Abstract
We investigated a case of metastatic adenocarcinoma of the lungs at the left proximal femur, masquerading as a primary pleomorphic sarcoma. A 72-year-old woman presented with pain in her left thigh in conjunction with a mass that had been gradually growing over a few months. She was being treated with gefitinib for lung adenocarcinoma positive for the epidermal growth factor receptor (EGFR) mutation L858R, and had multiple bone metastases. The lung adenocarcinoma and metastases had stabilized with the treatment. The metastatic lesions in the bone had also received radiation; however, a tumor in the proximal femur kept growing despite treatment. A biopsy specimen from the proximal femur revealed the proliferation of spindle-shaped cells without an epithelial glandular component. The patient underwent en bloc resection of the proximal femur that was replaced by prosthesis. Histologically, the resected tumor was entirely composed of pleomorphic cells and tumor giant cells exhibiting no apparent glandular structures. Tumor cells were diffusely positive for p53 and focally positive for epithelial markers and EGFR, but were negative for thyroid transcription factor-1, suggesting an initial diagnosis of primary pleomorphic sarcoma. Genetic examination revealed mutations in EGFR and p53 that were of the same type as the lung tumor, leading to the final diagnosis of the femoral mass as a sarcomatous transformation of metastatic lung adenocarcinoma. However, secondary genetic alterations that might explain the acquired resistance to gefitinib could not be found in the proximal femoral tumor. The patient remains alive and the remaining lesions are well controlled.
Keywords: Lung adenocarcinoma, EGFR, TP53, metastasis
Introduction
Non-small cell lung cancer (NSCLC) accounts for a significant number of cancer-related deaths in the world. Recently, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib, have provided a marked benefit for patients with NSCLC tumors harboring specific genetic alterations [1-3]. However, in spite of remarkable initial response, EGFR-mutated NSCLCs eventually acquire secondary resistance to these agents [4]. Several mechanisms of acquired resistance to EGFR-TKIs have been described, the most common of which is a secondary EGFR T790M point mutation in exon 20 [4,5]. Other molecular mechanisms of resistance are the upregulation of MET/HGF, HER2 mutations, HER3 overexpression, persistent activation of IGF-1R, mutation of PIK3CA/AKT, loss or downregulation of PTEN, and abnormal dimerization of STAT3 [5-8]. Additionally, epithelial to mesenchymal transition (EMT) has been reported as a cause of acquired resistance to EGFR-TKIs [5,9]. There were several studies and case reports describing changes in EMT status and transformation to small cell lung cancer (SCLC) [10-12]. However, we could not find a case that described a bone metastasis undergoing sarcomatous transformation after treatment with gefitinib. Herein, we describe a case of sarcomatous overgrowth of metastatic adenocarcinoma of the lungs at the left proximal femur, masquerading as a primary pleomorphic sarcoma. Detection of the same mutations of EGFR and TP53 in the both lung and femoral lesions led us to the final diagnosis of the latter as metastatic carcinoma.
Materials and methods
Immunohistochemistry
Immunohistochemical staining was performed with the following antibodies: thyroid transcription factor-1 (TTF-1) (DAKO, clonal; 8G7G3/1), p53 (Leica Biosystems, clonal; PAb 1801), CAM5.2 (Becton, Dickinson and Company, clonal; CAM5.2), epithelial membrane antigen (EMA) (Leica Biosystems, clonal; GP1.4), AE1/3 (Leica Biosystems, clonal; AE1 and AE3, mixed to a ratio of 20:1), desmin (Leica Biosystems, clonal; DE-R-11), SMA (DAKO, clonal; 1A4), M-actin (DAKO, clonal; HHF35), and EGFR (DAKO, clonal; 2-18C9).
Mutation analysis of the EGFR, TP53, KRAS, NRAS, HRAS, and PIK3CA
Genome DNA was extracted from formalin-fixed, paraffin-embedded blocks from which the tumor-bearing areas were dissected manually with a scalpel. DNA samples from the primary lung tumor as well as tumorous and non-tumorous tissue from the left femoral tumor were prepared for mutation analysis. Bidirectional sequencing of EGFR, TP53 KRAS, NRAS, HRAS, and PIK3CA were performed. The primer sequences used in this study are listed in Table 1. PCR cycling conditions were as follows: 94°C for 2 minutes followed by 40 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds, and a final hold at 72°C for 2 minutes.
Table 1.
Primer sequences used in this study
| Gene | Region | Forward/Reverse | Primer sequence | Size (bp) |
|---|---|---|---|---|
| EGFR | Ex 18 | F | 5’-CAA GTG CCG TGT CCT GGC ACC CAA GC-3’ | 381 |
| R | 5’-CCA AAC ACT CAG TGA AAC AAA GAG-3’ | |||
| Ex 19 | F | 5’-GCA CCA TCT CAC AAT TGC CAG TTA-3’ | 207 | |
| R | 5’-AAA AGG TGG GCC TGA GGT TCA-3’ | |||
| Ex 20 | F | 5’-GAA ACT CAA GAT CGC ATT CAT GC-3’ | 379 | |
| R | 5’-GCA AAC TCT TGC TAT CCC AGG AG-3’ | |||
| Ex21 | F | 5’-CAG CCA TAA GTC CTC GAC GTG G-3’ | 374 | |
| R | 5’-CAT CCT CCC CTG CAT GTG TTA AAC-3’ | |||
| p53 | Ex 5 | F | 5’-CTC TTC CTG CAG TAC TCC CCT GC-3’ | 211 |
| R | 5’-GCC CCA GCT GCT CAC CAT CGC TA-3’ | |||
| Ex 6 | F | 5’-GAT TGC TCT TAG GTC TGG CCC CTC-3’ | 182 | |
| R | 5’-GGC CAC TGA CAA CCA CCC TTA ACC-3’ | |||
| Ex 7 | F | 5’-GCT TGC CAC AGG TCT CCC CAA G-3’ | 192 | |
| R | 5’-AGG CTG GCA AGT GGC TCC TGA C-3’ | |||
| Ex 8 | F | 5’-TGG TAA TCT ACT GGG ACG GA-3’ | 134 | |
| R | 5’-GCT TAG TGC TCC CTG GGG GC-3’ | |||
| Ex 9 | F | 5’-GCC TCT TTC CTA GCA CTG CCC AAC-3’ | 101 | |
| R | 5’-CCC AAG ACT TAG TAC CTG AAG GGT G-3’ | |||
| Kras | Ex 2 | F | 5’-AAG GCC TGC TGA AAA TGA C-3’ | 166 |
| R | 5’-TGG TCC TGC ACC AGT AAT ATG-3’ | |||
| Ex 3 | F | 5’-GAG ACT GTG TTC TCC CTT CTC A-3’ | 131 | |
| R | 5’-CTC ATG TAC TGG TCC CTC ATT G-3’ | |||
| Ex 4 | F | 5’-TGG ACA GGT TTT GAA AGA TAT TTG-3’ | 381 | |
| R | 5’-ATT AAG AAG CAA TGC CCT CTC AAG-3’ | |||
| Nras | Ex 2 | F | 5’-GAA CCA AAT GGA AGG TCA CA-3’ | 301 |
| R | 5’-TGG GTA AAG ATG ATC CGA CA-3’ | |||
| Ex 3 | F | 5’-GGT GAA ACC TGT TTG TTG GA-3’ | 272 | |
| R | 5’-AAC CTA AAA CCA ACT CTT CCC A-3’ | |||
| Hras | Ex 2 | F | 5’-AGG AGA CCC TGT AGG AGG A-3’ | 169 |
| R | 5’-CGC TAG GCT CAC CTC TAT AGT G-3’ | |||
| Ex 3 | F | 5’-CTG CAG GAT TCC TAC CGG A-3’ | 160 | |
| R | 5’-ACT TGG TGT TGT TGA TGG CA-3’ | |||
| PIK3CA | Ex 9 | F | 5’-GCT AGA GAC AAT GAA TTA AGG GAA A-3’ | 122 |
| R | 5’-AGC ACT TAC CTG TGA CTC CA-3’ | |||
| Ex 20 | F | 5’-AAC TGA GCA AGA GGC TTT GG-3’ | 122 | |
| R | 5’-CTT TTC AGT TCA ATG CAT GCT G-3’ |
Case presentation
A 72-year-old woman presented with pain in her left thigh that had persisted over a few months concomitant with a gradually growing mass. The patient was receiving gefitinib to treat lung adenocarcinoma that had multiple bone metastases and was positive for the EGFR mutation L858R (Figure 1A-D). The patient had no respiratory symptoms, and she had never smoked. The primary lung adenocarcinoma and all metastatic lesions except one on the left proximal femur had remained stable during treatment (Figure 1E). The bone metastatic lesions had also received radiation; however, the proximal femoral tumor continued to grow despite treatment. A physical examination revealed swelling and pain at the left proximal thigh. Laboratory tests detected high levels of carcinoembryonic antigen, although gefitinib treatment decreased the levels from 446.2 ng/mL to 23.5 ng/mL prior to surgery. Radiography revealed an osteosclerotic lesion with osteolytic change in the left proximal femur (Figure 1F). Computed tomography revealed that the tumor inside of the left femur gradually enlarged, penetrated the cortex, and invaded the surrounding soft tissue (Figure 1G, 1H). Magnetic resonance imaging uncovered a mass with isointensity on T2-weighted images and with heterogenous intensity on fat-suppressed T2-weighted images (Figure 1I, 1J). A biopsy specimen from the proximal femur revealed the proliferation of spindle-shaped cells without an epithelial glandular component. With an initial diagnosis of pleomorphic sarcoma, the patient underwent en bloc resection of the left proximal femur where tumorous tissue was replaced with an artificial joint.
Figure 1.
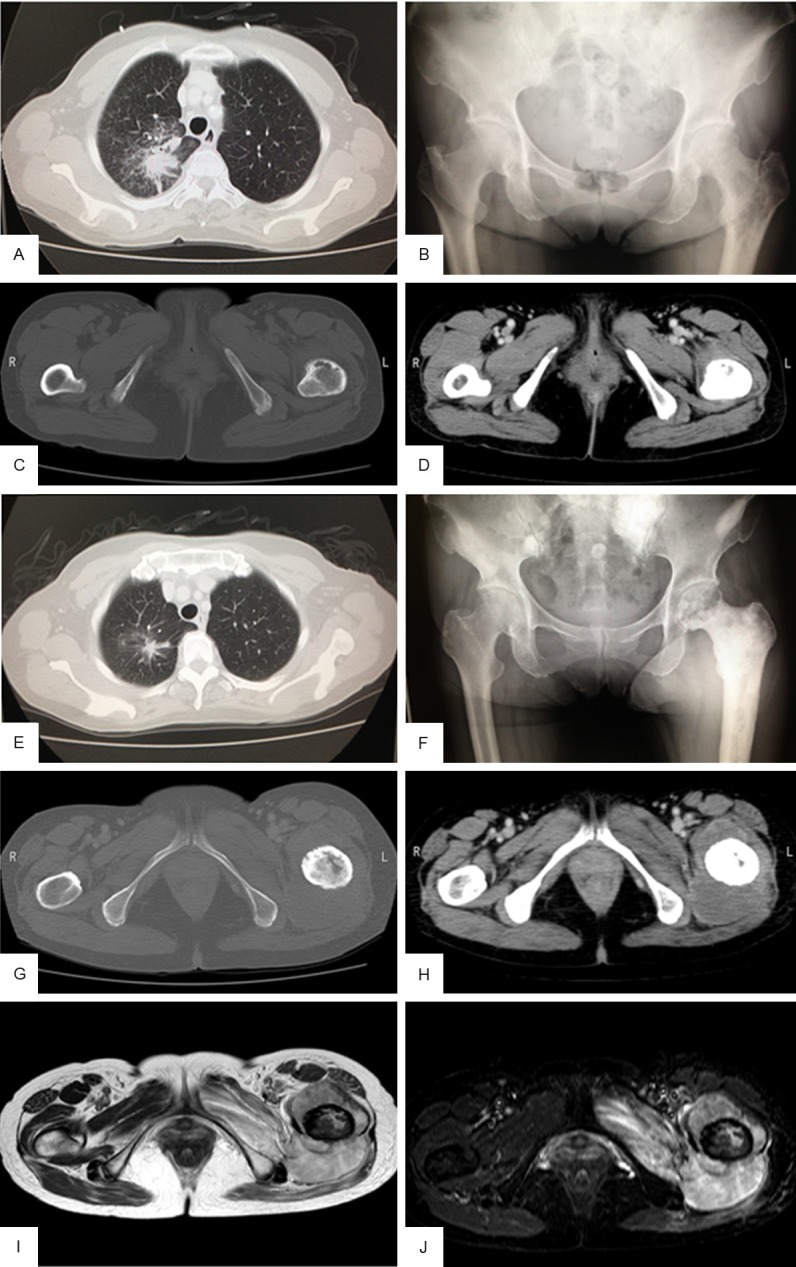
Presurgical imaging of the primary tumor and femoral metastasis. (A) Computed tomography (CT) of the chest showed a high-density nodular mass before gefitinib treatment. (B) Plain radiography revealed an osteolytic lesion in the left proximal femur before gefitinib treatment. (C, D) Contrast enhanced CT of the femurs and pelvis showed a thin cortex in the left femur with no soft tissue mass before gefitinib treatment. (E) Chest CT taken during gefitinib treatment and irradiation revealed marked shrinkage of the tumor. (F) Plain radiograph taken before surgery showed an osteosclerotic lesion with osteolytic change in the left proximal femur and decreased permeability into the surrounding soft tissue. (G, H) Enhanced CT taken before surgery revealed an isodense mass within the bone marrow and surrounding soft tissue. (I, J) Magnetic resonance imaging performed before surgery revealed a mass with isointensity on T2-weighted images (I) and with heterogenous intensity on fat-suppressed T2-weighted images (J) around the left femur.
Pathological examination
The biopsy specimen from the lung tumor revealed adenocarcinoma without a sarcomatous component (Figure 2A). Immunohistochemically, EGFR was not detected (Figure 2B) but diffuse TTF-1 (Figure 2C) and focal p53 (Figure 2D) staining were observed. The resected tumor was also entirely composed of spindle-shaped pleomorphic cells and tumor giant cells without glandular structures (Figure 2E, 2F). Focally, necrosis was observed (Figure 2G). Mitosis was also frequently detected (Figure 2H). Immunohistochemically, EGFR was expressed focally on the cell membrane (Figure 3A), but TTF-1 expression was not observed (Figure 3B). In addition, tumor cells were diffusely positive for p53 (Figure 3C), and focally positive for the epithelial markers CAM5.2, EMA, and AE1/AE3 (Figure 3D-F). Based on these findings, a diagnosis of primary pleomorphic sarcoma with epithelial differentiation was tentatively made; however, genetic testing for EGFR was performed to confirm the diagnosis. TP53, KRAS, HRAS, NRAS, and PIK3CA mutations were screened in both the lung and femoral tumors to identify any possible genetic alterations responsible for the acquired resistance to TKIs in the femoral tumor. Genetic testing revealed that the same types of mutationsin EGFR (L858R) and TP53 (R181P) were present in both the tumors (Figure 4A-F), confirming a common clonal origin of the two tumors and leading to the final diagnosis of sarcomatous overgrowth of metastatic lung adenocarcinoma. However, KRAS, NRAS, HRAS, and PIK3CA mutations were not detected. The patient remains alive and walks with the assistance of crutches; the remaining lesions are well controlled.
Figure 2.
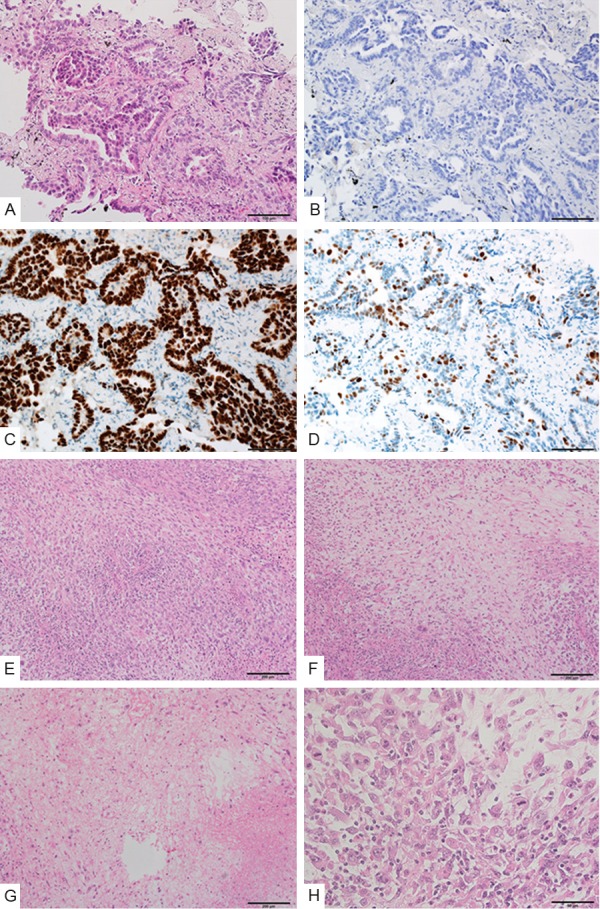
Histologies of the primary lung adenocarcinoma and femoral tumor. (A) Biopsy specimen shows adenocarcinoma without an apparent sarcomatous component. (B, C) Tumor cells in the lung adenocarcinoma are negative for EGFR staining (B), but positive for thyroid transcription factor-1 (TTF-1) staining (C). (D) Tumor cells show diffuse expression of p53. (E) The resected femoral tumor shows a proliferation of spindle-shaped pleomorphic cells. (F) The tumor contains myxoid area with lower cellularity. (G) The tumor includes a necrotic area. (H) Mitoses are frequently observed.
Figure 3.
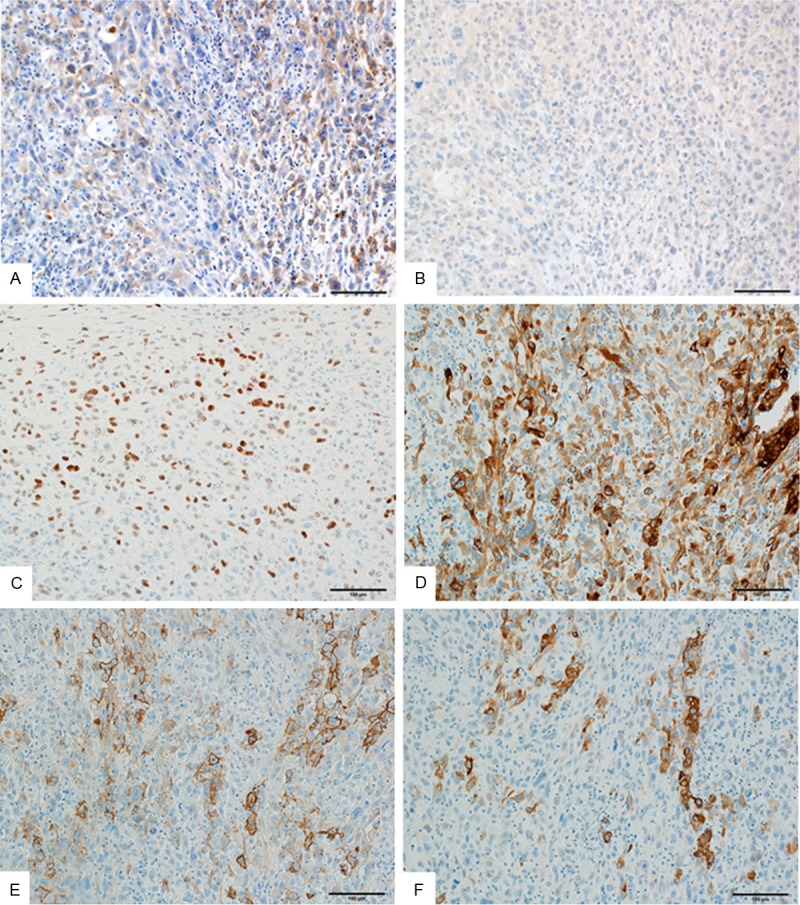
Immunohistochemistry of the left proximal femoral tumor. (A) The tumor cells show focal expression of EGFR. (B) The tumor cells are negative for TTF-1. (C) The tumor cells show diffuse expression of p53. (D-F) The tumor cells show focal expressions of CAM5.2 (D), epithelial membrane antigen (E), and AE1/AE3 (F).
Figure 4.
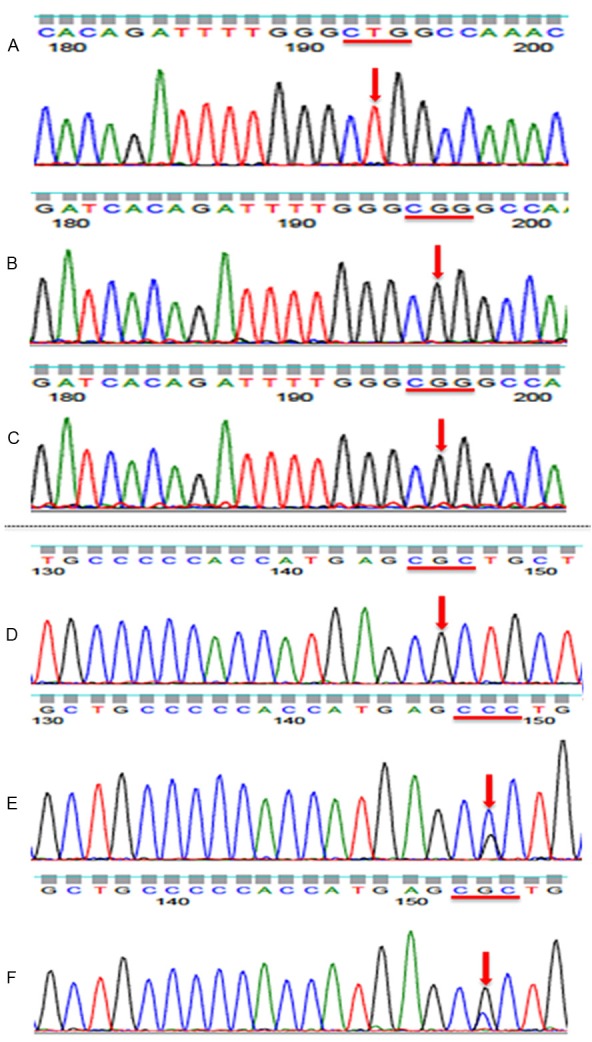
Genomic analysis of the primary lung adenocarcinoma and femoral tumor. Both the primary lung adenocarcinoma and the left femoral tumor contained the EGFR variant at codon 858 (CTG>CGG; L858R), and this was confirmed to be a tumor-specific mutation. (A) DNA sample from non-tumorous tissue, (B) DNA from the left femoral tumor, (C) DNA from the lung adenocarcinoma. Furthermore, both the primary lung adenocarcinoma and the left femoral tumor contained a TP53 variant at codon 181 (CGC>CCC; R181P), and this was confirmed to be tumor-specific mutation. (D) DNA sample from non-tumorous tissue, (E) DNA from the left femoral tumor, (F) DNA from the lung adenocarcinoma.
Discussion
When the patient was first admitted, two possibilities were considered regarding the femoral tumor. One was that it was a metastasis from the primary lung adenocarcinoma. A primary bone tumor was the other differential diagnosis because it was puzzling that only the femoral tumor grew progressively among the multiple metastatic lesions that were being identically treated. Biopsy from the proximal femur revealed that the tumor was entirely composed of a proliferation of sarcomatous pleomorphic cells without glandular structure, thus supporting the diagnosis of a primary bone tumor. Furthermore, histological examination of the surgical specimen showed features consistent with a diagnosis of primary pleomorphic sarcoma of the proximal femur. However, genetic testing of EGFR and TP53 was performed for further confirmation, and this analysis led us to revise the diagnosis to that of a metastasis due to identical genetic patterns in both the lung and femoral tumors. TTF-1 is used as a sensitive marker for lung adenocarcinoma, although loss of its expression has been reported to correlate with increased tumor aggressiveness [13]. In this case, TTF-1 expression was absent in the metastatic tumor despite its strong expression in the lung biopsy specimen. Hence, this patient illustrates the importance of genetic testing for resolving ambiguous cases.
As the patient had received radiation therapy (24 Gy) for metastases in the lumbar vertebrae and left femur 1 year previously, radiation-induced sarcoma or dedifferentiation of metastatic lung adenocarcinoma could also explain the femoral tumor. Several studies describe that radiation-related sarcoma commonly occurs after radiation therapy, although the interval between irradiation and detection of the second malignancy is at least 3-5 years [14-18]. Thus, the possibility of the radiation-related sarcoma was disregarded. Nakanishi et al. reported that TP53 mutations were one of the causative factors of radiation-induced sarcoma [18]. In the present case, the TP53 mutation R181P was detected in both the lung biopsy specimen and the left femoral tumor; thus, this alteration in TP53 was deemed not related to the radiation.
Various studies show that almost all EGFR-mutation-positive NSCLCs acquire resistance to EGFR-TKIs despite remarkably good responses initially. Recently, several mechanisms of acquired resistance to EGFR-TKIs in NSCLC have been described, the most common of which is a secondary EGFR T790M mutation [4,5]. Other molecular mechanisms of resistance include upregulation of HGF/MET, HER2 mutations, HER3 overexpression, persistent activation of IGF-1R, mutations of PIK3CA/AKT, loss or downregulation of PTEN, and abnormal dimerization of STAT3 [5-8]. Some secondary genetic alterations or histological changes occurred in all 11 cases of lung adenocarcinoma with acquired resistance to EGFR-TKIs in a study by Uramoto et al. [10]. Sequist et al. described that histological change were observed in 8 of 37 cases with drug-resistant NSCLCs carrying EGFR mutations, and EMT was described as the cause of drug resistance in 3 of these 8 cases [11]. The molecular mechanisms and the associated mesenchymal phenotypes underlying drug resistance to TKIs remain unknown, although it has been shown that cell lines undergoing EMT are intrinsically resistant to EGFR inhibitors [19-21]. In our case, secondary genetic alterations such as T790M in EGFR, or additional mutations in Kras, Nras, Hras, and PIK3CA, were not found. The femoral tumor in this case was entirely composed of pleomorphic cells and tumor giant cells with bizarre nuclei, and no apparent glandular structures were observed. It is not clear whether the lung tumor was pure adenocarcinoma or pleomorphic carcinoma from the small biopsy specimen. According to the WHO classification, a carcinoma is defined as pleomorphic if the sarcomatous component is greater than 10% [22,23]. EGFR mutation was reported to occur in 15-20% of pleomorphic carcinomas of the lungs, and EGFR-TKIs such as gefitinib and erlotinib have high efficiency against EGFR-mutated tumors including pleomorphic carcinoma [24-26]. Furthermore, metastases often arise from poorly differentiated components of tumors with pleomorphic features. However, the fact that the primary tumor as well as the remaining metastatic lesions remained stable due to TKI treatment suggests that the lung adenocarcinoma was a pure adenocarcinoma while EMT occurred only in the proximal femoral tumor. Thus, EMT could be the cause of acquired drug resistance to TKI in this case.
In conclusion, we investigated a metastatic adenocarcinoma of the lungs on the left proximal femur, masquerading as a primary pleomorphic sarcoma. Our results show that genetic testing is highly recommended in such cases when the histologic features of suspected metastases are markedly different from the primary lesion.
After acceptance of the manuscript, a new lesion of the right lung was noticed by the chest-CT and it has been gradually enlarged. The biopsy from this lesion revealed sarcomatous feature without apparent glandular structures. Because the original lesion of the right lung remained stable, the newly established lesion was considered to be metastasized from the femoral lesion.
Acknowledgements
This work was supported in part by a Grant-in-Aid for General Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (#26670286 to Tsuyoshi Saito and #25861342 to Yoshiyuki Suehara), Tokyo, Japan.
Disclosure of conflict of interest
None.
References
- 1.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 3.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T. North-East Japan Study Group Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y, Wang X, Jin H. EGFR-TKI resistance in NSCLC patients: mechanisms and strategies. Am J Cancer Res. 2014;4:411–435. [PMC free article] [PubMed] [Google Scholar]
- 6.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Jänne PA. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 7.Yano S, Wang W, Li Q, Matsumoto K, Sakurama H, Nakamura T, Ogino H, Kakiuchi S, Hanibuchi M, NIshioka Y, Uehara H, Mitsudomi T, Yatabe Y, Nakamura T, Sone S. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–9487. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto C, Basaki Y, Kawahara A, Nakashima K, Kage M, Izumi H, Kohno K, Uramoto H, Yasumoto K, Kuwano M, Ono M. Loss of PTEN expression by blocking nuclear translocation of EGR1 in gefitinib-resistant lung cancer cells harboring epidermal growth factor receptor-activating mutations. Cancer Res. 2010;70:8715–8725. doi: 10.1158/0008-5472.CAN-10-0043. [DOI] [PubMed] [Google Scholar]
- 9.Suda K, Tomizawa K, Fujii M, Murakami H, Osada H, Maehara Y, Yatabe Y, Sekido Y, Mitsudomi T. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011;6:1152–1161. doi: 10.1097/JTO.0b013e318216ee52. [DOI] [PubMed] [Google Scholar]
- 10.Uramoto H, Shimokawa H, Hanagiri T, Kuwano M, Ono M. Expression of selected gene for acquired drug resistance to EGFR-TKI in lung ade-nocarcinoma. Lung Cancer. 2011;73:361–365. doi: 10.1016/j.lungcan.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, Akhavanfard S, Heist RS, Temel J, Christensen JG, Wain JC, Lynch TJ, Vernovsky K, Mark EJ, Lanuti M, Iafrate AJ, Mino-Kenudson M, Engelman JA. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe S, Sone T, Matsui T, Yamamura K, Tani M, Okazaki A, Kurokawa K, Tambo Y, Takato H, Ohkura N, Waseda Y, Katayama N, Kasahara K. Transformation to small-cell lung cancer following treatment with EGFR tyrosine kinase inhibitors in a patient with lung adenocarcinoma. Lung Cancer. 2013;82:370–372. doi: 10.1016/j.lungcan.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Barletta JA, Perner S, Iafrate AJ, Yeap BY, Weir BA, Johnson LA, Johnson BE, Meyerson M, Rubin MA, Travis WD, Loda M, Chirieac LR. Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma. J Cell Mol Med. 2009;13:1977–1986. doi: 10.1111/j.1582-4934.2008.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel SR. Radiation-induced sarcoma. Curr Treat Options Oncol. 2000;1:258–261. doi: 10.1007/s11864-000-0037-6. [DOI] [PubMed] [Google Scholar]
- 15.Sabanas AO, Dahlin DC, Childs DS Jr, Ivins JC. Postradiation sarcoma of bone. Cancer. 1956;9:528–542. doi: 10.1002/1097-0142(195605/06)9:3<528::aid-cncr2820090316>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Cruz M, Coley BL, Stewart FW. Postradiation bone sarcoma; report of eleven cases. Cancer. 1957;10:72–88. doi: 10.1002/1097-0142(195701/02)10:1<72::aid-cncr2820100111>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Arlen M, Higinbotham NL, Huvos AG, Marcove RC, Miller T, Shah IC. Radiation-induced sarcoma of bone. Cancer. 1971;28:1087–1099. doi: 10.1002/1097-0142(1971)28:5<1087::aid-cncr2820280502>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi H, Tomita Y, Myoui A, Yoshikawa H, Sakai K, Kato Y, Ochi T, Aozasa K. Mutation of the p53 gene in postradiation sarcoma. Lab Invest. 1998;78:727–733. [PubMed] [Google Scholar]
- 19.Thomson S, Buck E, Petti F, Griffin G, Brown E, Ramnarine N, Iwata KK, Gibson N, Haley JD. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65:9455–9462. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- 20.Frederick BA, Helfrich BA, Coldren CD, Zheng D, Chan D, Bunn PA Jr, Raben D. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non-small cell lung carcinoma. Mol Cancer Ther. 2007;6:1683–1691. doi: 10.1158/1535-7163.MCT-07-0138. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs BC, Fujii T, Dorfman JD, Goodwin JM, Zhu AX, Lanuti M, Tanabe KK. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res. 2008;68:2391–2399. doi: 10.1158/0008-5472.CAN-07-2460. [DOI] [PubMed] [Google Scholar]
- 22.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. World Health Organization International Agency for Research on Cancer. IARC Press; 2004. WHO Classification of Tumours Pathology and Genetics of Tumours of the Lung, Thymus and Heart; pp. 53–62. [Google Scholar]
- 23.Travis WD. Sarcomatoid neoplasms of the lung and pleura. Arch Pathol Lab Med. 2010;134:1645–1658. doi: 10.5858/2010-0086-RAR.1. [DOI] [PubMed] [Google Scholar]
- 24.Kaira K, Horie Y, Ayabe E, Murakami H, Takahashi T, Tsuya A, Nakamura Y, Naito T, Endo M, Kondo H, Nakajima T, Yamamoto N. Pulmonary pleomorphic carcinoma: a clinicopathological study including EGFR mutation analysis. J Thorac Oncol. 2010;5:460–465. doi: 10.1097/JTO.0b013e3181ce3e3c. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Kim Y, Sun JM, Choi YL, Kim JG, Shim YM, Park YH, Ahn JS, Park K, Han JH, Ahn MJ. Molecular profiles of EGFR, K-ras, c-met, and FGFR in pulmonary pleomorphic carcinoma, a rare lung malignancy. J Cancer Res Clin Oncol. 2011;137:1203–1211. doi: 10.1007/s00432-011-0986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang YL, Wu CT, Shih JY, Lee YC. EGFR and p53 status of pulmonary pleomorphic carcinoma: implications for EGFR tyrosine kinase inhibitors therapy of an aggressive lung malignancy. Ann Surg Oncol. 2011;18:2952–2960. doi: 10.1245/s10434-011-1621-7. [DOI] [PubMed] [Google Scholar]


