Abstract
Less than 40 cases of primary pleural synovial sarcoma (SS) have been reported to date. Furthermore, only three cases of cystic SS have been documented in the English literature, including cases originating from sites other than the pleura. Herein, we present an exceedingly rare case of cystic SS originating from the mediastinal side of the visceral pleura in an asymptomatic 47-year-old man, which was detected during a checkup. On contrast-enhanced computed tomography, distinguishing between cystic SS and cystic thymoma was difficult because the tumor was attached to the anterior mediastinum where the latter type of malignancy is more often detected. Histopathological examination showed tumor cells with spindled morphology showing hypercellularity and moderate nuclear atypia, with less than one mitotic figure per high-power field. As these features are associated with both monophasic fibrous SS and type A thymoma, more data was required to determine proper diagnosis, and therefore, immunohistochemistry was performed. Along with a conventional panel of markers, the SS-specific marker integrase interactor 1 (INI-1) was applied and found to be decreased; decreased expression of INI-1 is characteristic of SS. A diagnosis of SS was confirmed by detection of the SYT-SSX fusion gene via fluorescence in situ hybridization. Given the relatively common availability of INI-1 testing in departments of pathology, this protein would be helpful incorporated into the standard panel of markers for diagnosing SS.
Keywords: Synovial sarcoma, cystic, pleura, SYT-SSX, INI-1
Introduction
Common sites of origin of synovial sarcoma (SS) are the thigh, knee, ankle, foot, and upper extremities [1]. In rare instances, SS arises in the thorax [2]. Primary SS of the pleura was first reported by Gaertner et al. in 1996 [3], and to date, less than 40 cases of this type of SS have been reported [4,5]. Historically, SS was thought to be related to the synovium; however, it was subsequently determined that no demonstrable relationship to synovial tissue exists [6]. While the histogenesis of SS has not yet been clarified, it is speculated to originate from a pluripotent mesenchymal cell, enabling epithelial differentiation [7,8]. SS is a malignant soft-tissue tumor of uncertain differentiation [9] and is classified histologically into monophasic fibrous, biphasic, and poorly differentiated categories [9]. These histologic patterns of SS present a wide range of differential diagnoses including spindle cell and/or small round cells neoplasms. If it takes the form of spindle cell morphology near the mediastinum, thymoma (especially type A) [10] is included in the differential diagnosis.
Cystic SS is extremely rare, although focal cystic change is often present in SS [1]. To the best of our knowledge, there have been only three documented cases in the English literature, including cases originating from other than the pleura [1,11,12]. On the other hand, cystic change is also encountered in thymomas; it has been reported that 40% of these tumors undergo focal cystic change [13]. Although more cases of cystic thymoma are documented than cystic SS [13-17], it is a relatively rare subset of the total number of thymoma cases.
Herein, we present an extremely rare case of cystic SS due to intratumoral hemorrhage in the anterior mediastinum, which was initially presumed to be a cystic thymoma radiologically. Even during intraoperative histopathologcial examination, the possibility of thymoma could not be ruled out owing to the spindled morphology of the tumor cells. Performing immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) in addition to morphological analysis enabled us to determine the correct diagnosis.
Case report
Clinical summary
A 47-year-old man presented at another facility with abnormities found on a chest radiograph obtained during a checkup. Computed tomography (CT) was performed, and an anterior mediastinal lesion, measuring approximately 10 cm at the maximal diameter, was found. The patient was then referred to our hospital. His laboratory tests revealed no remarkable findings. On contrast-enhanced CT, there was a cystic lesion in the anterior mediastinum, 98 × 74 × 72 mm in size, with irregular wall thickening containing a solid and bulky mural nodule that measured 36 × 32 × 30 mm. The wall of the lesion was enhanced, especially at the mural nodule and thickened areas (Figure 1A-C). A tumorous lesion, namely cystic thymoma, was suspected, and surgery was performed. The wall of the cystic lesion was ruptured during the operation and bloody material was discharged. Part of the wall was submitted for intraoperative histopathological examination, which revealed that the tissue was composed of atypical spindled cells, resulting in the differential diagnoses of a thymoma or sarcoma. The tumor was found to be originating from the mediastinal aspect of the visceral pleura and was firmly adhered to the upper lobe of the right lung. Resection of this lobe, including the entire tumor, was thus performed, separating it from the mediastinal pleura. Postoperative course was uneventful for a period of time without neoadjuvant chemotherapy. However, recurrence manifested 8 months after surgery in the form of pleural dissemination with increased pleural effusion, which was easily detectable after contrast enhancement (Figure 1D, 1E). Sample obtained by drainage of the effusion was submitted for cytopathological evaluation, which revealed atypical spindled cells and thus confirmed the pleural dissemination of the tumor.
Figure 1.
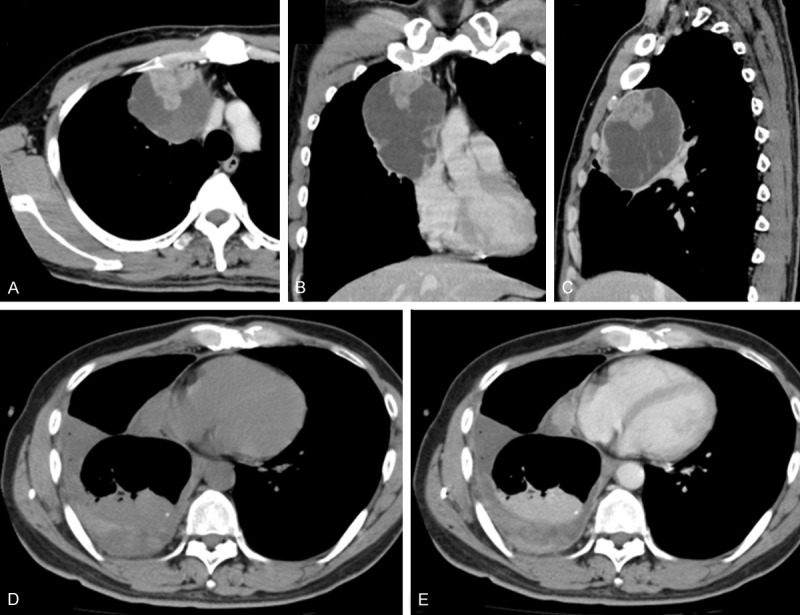
(A-C) Computed tomography (CT) findings with contrast enhancement before the operation. (A) Axial; (B) Coronal; (C) Sagittal. There was a cystic lesion in the anterior mediastinum, 98 × 74 × 72 mm in size, with irregular wall thickening containing a solid and bulky mural nodule that measured 36 × 32 × 30 mm. The wall of the lesion, especially the mural nodule and thickened areas, were enhanced. (D-E) CT findings during the postoperative follow-up. Recurrence in the form of pleural dissemination with increased pleural effusion was observed. It was ambiguous before contrast enhancement (D). However, it was obvious after contrast enhancement (E).
Pathological findings
Macroscopically, the content of the tumoral cyst had already been discharged because of its wall rupturing during surgery; the cyst was immersed in formalin. Blood clots attached to the wall remained. The cyst was firmly adhered to the upper lobe of the right lung (Figure 2A). On the cut surface, the cyst wall was irregularly thickened, and a solid, bulky mural nodule was observed; the wall was predominately flat otherwise. The border of the tumoral cyst was well demarcated, and invasion into the lungs was not grossly apparent (Figure 2B).
Figure 2.
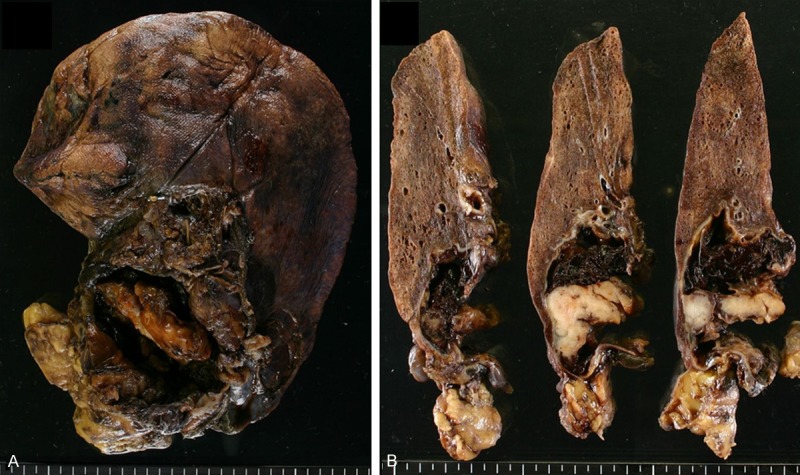
Surgically resected specimen. A. The cyst was firmly attached to the upper lobe of the right lung. B. The wall of the cyst was irregularly thickened, and a solid, bulky mural nodule was observed although the wall was predominantly flat. The border of the tumoral cyst was well demarcated, and invasion into the lung was not apparent. Of note, blot clotting was abundantly present.
Histopathologically, tumor cells were present on the entire wall that was firmly attached to the pleura; the border between the tumorous tissue and the lung was well demarcated. The solid and bulky mural nodule was comprised only of tumorous tissue (Figure 3A). Even in the flat section of the wall, a microscopic band-like layer composed of tumor cells was observed throughout; the surface of this layer was smooth (Figure 3B). Tumor cells with spindled shapes formed interlacing fascicles as they grew (Figure 3C). They had mildly enlarged hyperchromatic nuclei without prominent nucleoli. Although nuclear atypia was moderate, cellularity was very high (Figure 3D). Mitotic figures were approximately 8 per 10 high-power fields. Necrosis was not apparent.
Figure 3.
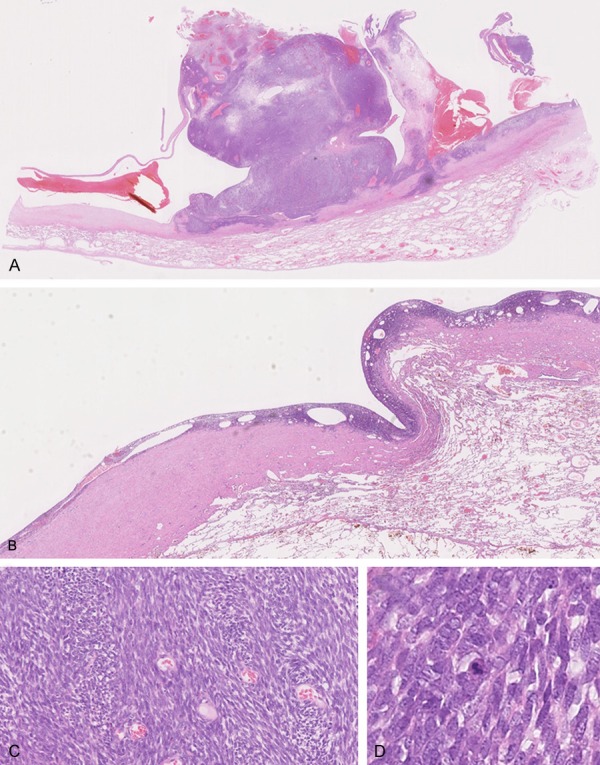
Microscopic findings. A. Tumor cells were present on the entire wall firmly attached to the pleura; the border between tumorous tissue and the lung was well demarcated. The solid and bulky mural nodule was composed only of tumorous tissue (× 20). B. In the flat part of the cyst wall, a microscopic band-like layer composed of tumor cells was observed throughout the wall; the surface of this layer was smooth (× 40). C. Tumor cells with spindled shape grow forming interlacing fascicles (× 200). D. Tumor cells had mildly enlarged hyperchromatic nuclei without prominent nucleoli. Although nuclear atypia was moderate, cellularity was very high. Of note, a mitotic figure is present at the center of the field (× 400).
Upon IHC, tumor cells positive for CK7 (OV-TL 12/30, 1:50; Dako, Glostrup, Denmark), were sparsely present (Figure 4A). Tumor cells positive for epithelial membrane antigen (EMA) (E29, 1:50; Dako) were more numerous, yet still comprised a minority of tumor cells (Figure 4B). Tumor cells were positive for BCL-2 (124, 1:50; Dako) and CD99 (12E7, 1:50, Dako). Cells were focal and weakly positive for S100 protein (polyclonal, 1:1000, Dako), and negative for AE1/AE3 (AE1/AE3, 1:50; Dako) and CD34 (QBEnd 10, 1:50; Dako) (data not shown). Decreased expression of integrase interactor 1 (INI-1; 25/BAF47, 1:200; BD Transduction Laboratories, Franklin Lakes, NJ) was observed compared with endothelial cells (Figure 4C). The Ki-67 (MIB-1, 1:100; Dako) labeling index was 21% in the area with the highest density of positive cells (210 positive cells per 1000 counted cells) (Figure 4D).
Figure 4.
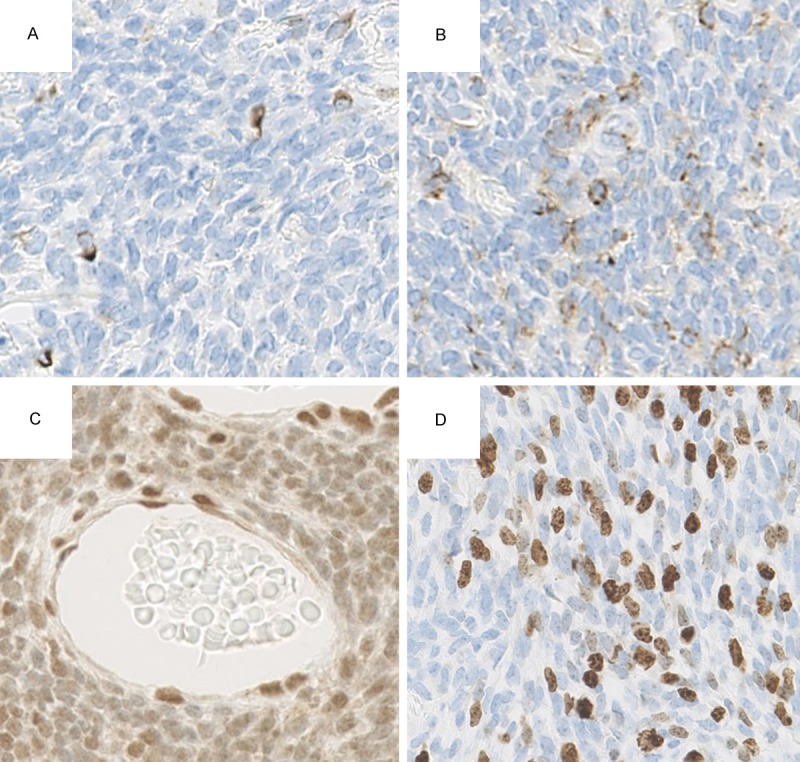
Immunohistochemical findings. A. Sparse positivity for CK7 (× 400). B. Sparse positivity but more numerous than CK7 for EMA (× 400). C. Decreased expression of INI-1 in tumor cells compared with endothelial cells (× 400). D. Ki-67 labeling index was 21% in the area with the highest density of positive cells (× 400).
The diagnosis of monophasic fibrous SS was considered based on morphological and immunohistochemical characteristics.
FISH was conducted on interphase nuclei present on formalin-fixed paraffin-embedded sections (4 μm thick). A break-apart probe was used to evaluate rearrangement of the SYT (synonym of the SS18) gene (Vysis SS18 Break Apart FISH Probe Kit; Abbott Molecular, Des Plaines, Illinois, USA). Almost all the observed tumor cells harbored rearrangement of SYT, indicated by the split signal of the gene (Figure 5). The diagnosis of SS was thus confirmed.
Figure 5.
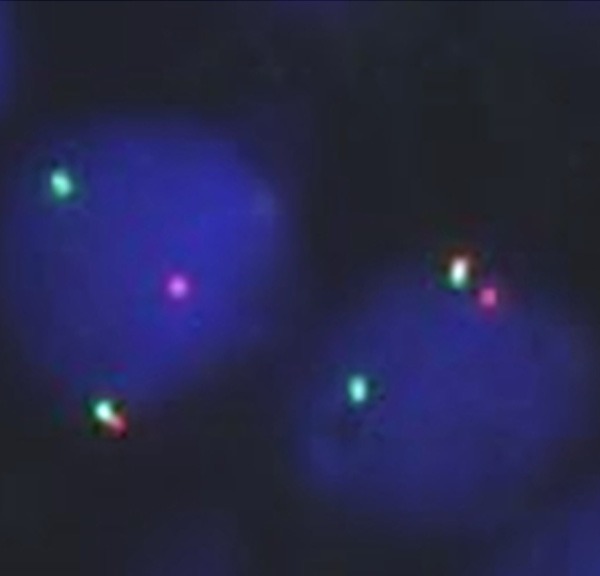
Fluorescence in situ hybridization using SYT break-apart probe. One probe labeled in SpectrumOrange lies distal to the SS18 gene. The other probe labeled in SpectrumGreen lies proximal to the SS18 gene. One fusion signal shown in yellow color was present in these two nuclei. One orange and one green signal were also observed, indicating rearrangement of SYT gene (× 1000).
Discussion
After the discovery of the t(X;18) translocation specific for SS [18], molecular analyses have been applied to distinguish between SS and its histological mimics. This translocation results in the fusion of the SYT gene to either the SSX1, SSX2, or SSX4 gene [9]. The SYT-SSX fusion gene is found in more than 90% of SS, the presence of which can be detected by FISH [19]. In the present case, a split signal of SYT was observed, but this did not indicate which gene was fused to it. Regardless, rearrangement of SYT detected by FISH is indicative of SS.
Without cytogenetic information, differentiating between SS and type A thymoma is difficult when both consist of tumor cells showing spindled morphology [9,10]. IHC was employed to help identify the tumor in this case. Sparse positivity for CK7 and EMA alone could not completely eliminate the possibility of thymoma. However, negativity for AE1/AE3 is one criterion to rule out thymoma, as this protein is consistently expressed in thymomas [20]. As a focal area of cystic change is also found in a solitary fibrous tumor (SFT), a type of tumor well known to occur in the pleura and possesses spindled morphology, the possibility of a SFT should also be eliminated [21,22]. BCL-2 and CD99, which were expressed in our case, are frequently expressed by SFTs. However, negativity for CD34 along with scattered cells positive for CK7 and EMA did not match the criteria for SFT diagnosis [23].
Decreased expression of INI-1 is another piece of evidence supporting the diagnosis of SS, since downregulation of this protein appears to be a feature completely unique to SS among it and its mimickers [24,25], and is thus an extremely useful observation to reach its correct diagnosis. Kohashi et al. [24] determined the incidence of INI-1 downregulation in SS to be 69% (66 out of 95 cases), while Arnold et al. [25] determined the incidence to be 86% (42 out of 49 cases). In the present case, INI-1 expression was downregulated, and thus the diagnosis of SS was essentially confirmed before the result of FISH was known. IHC for testing INI-1 expression is thus a convenient and cost-effective diagnostic method. Paradoxically, INI-1 mRNA levels were upregulated in SS tumors with downregulated protein [24]. It is thus postulated that a post-transcriptional mechanism exists whereby SYT-SSX indirectly regulates INI-1 through interaction of SYT with a chromatin remodeling pathway that could affect INI-1 [26,27]. There appears to be no prognostic difference between cases where INI-1 is decreased and cases where it is not [24].
To the best of our knowledge, cystic changes of malignant peripheral nerve sheath tumors (MPNSTs), which resemble SS, have not been focused upon in the English literature. However, any tumor can undergo cystic change due to degeneration or hemorrhage. Therefore, knowledge of how to differentiate SS from MPNST via IHC is important. Both these tumor types usually express S100 protein [28] making its measurement of limited diagnostic value for differentiating between them. Gene expression profiling has revealed consistent overexpression of the transducer-like enhancer of split 1 (TLE1) in SS [29], which encodes a transcriptional corepressor involved in epithelial and neuronal differentiation [30]. TLE1 protein was detected on IHC in most SS cases (82%; 60 out of 73 cases) and in many MPNSTs (47%; 7 out of 15 cases); however, its expression in MPNST is weak and distinguishable from that of SS [31]. Conversely, a more specific marker for MPNST is SRY-related HMG box 10 (SOX10), a transcription factor involved in neural crest differentiation [32] which has been shown to be a sensitive and specific marker for schwannian tumors on IHC [33]. The incidence of SOX10 expression in MPNST is 67% (32 out of 48 cases), but is only 7% (7 out of 97 cases) in SS. Specificity of SOX10 to MPNST in comparison to SS is 93%. When considering the aforementioned decrease in INI-1 expression and its 100% specificity when distinguishing SS from its mimickers, and taking into account its easier accessibility compared to TLE1 and SOX10 in departments of pathology, INI-1 would be the protein of choice to add to conventional marker screening panels.
SS is an aggressive tumor with a poor prognosis. Recurrences are common and patients may undergo consecutive surgical resections. The five-year disease-free survival was 20.9% in one study [34]. Since our patient showed inoperable recurrence, chemotherapy, radiotherapy, and radiofrequency thermal ablation [35] may be recommended in cases such as this.
In conclusion, this is an exceedingly rare case of cystic SS. Because of its occurrence in the visceral pleura near the anterior mediastinum and its adherence to the mediastinal pleura, distinguishing between cystic SS and cystic thymoma (the latter of which was more often encountered) was difficult on contrast-enhanced CT. Histopathological examination showed a tumor composed of spindled cells. This feature alone could not differentiate between the two suspected tumor types because monophasic fibrous SS and type A thymoma both take the form of spindled cells. Therefore, IHC was invaluable for determining the tumor type. Decreased expression of INI-1 is specific to SS and distinguishes it from other similar tumors. Assuming that antibodies for INI-1 are relatively prevailing across pathology departments, INI-1 should hence be included in the conventional panel of markers for diagnosing SS.
Disclosure of conflict of interest
None.
References
- 1.Murphey MD, Gibson MS, Jennings BT, Crespo-Rodriguez AM, Fanburg-Smith J, Gajewski DA. From the archives of the AFIP: Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006;26:1543–1565. doi: 10.1148/rg.265065084. [DOI] [PubMed] [Google Scholar]
- 2.Gladish GW, Sabloff BM, Munden RF, Truong MT, Erasmus JJ, Chasen MH. Primary thoracic sarcomas. Radiographics. 2002;22:621–637. doi: 10.1148/radiographics.22.3.g02ma17621. [DOI] [PubMed] [Google Scholar]
- 3.Gaertner E, Zeren EH, Fleming MV, Colby TV, Travis WD. Biphasic synovial sarcomas arising in the pleural cavity. A clinicopathologic study of five cases. Am J Surg Pathol. 1996;20:36–45. doi: 10.1097/00000478-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kang MK, Cho KH, Lee YH, Han IY, Yoon YC, Park KT, Kang do K, Kim BM. Primary synovial sarcoma of the parietal pleura: a case report. Korean J Thorac Cardiovasc Surg. 2013;46:159–161. doi: 10.5090/kjtcs.2013.46.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandeepa HS, Kate AH, Chaudhari P, Chavan V, Patole K, Lokeshwar N, Chhajed PN. Primary pleural synovial sarcoma: a rare cause of hemorrhagic pleural effusion in a young adult. J Cancer Res Ther. 2013;9:517–519. doi: 10.4103/0973-1482.119367. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen M, Virtanen I. Synovial sarcoma-a misnomer. Am J Pathol. 1984;117:18–25. [PMC free article] [PubMed] [Google Scholar]
- 7.Dickersin GR. Synovial sarcoma: a review and update, with emphasis on the ultrastructural characterization of the nonglandular component. Ultrastruct Pathol. 1991;15:379–402. doi: 10.3109/01913129109016247. [DOI] [PubMed] [Google Scholar]
- 8.Fisher C. Synovial sarcoma: ultrastructural and immunohistochemical features of epithelial differentiation in monophasic and biphasic tumors. Hum Pathol. 1986;17:996–1008. doi: 10.1016/s0046-8177(86)80083-1. [DOI] [PubMed] [Google Scholar]
- 9.Suurmeijer AJ, De Bruijn DR, Geurts van Kessel A. Synovial sarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F, editors. WHO Classification of Tumours of Soft Tissue and Bone. 4th edition. Lyon, France: IARC Press; 2013. pp. 213–215. [Google Scholar]
- 10.Marx A, Strobel P, Badve SS, Chalabreysse L, Chan JK, Chen G, de Leval L, Detterbeck F, Girard N, Huang J, Kurrer MO, Lauriola L, Marino M, Matsuno Y, Molina TJ, Mukai K, Nicholson AG, Nonaka D, Rieker R, Rosai J, Ruffini E, Travis WD. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol. 2014;9:596–611. doi: 10.1097/JTO.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 11.Morrison C, Wakely PE Jr, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001;5:48–56. doi: 10.1053/adpa.2001.21479. [DOI] [PubMed] [Google Scholar]
- 12.Teng YS, Lin ZH, Li Y, Cao XL, Lin FC, Xiang JJ. Synovial sarcoma of the neck masquerading as a malignant second branchial cleft cyst. Int J Clin Exp Pathol. 2013;6:2257–2262. [PMC free article] [PubMed] [Google Scholar]
- 13.Suster S, Rosai J. Cystic thymomas. A clinicopathologic study of ten cases. Cancer. 1992;69:92–97. doi: 10.1002/1097-0142(19920101)69:1<92::aid-cncr2820690117>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Moran CA, Suster S. Thymoma with prominent cystic and hemorrhagic changes and areas of necrosis and infarction: a clinicopathologic study of 25 cases. Am J Surg Pathol. 2001;25:1086–1090. doi: 10.1097/00000478-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Rieker RJ, Aulmann S, Schnabel PA, Sack FU, Otto HF, Mechtersheimer G, Schirmacher P, Blaker H. Cystic thymoma. Pathol Oncol Res. 2005;11:57–60. doi: 10.1007/BF03032408. [DOI] [PubMed] [Google Scholar]
- 16.Bozok S, Yavasi O, Ilhan G, Gurbuz A. Unusual cause of cardiac compression in a trauma patient: cystic thymoma. West J Emerg Med. 2012;13:527–528. doi: 10.5811/westjem.2012.3.11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raad RA, Suh J, Ko JP. Case of the season: cystic thymoma. Semin Roentgenol. 2013;48:290–294. doi: 10.1053/j.ro.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Turc-Carel C, Dal Cin P, Limon J, Li F, Sandberg AA. Translocation X; 18 in synovial sarcoma. Cancer Genet Cytogenet. 1986;23:93. doi: 10.1016/0165-4608(86)90153-6. [DOI] [PubMed] [Google Scholar]
- 19.Aubry MC, Bridge JA, Wickert R, Tazelaar HD. Primary monophasic synovial sarcoma of the pleura: five cases confirmed by the presence of SYT-SSX fusion transcript. Am J Surg Pathol. 2001;25:776–781. doi: 10.1097/00000478-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Sato J, Fujiwara M, Kawakami T, Sumiishi A, Sakata S, Sakamoto A, Kurata A. Fascin expression in dendritic cells and tumor epithelium in thymoma and thymic carcinoma. Oncol Lett. 2011;2:1025–1032. doi: 10.3892/ol.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XM, Reng J, Zhou P, Cao Y, Cheng ZZ, Xiao Y, Xu GH. Solitary fibrous tumors in abdomen and pelvis: imaging characteristics and radiologic-pathologic correlation. World J Gastroenterol. 2014;20:5066–5073. doi: 10.3748/wjg.v20.i17.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu Arab W. Solitary fibrous tumours of the pleura. Eur J Cardiothorac Surg. 2012;41:587–597. doi: 10.1093/ejcts/ezr009. [DOI] [PubMed] [Google Scholar]
- 23.Bisceglia M, Galliani C, Giannatempo G, Lauriola W, Bianco M, D'Angelo V, Pizzolitto S, Vita G, Pasquinelli G, Magro G, Dor DB. Solitary fibrous tumor of the central nervous system: a 15-year literature survey of 220 cases (August 1996-July 2011) Adv Anat Pathol. 2011;18:356–392. doi: 10.1097/PAP.0b013e318229c004. [DOI] [PubMed] [Google Scholar]
- 24.Kohashi K, Oda Y, Yamamoto H, Tamiya S, Matono H, Iwamoto Y, Taguchi T, Tsuneyoshi M. Reduced expression of SMARCB1/INI1 protein in synovial sarcoma. Mod Pathol. 2010;23:981–990. doi: 10.1038/modpathol.2010.71. [DOI] [PubMed] [Google Scholar]
- 25.Arnold MA, Arnold CA, Li G, Chae U, El-Etriby R, Lee CC, Tsokos M. A unique pattern of INI1 immunohistochemistry distinguishes synovial sarcoma from its histologic mimics. Hum Pathol. 2013;44:881–887. doi: 10.1016/j.humpath.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 26.de Bruijn DR, dos Santos NR, Thijssen J, Balemans M, Debernardi S, Linder B, Young BD, Geurts van Kessel A. The synovial sarcoma associated protein SYT interacts with the acute leukemia associated protein AF10. Oncogene. 2001;20:3281–3289. doi: 10.1038/sj.onc.1204419. [DOI] [PubMed] [Google Scholar]
- 27.DiMartino JF, Ayton PM, Chen EH, Naftzger CC, Young BD, Cleary ML. The AF10 leucine zipper is required for leukemic transformation of myeloid progenitors by MLL-AF10. Blood. 2002;99:3780–3785. doi: 10.1182/blood.v99.10.3780. [DOI] [PubMed] [Google Scholar]
- 28.Karamchandani JR, Nielsen TO, van de Rijn M, West RB. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol. 2012;20:445–450. doi: 10.1097/PAI.0b013e318244ff4b. [DOI] [PubMed] [Google Scholar]
- 29.Baird K, Davis S, Antonescu CR, Harper UL, Walker RL, Chen Y, Glatfelter AA, Duray PH, Meltzer PS. Gene expression profiling of human sarcomas: insights into sarcoma biology. Cancer Res. 2005;65:9226–9235. doi: 10.1158/0008-5472.CAN-05-1699. [DOI] [PubMed] [Google Scholar]
- 30.Chen G, Courey AJ. Groucho/TLE family proteins and transcriptional repression. Gene. 2000;249:1–16. doi: 10.1016/s0378-1119(00)00161-x. [DOI] [PubMed] [Google Scholar]
- 31.Foo WC, Cruise MW, Wick MR, Hornick JL. Immunohistochemical staining for TLE1 distinguishes synovial sarcoma from histologic mimics. Am J Clin Pathol. 2011;135:839–844. doi: 10.1309/AJCP45SSNAOPXYXU. [DOI] [PubMed] [Google Scholar]
- 32.Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol. 2008;32:1291–1298. doi: 10.1097/PAS.0b013e3181658c14. [DOI] [PubMed] [Google Scholar]
- 34.Begueret H, Galateau-Salle F, Guillou L, Chetaille B, Brambilla E, Vignaud JM, Terrier P, Groussard O, Coindre JM. Primary intrathoracic synovial sarcoma: a clinicopathologic study of 40 t(X;18)-positive cases from the French Sarcoma Group and the Mesopath Group. Am J Surg Pathol. 2005;29:339–346. doi: 10.1097/01.pas.0000147401.95391.9a. [DOI] [PubMed] [Google Scholar]
- 35.Lee HK, Kwon HJ, Lee HB, Jin GY, Chung MJ, Lee YC. Radiofrequency thermal ablation of primary pleural synovial sarcoma. Respiration. 2006;73:250–252. doi: 10.1159/000087153. [DOI] [PubMed] [Google Scholar]


