Abstract
Purpose: This study aimed to explore effects of dexmedetomidine pretreatment on heme oxygenase-1 (HO-1) expression and oxidative stress during one-lung ventilation (OLV) in lung cancer patients. Methods: Fifty patients with lung carcinoma (ASA I-II, 40-65 years old, body mass index [BMI] < 30 kg/m2) undergoing pulmonary lobectomy were enrolled. They were divided randomly into two equal groups before anaesthesia induction to receive either intravenous injection of 1 μg/kg dexmedetomidine for 20 min (Dexmedetomidine) or not (Control). Results: The results showed no difference in heart rate (HR), mean arterial pressure (MAP) and bispectral index (BIS) between the two groups, as well as liquid intake and output volume (LIO), duration of OLV and time from surgery beginning to excision of pathological tissues (P > 0.05). Levels of tumor necrosis factor (TNF-α) and malondialdehyde (MDA) in Dexmedetomidine group were lower than that of Control at OLV 60 and 90 (P < 0.05). Superoxide dismutase (SOD) activity and the expression level of HO-1 were higher in Dexmedetomidine group than in Control (P < 0.05). Conclusions: Dexmedetomidine pretreatment could upregulated expression of HO-1 in lung tissue and reduce oxidative stress and inflammation during OLV. Thus dexmedetomidine played a role in protecting lung injury by promoting HO-1 expression.
Keywords: Dexmedetomidine, one-lung ventilation, heme oxygenase-1, oxidative stress
Introduction
Despite its wide application in thoracic surgery, one lung ventilation (OLV) also has some negative effects on patients, such as hypoxemia, acute hypoxic pulmonary vasoconstriction, and ischemia-reperfusion injury [1-4]. Among them, lung ischemia/reperfusion injury is often related to enhanced oxidative stress, increased oxygen free radicals, strengthened lipid peroxidation and declined ability to antioxidant of body [5]. The accumulated oxygen free radicals cause damages to pulmonary capillary endothelial cells and alveolar epithelial cells, leading to lung exudation and edema [6]. Furthermore, longtime inhalation of high concentration of oxygen can also cause oxidative stress and lead to lung damage [7].
The stress response caused by ischemia/reperfusion not only leads to cell damage and death, but also induces the expression of various of special genes and synthesis of the stress-related proteins [8], including heme oxygenase (HO), which plays a protective role in stress reaction to such as ischemia and reperfusion [9]. One protein HO-1 has been proved with a protective effect by anti-inflammatory, anti-oxidant and anti-apoptosis [9].
In recent years, it has been recognized that there was a close relationship between oxygen free radicals and anesthesia [10]. Dexmedetomidine as a sedative, has many advantages of inhibiting sympathetic activities, stabilizing haemodynamics [11-13], anti-inflammatory and organ protection [14,15]. Therefore, it was hypothesis that dexmedetomidine have an effect on the oxidative stress by HO-1 regulation. In this study, a randomized controlled trial was designed to evaluate the effects of dexmedetomidine pretreatment on the expression of HO-1 and oxidative stress during OLV in lung cancer patients.
Methods
Patients
A total of 50 patients with lung carcinoma (ASA I-II, 40-65 years old, body mass index [BMI] < 30 kg/m2) undergoing elective pulmonary lobectomy were enrolled in this study. All patients required open thoracotomy for lung surgery and OLV. Exclusion criteria included uncontrolled hypertension (baseline blood pressure ≥ 160/100 mmHg), serious liver and kidney dysfunction, heart diseases (such as severe coronary heart disease, cardiac arrhythmias), history of neurological and psychiatric disease, endocrine and immune system disease history, and history of alcoholism or drug abuse. All the patients received no medication before operation, and the ones with pulse oxygen saturation (SPO2) < 95% after OLV were also excluded. This study was approved by the Ethics Committee of Taian City Central Hospital and all the patients signed the informed consents.
Anesthesia method
All patients received 0.07 mg/kg i.m. midazolam half an hour before surgery. The vein of left-side upper extremity was opened. Routine monitoring was applied preoperatively including electrocardiogram (EGG), a right internal jugular vein catheter for continuous central venous pressure (CVP) monitoring, a left radial artery catheter for blood pressure monitoring and arterial blood gas sampling, heart rate (HR) and bispectral index (BIS). These patients were divided randomly into two equal groups before induction of general anaesthesia to receive either intravenous injection of 1 μg/kg dexmedetomidine (4 μg/ml; Batch No. 12101934; Hengrui Medical Co., LTD, China.) for 20 min (Dexmedetomidine group) or not (Control group). Then general anesthesia was induced with 0.4 μg/kg sufentanil and 2 mg/kg propofol. When BIS value decreased to 50-55, 0.6 mg/kg rocuronium bromide was injected. A left-side double lumen tube (Robertshaw, USA) was inserted after 90 s and connected to an anesthesia apparatus (Drager, Fabius, Germany) for mechanical ventilation after confirmed position by fiber bronchoscope (BFS) and auscultation. Parameter settings included tidal volume (VT) of 8-9 ml/kg, respiratory frequency (f) of 10-12 breaths/min, inspiratory/expiratory ratio (I:E) of 1:2, oxygen flow of 2 L/min, fraction of inspired oxygen (FiO2) of 100%, and end-tidal CO2 partial pressure (PETCO22) of 35-45 mmHg.
Anesthesia was maintained with a continuous intravenous infusion of 0.10-0.20 μg/(kg min) remifentanil and 3-8 mg/(kg h) propofol, and intravenous infusion of 0.15 mg/kg rocuronium bromide was given discontinuously for muscle relaxation. At the beginning of OLV, respiration parameters were regulated to VT of 5-7 ml/kg, f of 14-18 breaths/min, SPO2 maintained at 95-100%, PETCO2 maintained at 35-45 mmHg, FiO2 of 100%, and BIS maintained at 45-65. During the operation, HR was maintained at 50-100 breaths/min. The variations in mean arterial pressure (MAP) were maintained within 20% of baseline values, and atropine, urapidil and ephedrine were used when necessary.
Conventional indices observation
The values of MAP, HR and BIS were recorded respectively at baseline (5 min after arrival to the operating room), the time immediately before intubation, 1 min after intubation, the beginning and the end of OLV. The duration of OLV, the time and liquid intake and output volume (LIO) from surgery beginning to excision of pathological tissues were also recorded.
Determination of oxidative stress indicators
The blood samples were collected from jugular vein at 5 min after arrival to the operating room (Baseline), 30 (OLV 30), 60 (OLV 60) and 90 min (OLV 90) after OLV, with the supernatant to be tested. Analyses for superoxide dismutase activity (SOD) and malondialdehyde (MDA) were conducted using colorimetric procedures with commercially available kits (Nanjing Jian Cheng institute of Bio-engineering, Nanjing, China) according to previous research [16,17]. The concentration of tumor necrosis factor-α (TNF-α) was measured by ELISA [18].
Measurement of HO-1 expression
The abnormal lung tissue from excisional pathological lung tissues during surgery was cut off at the size of 3 cm × 3 cm × 3 cm above 5 cm away from the tumor. The tissue was splited with cell lysate followed by ultrasonication. The supernatant was obtained by centrifugation at 4°C and detected for protein by Lowry method [19]. Western blot was performed to detect the expression of HO-1 in the abnormal lung tissue. The relative expression level was shown as the ratio of HO-1 and GAPDH (reference) average gray values.
Statistical analyses
All the data were expressed as mean ± standard deviation (SD). Data at the same time point were analyzed by t test for intergroup comparisons. Data at different time points were analyzed by one-way analysis of variance (ANOVA) for intragroup comparisons followed by post hoc pairwise comparisons using LSD test. All analyses were performed using SPSS 15.0 (SPSS, Inc., Chicago, IL, USA) and differences were considered statistically significant with P < 0.05.
Results
Conventional indices analysis
There was no difference in demographic characteristics between Dexmedetomidine and Control groups, including patients’ age, gender and BMI (P > 0.05, data not shown). Meanwhile, dexmedetomidine pretreatment had no effects on the MAP, HR and BIS values at each time point compared with Control group (P > 0.05, Table 1). Furthermore, no difference was observed in the duration of OLV, the time and LIO from surgery beginning to excision of pathological tissues between the two groups (P > 0.05, Table 2).
Table 1.
Changes in mean arterial pressure (MAP), heart rate (HR) and bispectral index (BIS) values during operation
| Monitoring indices | Groups (n = 50) | Baseline | Before intubation | After intubation | Beginning of OLV | End of OLV |
|---|---|---|---|---|---|---|
| MAP (mmHg) | Dexmedetomidine (n = 25) | 101 ± 8 | 93 ± 10 | 102 ± 6 | 98 ± 9 | 94 ± 11 |
| Control (n = 25) | 96 ± 7 | 95 ± 9 | 105 ± 10 | 94 ± 8 | 97 ± 12 | |
| HR (breaths/min) | Dexmedetomidine (n = 25) | 73 ± 8 | 62 ± 10 | 74 ± 11 | 70 ± 9 | 68 ± 7 |
| Control (n = 25) | 75 ± 6 | 68 ± 9 | 78 ± 12 | 74 ± 7 | 70 ± 5 | |
| BIS value | Dexmedetomidine (n = 25) | 93.21 ± 2.2 | 51.34 ± 3.1 | 54.10 ± 1.1 | 52.34 ± 2.1 | 49.25 ± 3.0 |
| Control (n = 25) | 92.18 ± 1.16 | 53.18 ± 2.10 | 55.45 ± 2.11 | 54.21 ± 1.18 | 50.28 ± 2.34 |
OLV: One lung ventilation. A t test was used to analyze the differences between Dexmedetomidine and Control groups at the same time point. There was no difference in the MAP, HR and BIS values between the two groups (P > 0.05).
Table 2.
Duration of OLV, the time and LIO from surgery beginning to excision of pathological tissues between the Dexmedetomidine and Control groups during operation
| Item | Groups (n = 50) | |
|---|---|---|
|
| ||
| Dexmedetomidine (n = 25) | Control (n = 25) | |
| Duration of OLV (min) | 62 ± 11 | 58 ± 13 |
| Time from surgery beginning to excision of pathological tissues (min) | 74 ± 12 | 75 ± 9 |
| LIO (mL) | 740 ± 30 | 780 ± 20 |
OLV: One lung ventilation. LIO: Liquid intake and output volume. A t test was used to analyze the differences between Dexmedetomidine and Control groups. There was no difference in the duration of OLV, the time and LIO from surgery beginning to excision of pathological tissues between the two groups (P > 0.05).
Detection of TNF-α, MDA, SOD and HO-1 levels
Despite significant increase in TNF-α and MDA levels of both Dexmedetomidine and Control groups at OLV 60 and 90 compared with Baseline (P < 0.05), TNF-α and MDA levels of Dexmedetomidine group were lower than that of Control at the two time points (P < 0.05, Table 3). Both Dexmedetomidine and Control groups had decreased SOD activities during OLV (P < 0.05) compared with Baseline, however, SOD activities in Dexmedetomidine group was higher than Control at each time point of OLA, significantly at OLV 60 and 90 (P < 0.05). As shown in Figure 1, the relative expression level of HO-1 in Dexmedetomidine group (0.334 ± 0.05) was significantly higher than that of Control (0.125 ± 0.15, P < 0.05).
Table 3.
Tumor necrosis factor-α (TNF-α), superoxide dismutase activity (SOD) and malondialdehyde (MDA) at different time points
| Monitoring Index | Groups (n = 50) | Baseline | OLV 30 | OLV 60 | OLV 90 |
|---|---|---|---|---|---|
| TNF-α (ng/L) | Dexmedetomidine (n = 25) | 16 ± 4 | 20 ± 6 | 24 ± 5*,# | 26 ± 7*,# |
| Control (n = 25) | 14 ± 5 | 18 ± 3 | 34 ± 7* | 38 ± 4* | |
| MDA (nmol/mL) | Dexmedetomidine (n = 25) | 4.85 ± 0.19 | 4.92 ± 0.20 | 5.80 ± 0.45*,# | 7.01 ± 0.38*,# |
| Control (n = 25) | 4.17 ± 0.17 | 4.89 ± 0.18 | 6.75 ± 1.10* | 8.11 ± 1.20* | |
| SOD (U/mL) | Dexmedetomidine (n = 25) | 465 ± 35 | 444 ± 47 | 421 ± 34*,# | 409 ± 36*,# |
| Control (n = 25) | 453 ± 42 | 438 ± 55 | 366 ± 54* | 312 ± 33* |
Compared with Baseline, P < 0.05.
Compared with Control group, P < 0.05.
Data at the same time point were analyzed by t test for between-group comparisons. Data at different time points were analyzed by one-way analysis of variance (ANOVA) for intro-group comparisons.
Figure 1.
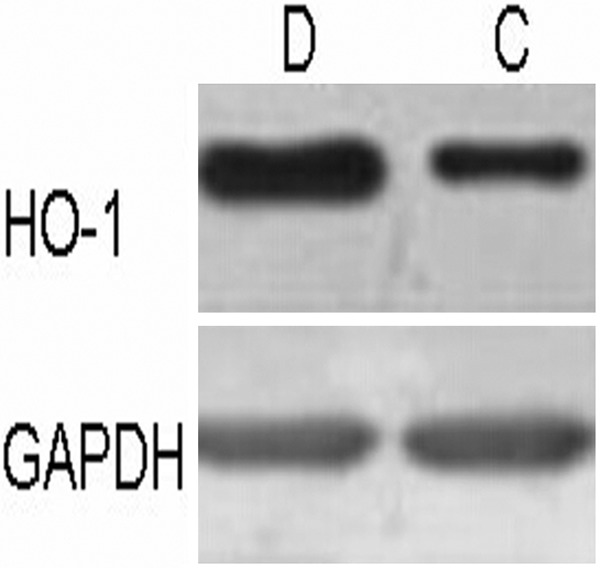
The relative expression levels of heme oxygenase-1 (HO-1) protein in the abnormal lung tissue of Dexmedetomidine (D) and Control (C) groups. Data were shown as the ratio of HO-1 and GAPDH (reference) average gray values.
Discussion
During OLV, hypoxemia and ischemia-reperfusion injury often occur, and cause enhanced oxidative stress and secretion of many kinds of cytokines [5]. As previous researches reported a protective effect of HO-1 and its metabolites by anti-inflammatory, anti-oxidant and anti-apoptosis [9,20] and a close relationship between oxygen free radicals and anesthesia [10], this study evaluated the effects of dexmedetomidine pretreatment on oxidative stress reaction and HO-1 expression during OLV in lung cancer patients, in order to explore the mechanisms by which dexmedetomidine protect organ.
TNF-α is an important inflammatory cytokine and plays an initiative role in the development of inflammatory reactions [21,22]. MDA content and SOD activity are the indices of oxidation-antioxidant balance, which could reflect the degree of oxidative stress in the process of cerebral ischemia/reperfusion injury [23]. In this study, the levels of TNF-α and MDA in Dexmedetomidine group were lower than that of Control group at OLV 60 and 90, and the SOD activity was also higher than Control at each time point of OLV. Our results suggested that dexmedetomidine pretreatment could alleviate the inflammatory reaction and oxidative stress reaction caused by OLV, which was consistent with previous studies [24]. Therefore, it seemed that dexmedetomidine could alleviate the lung ischemia/reperfusion injury and protect the lung tissue.
HO-1 is the only inducible enzyme among the three HO isozymes and could be induced by many factors, such as hemoglobin, heavy metal, cytokines, hypoxia, H2O2, high temperature and ultraviolet radiation [25], all of which could cause oxidative stress [26]. It has been found that HO-1 over-expression could reduce the ischemia/reperfusion injury, and the animal with HO-1 gene knockout is more sensitive to ischemia/reperfusion injury [27]. The results showed that the HO-1 expression level in abnormal lung tissue of Dexmedetomidine group was significantly higher than that of Control, indicating that dexmedetomidine could protect against inflammatory and oxidative stress reactions by promoting the expression of HO-1. Deng et al [28] also explored the levels of IL-1 beta, IL-10, TNF-α, MDA, myeloperoxidase (MPO) and xanthine oxidase (XOD) during OLV and found that dexmedetomidine showed protection effects on lung via limiting extent of inflammatory and redox reactions, which was consistent with our result. Another study proved that dexmedetomidine might protect the lung from ischemia-reperfusion injury in patients undergoing OLV through decreasing the activity of plasma XOD and MPO and the number of polymorphonuclear leukocytes (PMN) [29]. However, the specific mechanism of dexmedetomidine during OLV was still controversial. Hofer S et al [30] reported that dexmedetomidine inhibited sympathetic activity by activating α2 adrenergic receptors and cholinergic anti-inflammatory pathway. Gu J et al [31] found that dexmedetomidine could reduce the expression of intercellular adhesion molecule-1 (ICAM-1) and MPO activity to relieve the lung damage caused by renal ischemia/reperfusion injury. They also suggested that dexmedetomidine could target on phosphatidyl inositol-3 kinase (PI3K-Akt) signaling pathway through α2 receptor to reduce the apoptosis, inhibit the release of high mobility family protein B1, and then inhibit the Toll-like receptor-4 signal transduction, to protect against renal ischemia/reperfusion injury. In our study, it was demonstrated that dexmedetomidine played a role in protecting lung injury by promoting the expression of HO-1. Thus, further researches were needed to explore the functional mechanism and pathway of dexmedetomidine during OLV.
Several limitations to this study must be addressed. First, the cases of patients were insufficient, and the statistical accuracy might exist. Second, our single-hospital experience wasn’t generalized to the broader community. Hence, further experimental research might be needed on a larger number of patients with lung carcinoma from several hospitals.
In conclusion, dexmedetomidine pretreatment could promote the expression of HO-1 in lung tissue and reduce oxidative stress and inflammation during OLV. Therefore, it seemed that dexmedetomidine has a protective effect on lung ischemia/reperfusion injury caused by OLV.
Acknowledgements
We wish to express our warm thanks to Fenghe (Shanghai) Information Technology Co., Ltd. Their ideas and help gave a valuable added dimension to our research.
Disclosure of conflict of interest
None.
References
- 1.Misthos P. The degree of oxidative stress is associated with major adverse effects after lung resection: a prospective study. Eur J Cardiothorac Surg. 2006;29:591–595. doi: 10.1016/j.ejcts.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Birdas TJ. Oxidative stress and one-lung ventilation. Eur J Cardiothorac Surg. 2006;30:412–413. doi: 10.1016/j.ejcts.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Yuluğ E, Tekinbas C, Ulusoy H, Alver A, Yenilmez E, Aydin S, Cekiç B, Topbas M, İmamoğlu M, Arvas H. The effects of oxidative stress on the liver and ileum in rats caused by one-lung ventilation. J Surg Res. 2007;139:253–260. doi: 10.1016/j.jss.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 4.Huang CH, Wang YP, Wu PY, Chien CT, Cheng YJ. Propofol infusion shortens and attenuates oxidative stress during one lung ventilation. Acta Anaesthesiol Taiwan. 2008;46:160–165. doi: 10.1016/S1875-4597(09)60003-5. [DOI] [PubMed] [Google Scholar]
- 5.Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biology. 2014;2:702–14. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halladin N, Ekeløf S, Alamili M, Bendtzen K, Lykkesfeldt J, Rosenberg J, Gögenur I. Lower limb ischaemia and reperfusion injury in healthy volunteers measured by oxidative and inflammatory biomarkers. Perfusion. 2015;30:94–70. doi: 10.1177/0267659114530769. [DOI] [PubMed] [Google Scholar]
- 7.Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, Roberts LJ, Arduini A, Escobar JJ, Sastre J. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. 2009;124:e439–e449. doi: 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- 8.Bogoyevitch MA, Gillespie-Brown J, Ketterman AJ, Fuller SJ, Ben-Levy R, Ashworth A, Marshall CJ, Sugden PH. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogenactivated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ Res. 1996;79:162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi T, Shimizu H, Morimatsu H, Maeshima K, Inoue K, Akagi R, Matsumi M, Katayama H, Morita K. Heme oxygenase-1 is an essential cytoprotective component in oxidative tissue injury induced by hemorrhagic shock. J Clin Biochem Nutr. 2009;44:28. doi: 10.3164/jcbn.08-210-HO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson JX, Gelb AW. Free radicals, antioxidants, and neurologic injury: possible relationship to cerebral protection by anesthetics. J Neurosurg Anesthesiol. 2002;14:66–79. doi: 10.1097/00008506-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J Dexmedetomidine for Long-Term Sedation Investigators. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307:1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 12.SHI M, Huang QQ. Research advance on clinical application of dexmedetomidine. Modern Medicine Health. 2011;7:042. [Google Scholar]
- 13.Blake D. Dexmedetomidine and hemodynamic responses to simulated hemorrhage in experimental heart failure. Anesth Analg. 2000;91:1112–1117. doi: 10.1097/00000539-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Can M, Gul S, Bektas S, Hanci V, Acikgoz S. Effects of dexmedetomidine or methylprednisolone on inflammatory responses in spinal cord injury. Acta Anaesthesiol Scand. 2009;53:1068–1072. doi: 10.1111/j.1399-6576.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XY, Liu ZM, Wen SH, Li YS, Li Y, Yao X, Huang WQ, Liu KX. Dexmedetomidine administration before, but not after, ischemia attenuates intestinal injury induced by intestinal ischemia-reperfusion in rats. Anesthesiology. 2012;116:1035–1046. doi: 10.1097/ALN.0b013e3182503964. [DOI] [PubMed] [Google Scholar]
- 16.Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s00425-017-2699-3. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Sun CB, Tong Y, Qu J. [Evaluation of serum levels of SOD and MDA in patients with Leber’s hereditary optic neuropathy carrying the mitochondrial DNA G11778A mutation] . Zhonghua Yan Ke Za Zhi. 2009;45:719–723. [PubMed] [Google Scholar]
- 18.Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54:M357–364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- 19.Nalinanon S, Benjakul S, Kishimura H, Shahidi F. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 2011;124:1354–1362. [Google Scholar]
- 20.Tsuchihashi SI, Zhai Y, Bo Q, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 mediated cytoprotection against liver ischemia and reperfusion injury: inhibition of type-1 interferon signaling. Transplantation. 2007;83:1628–1634. doi: 10.1097/01.tp.0000266917.39958.47. [DOI] [PubMed] [Google Scholar]
- 21.Xie GQ, Jiang JX, Chen YH, Liu DW, Zhu PF, Wang ZG. Induction of acute hepatic injury by endotoxin in mice. Hepatobiliary Pancreat Dis Int. 2002;1:558–564. [PubMed] [Google Scholar]
- 22.Kim GY, Roh SI, Park SK, Ahn SC, Oh YH, Lee JD, Park YM. Alleviation of experimental septic shock in mice by acidic polysaccharide isolated from the medicinal mushroom Phellinus linteus. Biol Pharm Bull. 2003;26:1418–1423. doi: 10.1248/bpb.26.1418. [DOI] [PubMed] [Google Scholar]
- 23.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Z JQ, Meng FM. Effect of dexmedetomidine on inflammatory response and oxidative stress during one-lung ventiliation. J Clin Anesthesiol. 2013;29:229–231. [Google Scholar]
- 25.Takahashi T, Morita K, Akagi R, Sassa S. Heme oxygenase-1: a novel therapeutic target in oxidative tissue injuries. Curr Med Chem. 2004;11:1545–1561. doi: 10.2174/0929867043365080. [DOI] [PubMed] [Google Scholar]
- 26.Lee HZ, Liu WZ, Hsieh WT, Tang FY, Chung JG, Leung HW. Oxidative stress involvement in Physalis angulata-induced apoptosis in human oral cancer cells. Food Chem Toxicol. 2009;47:561–570. doi: 10.1016/j.fct.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida T, Maulik N, Ho YS, Alam J, Das DK. Hmox-1 constitutes an adaptive response to effect antioxidant cardioprotection: A study with transgenic mice heterozygous for targeted disruption of the heme oxygenase-1 gene. Circulation. 2001;103:1695–1701. doi: 10.1161/01.cir.103.12.1695. [DOI] [PubMed] [Google Scholar]
- 28.Deng RX, Zhang S, Zhang JZ, Wu XH. Dexmedetomidine in protection of inflammation and lung injury during general anesthesia. Chinese Journal of Nosocomiology. 2013;10:035. [Google Scholar]
- 29.Yanan L, Yisa S, Rongzhi Z. The effect of dexmedetomidine on ischemia-reperfusion injury in patients undergoing one lung ventilation. Modern Medicine Journal of China. 2013;3:010. [Google Scholar]
- 30.Hofer S, Steppan J, Wagner T, Funke B, Lichtenstern C, Martin E, Graf BM, Bierhaus A, Weigand MA. Central sympatholytics prolong survival in experimental sepsis. Crit Care. 2009;13:R11. doi: 10.1186/cc7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu J, Sun P, Zhao H, Watts HR, Sanders RD, Terrando N, Xia P, Maze M, Ma D. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15:R153. doi: 10.1186/cc10283. [DOI] [PMC free article] [PubMed] [Google Scholar]


