Abstract
The aim of the study is to determine the levels of E-cadherin, vimentin expression in tumor tissues from patients with oral squamous cell carcinoma (OSCC), and the relationship between the expression of E-cadherin, vimentin and epithelial-mesenchymal transition, in order to explore its values for predicting the invasion and metastasis of oral squamous cell carcinoma, short survival of patients in many types of cancer. E-cadherin and vimentin expression of 10 benign and 42 OSCC tumor tissues was examined by immunohistochemical staining. E-cadherin is positively expressed in normal oral mucosa epithelium, but vimentin expression is not found in normal oral mucosa epithelia; the E-cadherin and vimentin were expressed in 26 of 42 (61.9%) and 16 of 42 (38.1%), respectively. No statistically difference was found for E-cadherin and vimentin expression in patients with different age, gender and tumor location, E-cadherin and vimentin expression was significantly associated with lymph node metastasis and tissue location (P < 0.05); E-cadherin expression was also significantly associated with tumor stage (P < 0.05); there are significantly difference between infiltrative margin and central area in patients with oral squamous cell carcinoma for E-cadherin and vimentin positive expression (P < 0.05). E-cadherin and vimentin positive expression was associated with tumor metastasis of oral squamous cell carcinoma. Our study preliminarily confirmed that EMT phenomenon is existed during the development of oral squamous cell carcinoma. Co-evaluation of E-cadherin and vimentin might be a valuable tool for predicting OSCC patient outcome.
Keywords: Oral squamous cell carcinoma, immunohistochemistry, E-cadherin, vimentin, epithelial-mesenchymal transition
Introduction
Head and neck squamous cell carcinoma (HNSCC) ranks sixth among the most common cancers worldwide with an incidence of over 500,000 new cases each year [1]. Oral squamous cell carcinoma (OSCC) is the most prevalent malignancy in oral cavity [2], which is a disease found particularly in low income communities and mainly a problem of older men, 90% being in > 45 year age group who are exposed to the known risk factors of tobacco and/or alcohol (IARC 2004) [3].
Tumor metastasis is a complex dynamic process of multi factors, multi steps, and tumor invasion is the first implementation of metastasis. EMT may lead to adhesion between epithelial cells decrease and loss, also get the phenotypic characteristics of the interstitial cells, thus giving the migration of tumor cells enhanced the invasive ability, but also provides an idea of explanation of the mechanism of tumor metastasis. Due to the different parts of the tumor tissue is often great heterogeneity, Bryne proposed the tumor invasive front (ITF) theory, and it refers to the tumor host junction in the forefront of the 3 to 6 layers of tumor cells or dispersed cell group [4,5], there exist great differences in the morphological characteristics, cell differentiation degree and biological behavior of tumor cells, which are more objective to assess recurrence and prognosis of the tumor.
The concept of epithelial and mesenchymal transition (EMT) was first proposed by Greenberg et al. [6]. EMT may play a key role in tumor invasion and metastasis; in recent years, a growing number of studies have shown that many malignant tumor cell migration ability is achieved by EMT.
E-cadherin (Epitheia-cadhein) is a calcium-dependent transmembrane glycoprotein located in the epithelial tissue, which is an important cell adhesion molecule and signal transduction factor, can direct the formation of protein complexes attached to the actin cytoskeleton in combination with beta-catenin formation which can prevent and reduce tumor cell adhesion. E-cadherin is an important symbol of occurrence of the loss of EMT. Vimentin is a cytoskeletal protein, not expressed in normal epithelial cells, but widely distributed in fibroblasts, endothelial cells, and lymphocytes in the interstitial cells. Several studies have found that the abnormal expression of vimentin was also observed in a variety of epithelial tumors, and had close relationship with differentiation, invasion and metastasis of cancer cell.
The aims of this study were as follows: 1) to detect the expression of E-cadherin and vimentin in normal oral mucosa and oral squamous cell carcinoma; 2) to compare the expression level of E-cadherin and vimentin between the center and infiltrating margin in oral squamous cell carcinoma; 3) to confirm whether the expression of E-cadherin and vimentin are associated with pathological grade, lymph node metastasis, EMT and clinical factors, Which provide basis for the mechanism of metastasis and recurrence in further study.
Subjects and methods
A total of 42 individual oral SCCs specimens were taken at Yijishan Hospital of Wannan Medical College from 2010 to 2012, which consist of 23 male and 19 female. The age of the study patients was from 34 to 86 years at the time of diagnosis. Of these 42 patients, 23 cases of tongue cancer, 6 patients with buccal carcinoma, 5 cases with gingival carcinoma, 3 cases with mouth floor carcinoma, 2 cases with palate carcinoma, 1 case with lip and chin carcinoma. Another 10 cases of normal oral mucosa tissues as control, all the normal oral mucosa tissues were derived from oral mucosa which was more than 2 cm from the tumor edge, and confirmed by pathological examination of oral mucosal negative. Two investigators assessed the slides without knowledge of the clinicopathological features and were blinded to each other’s evaluation. They were in agreement on all the slides examined. The diagnosis, differential diagnosis and pathological classification of oral squamous cell carcinoma are based on the seventh edition “oral histopathology and pathology” [7].
Antibodies
Mouse anti-human monoclonal antibody to vimentin was purchased from Wuhan Boster Biological Engineering Co., Ltd (China). Mouse anti-human monoclonal antibody to E-cadherin was purchased from Fuzhou Maixin biological Technology Development Company (Fuzhou, China).
Experimental methods
SP immunohistochemical staining was conducted strictly in accordance with the methods which were offered by Maixin Co. E-cadherin and vimentin positive plate were used for positive control, and PBS instead of the first antibody for negative control.
Result determination
E-cadherin staining was mainly localized in the cell membrane, the positive expression of yellow or brown/brown coloring; vimentin staining was localized in the cytoplasm, by the appearance of clear brown (dark brown) color particles determined as positive. Immunohistochemical staining results were determined by semi quantitative scoring method, based on the expression of intensity integration: no coloring is 0; the yellow is 1; brown is 2; dark brown is 3. At low magnification, selected specimens positive cells and uniform distribution area, the mean percentage of random counting 5 unique, non overlapping high power field of positive cells, according to the percentage of positive cell integral: 0~5% to 0; 6~25% to 1; 26~50% to 2; 51 to 75% to 3 more than 75% to 4. Finally, the two integral multiplications, the integral is greater than or equal to 2 divided into positive.
Statistical analysis
Statistical analysis was performed by R program language, percent of gender, age, tumor location, lymph node metastasis, differential degree, tissue location, positive expression of E-cadherin and vimentin between two groups was conducted by χ2 test or Fisher’s exact test. A P-value of less than 0.05 was considered to be statistically significant.
Results
Expression of E cadherin and vimentin in normal oral mucosa
E-cadherin positive expression was found on cell membrane of normal oral mucosa epithelial tissues but vimentin. However, vimentin positive expression was observed in submucous connective tissue and in cytoplasm of mesenchymal cells. As showed in Figure 1A and 1B.
Figure 1.
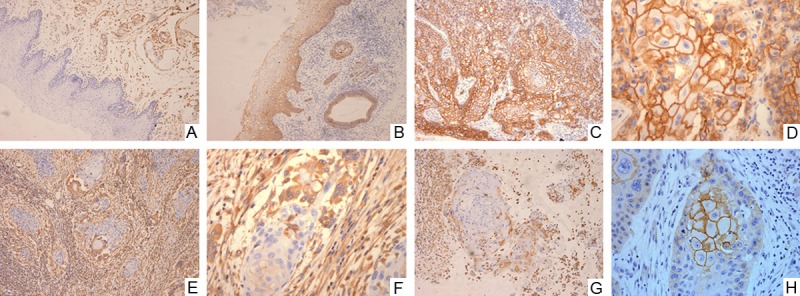
Vimentin was detected in the cytoplasm of the connective tissue mesenchymal cells of the normal oral mucosal tissue but not in the normal squamous epithelium (A×100); E-cadherin strong positive expression was observed in the cell membrane in normal oral mucous membrane (B×100). E-cadherin strong positive expression was observed in the cell membrane in oral squamous cell carcinoma tissue (C×100; D×400). vimentin strong positive expression was observed in the infiltrative margin in oral squamous cell carcinoma tissue (E×100; F×400). Vimentin strong positive expression was observed in cytoplasm in oral squamous cell carcinoma infiltrative margin tissue, vimentin expression was not detected in the central area. (G×100); E-cadherin strong positive expression was observed in the cell membrane in the central area in oral squamous cell carcinoma tissue, vimentin expression was not detected in the tumor periphery (H×400).
Association between E-cadherin, vimentin expressions and clinic pathologic feature
E-cadherin has 83.3% (10/12), 61.9% (13/21) and 33.3% (3/9) positive expression rate in well-, moderate- and poor-differentiation of oral squamous cell carcinomas respectively (Figure 1C and 1D). Vimentin has 25% (3/12), 33.3% (7/21) and 66.7% (6/9) positive expression rate in high, medium and low differentiation of oral squamous cell carcinomas (Figure 1E and 1F). Trend chi-square test results show that E-cadherin expression was associated with differentiation degree of oral squamous cell carcinomas (P < 0.05), while there were no significant difference on vimentin positive expression among different differentiation degree (P > 0.05) (Figure 1C and 1D). In addition to these two kinds of protein expression are not associated with clinical pathological feature (patients’ age and gender) (P > 0.05), but is significantly associated with lymph node metastasis and parts of the organization (P < 0.05), E-cadherin positive expression rate was higher in tissue without lymph node metastasis as compared to lymph node metastasis (P < 0.05). Positive rate of vimentin expression was lower in tissue without lymph node metastasis as compared to lymph node metastasis (P < 0.05). E-cadherin positive expression rate was higher in center area as compared to infiltrative margin (P < 0.05). Vimentin positive expression rate was lower in center area as compared to infiltrative margin (P < 0.05) (Figure 1G and 1H). The details are shown in Table 1.
Table 1.
Expression of E-cadherin, vimentin in oral squamous cell carcinoma
| Variable | E-cadherin | Vimentin | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Positive | Negative | χ2/Z | P | Positive | Negative | χ2/Z | P | |
| gender | ||||||||
| Male (23) | 15 | 8 | 0.237 | 0.627 | 8 | 15 | 0.237 | 0.627 |
| Female (19) | 11 | 8 | 8 | 11 | ||||
| Age (years) | ||||||||
| ≤ 50 (9) | 6 | 3 | 0.000A | 1.000 | 2 | 7 | 1.826A | 0.177 |
| > 50 (33) | 20 | 13 | 14 | 19 | ||||
| Tumor location | ||||||||
| Tongue (23) | 16 | 7 | 0.173B | 0.678 | 9 | 14 | 0.040B | 0.841 |
| Buccal (7) | 3 | 4 | 2 | 4 | ||||
| Gingival (5) | 2 | 3 | 1 | 4 | ||||
| Other | 5 | 2 | 4 | 4 | ||||
| Lymph node metastasis | ||||||||
| Yes (17) | 5 | 12 | 12.786 | 0.000 | 11 | 6 | 8.576 | 0.003 |
| No (25) | 21 | 4 | 5 | 20 | ||||
| Differentiated degree | ||||||||
| High (12) | 10 | 2 | 5.267B | 0.022 | 3 | 9 | 3.418B | 0.064 |
| Medium (21) | 13 | 8 | 7 | 14 | ||||
| Low (9) | 3 | 6 | 6 | 3 | ||||
| Tissue location | ||||||||
| Central area | 24 | 18 | 14.775 | 0.000 | 5 | 37 | 7.683 | 0.006 |
| Infiltrative margin | 7 | 35 | 16 | 26 | ||||
For value of continuity correction chi-square;
A for value of linear-by-linear association chi-square.
Relationship between E-cadherin and vimentin expressions in the infiltrative margin and the central area in oral squamous cell carcinoma
Further observed that vimentin positive expression was detected in 3 of 24 case with E-cadherin positive expression in central area, and E-cadherin positive expression was detected in 3 of 16 case with vimentin positive expression in infiltrative margin, Spearman’s analysis revealed that the expressions of E-cadherin were negatively correlated with vimentin expression in both central area and infiltrative margin (P < 0.05), as showed in Table 2.
Table 2.
Relationship between E-cadherin and vimentin expressions in the infiltrative margin and the central area in oral squamous cell carcinoma
| E-cadherin (central area) | Vimentin (central area) | E-cadherin (infiltrative margin) | Vimentin (infiltrative margin) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Positive | Negative | P | Positive | Negative | P | |||
| Positive | 3 | 21 | 0.000 | Positive | 3 | 4 | 0.049 | |
| Negative | 2 | 16 | Negative | 13 | 22 | |||
Discussions
The present study found that E-cadherin is positive expressed in normal oral mucosa epithelium, but vimentin expression is not found in normal oral mucosa epithelial, E-cadherin and vimentin expression was significantly associated with lymph node metastasis and tissue location (P < 0.05), and E-cadherin expression was also significantly associated with tumor stage (P < 0.05), which is inconsistent with previous study.
Previous study found that there are decreasing or no expression of E-cadherin and higher expression of Vimentin among intrahepatic bile duct cancer [8] and breast cancer [9], and a in vitro tests of OSCC cell line detected that expression of E-cadherin was down regulated and vimentin up-regulation [10]. Miyazawa found that there are high mobility group protein A-2 (HMGA-2) expression up-regulation in the OSCC infiltration, which is associated with EMT related factor [11]. The change expression of E-cadherin/β-catenin [12], vimentin [13] were also observed in the front infiltration of OSCC; Nguven et al. [14] using immunohistochemical method confirmed the high expression of N-cadherin and the metastasis and invasion of cancer cells are associated with HNSCC patients, Nijkamp et al. also reported low expression of E-cadherin in head and neck squamous cell carcinoma more prone to transfer than high expression group [15].
In conclusion, expression of E-cadherin and vimentin in oral squamous cell carcinoma has a contribution to judge whether there are EMT occurrence in the process of the development. The combined detection of E-cadherin and vimentin has a prognosis value for the patients with oral squamous cell carcinoma judgment. There are also some limitation in this study, for example, sample size is small, especially the lack of cases with low differentiation and high differentiation, and the lack of clinical staging and postoperative follow-up data of the selected cases. Thus, increasing sample is need in future study, and conduct a long-term follow-up to further confirm the relationship between EMT and the prognosis of the patients with oral squamous cell carcinoma.
Acknowledgements
This work was supported by Provincial Natural Science Research Project of Anhui Colleges (No. KJ2014A269).
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Cao ZG, Li CZ. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter enhances oral squamous cell carcinoma susceptibility in a Chinese population. Oral Oncol. 2006;42:32–8. doi: 10.1016/j.oraloncology.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Scully C, Bagan J. Oral squamous cell carcinoma: overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009;15:388–99. doi: 10.1111/j.1601-0825.2009.01563.x. [DOI] [PubMed] [Google Scholar]
- 4.Bryne M, Boysen M, Alfsen CG, Abeler VM, Sudbø J, Nesland JM, Kristensen GB, Piffko J, Bankfalvi A. The invasive front of carcinomas. The most important area for tumour prognosis? Anticancer Res. 1998;18:4757–64. [PubMed] [Google Scholar]
- 5.Piffkò J, Bànkfalvi A, Ofner D, Bryne M, Rasch D, Joos U, Böcker W, Schmid KW. Prognostic value of histobiological factors (malignancy grading and AgNOR content) assessed at the invasive tumour front of oral squamous cell carcinomas. Br J Cancer. 1997;75:1543–6. doi: 10.1038/bjc.1997.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95:333–9. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shifeng Y. Oral histopathology and pathology. Beijing: People’s Medical Publishing House; 2012. [Google Scholar]
- 8.Yao X, Wang X, Wang Z, Dai L, Zhang G, Yan Q, Zhou W. Clinicopathological and prognostic significance of epithelial mesenchymal transition-related protein expression in intrahepatic cholangiocarcinoma. Onco Targets Ther. 2012;5:255–61. doi: 10.2147/OTT.S36213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Zhang X, Shang M, Zhang Y, Xia B, Niu M, Liu Y, Pang D. Dysregulated expression of Slug, vimentin, and E-cadherin correlates with poor clinical outcome in patients with basal-like breast cancer. J Surg Oncol. 2013;107:188–94. doi: 10.1002/jso.23240. [DOI] [PubMed] [Google Scholar]
- 10.Krisanaprakornkit S, Iamaroon A. Epithelial-mesenchymal transition in oral squamous cell carcinoma. ISRN Oncol. 2012;2012:681469. doi: 10.5402/2012/681469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyazawa J, Mitoro A, Kawashiri S, Chada KK, Imai K. Expression of mesenchyme-specific gene HMGA2 in squamous cell carcinomas of the oral cavity. Cancer Res. 2004;64:2024–9. doi: 10.1158/0008-5472.can-03-1855. [DOI] [PubMed] [Google Scholar]
- 12.Mehendiratta M, Solomon MC, Boaz K, Guddattu V, Mohindra A. Clinico-pathological correlation of E-cadherin expression at the invasive tumor front of Indian oral squamous cell carcinomas: An immunohistochemical study. J Oral Maxillofac Pathol. 2014;18:217–22. doi: 10.4103/0973-029X.140753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu LK, Jiang XY, Zhou XX, Wang DM, Song XL, Jiang HB. Upregulation of vimentin and aberrant expression of E-cadherin/beta-catenin complex in oral squamous cell carcinomas: correlation with the clinicopathological features and patient outcome. Mod Pathol. 2010;23:213–24. doi: 10.1038/modpathol.2009.160. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen PT, Kudo Y, Yoshida M, Kamata N, Ogawa I, Takata T. N-cadherin expression is involved in malignant behavior of head and neck cancer in relation to epithelial-mesenchymal transition. Histol Histopathol. 2011;26:147–56. doi: 10.14670/HH-26.147. [DOI] [PubMed] [Google Scholar]
- 15.Nijkamp MM, Span PN, Hoogsteen IJ, van der Kogel AJ, Kaanders JH, Bussink J. Expression of E-cadherin and vimentin correlates with metastasis formation in head and neck squamous cell carcinoma patients. Radiother Oncol. 2011;99:344–8. doi: 10.1016/j.radonc.2011.05.066. [DOI] [PubMed] [Google Scholar]


