Prev. 2012;13:3821–4. doi: 10.7314/apjcp.2012.13.8.3821. [DOI] [PubMed] [Google Scholar]
- 20.Deloia JA, Bhagwat NR, Darcy KM, Strange M, Tian C, Nuttall K, Krivak TC, Niedernhofer LJ. Comparison of ERCC1/XPF genetic variation, mRNA and protein levels in women with advanced stage ovarian cancer treated with intraperitoneal platinum. Gynecol Oncol. 2012;126:448–54. doi: 10.1016/j.ygyno.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan I, Salazar J, Majem M, Pallarés C, Del Río E, Páez D, Baiget M, Barnadas A. Pharmacogenetics of the DNA repair pathways in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Cancer Lett. 2014;353:160–6. doi: 10.1016/j.canlet.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Xu TP, Shen H, Liu LX, Shu YQ. Association of ERCC1-C118T and -C8092A polymorphisms with lung cancer risk and survival of advanced-stage non-small cell lung cancer patients receiving platinum-based chemotherapy: a pooled analysis based on 39 reports. Gene. 2013;526:265–74. doi: 10.1016/j.gene.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Kornguth DG, Garden AS, Zheng Y, Dahlstrom KR, Wei Q, Sturgis EM. Gastrostomy in oropharyngeal cancer patients with ERCC4 (XPF) germline variants. Int J Radiat Oncol Biol Phys. 2005;62:665–71. doi: 10.1016/j.ijrobp.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Fareed KR, Al-Attar A, Soomro IN, Kaye PV, Patel J, Lobo DN, Parsons SL, Madhusudan S. Tumour regression and ERCC1 nuclear protein expression predict clinical outcome in patients with gastro-oesophageal cancer treated with neoadjuvant chemotherapy. Br J Cancer. 2010;102:1600–7. doi: 10.1038/sj.bjc.6605686. [DOI] [PMC free article] [PubMed] [Google Scholar]


