Abstract
Lymphoepithelioma-like hepatocellular carcinoma is rare, which has been recognized as a variant of hepatocellular carcinoma. Here we report a locally advanced case of lymphoepithelioma-like hepatocellular carcinoma. A 50-year-old man with chronic hepatitis B virus infection presented with a single mass in the liver and two enlarged lymph nodes in retroperitoneum suspected to be hepatocellular carcinoma with lymph node metastasis. After discussion by multidisciplinary team, the patient underwent hepatectomy of VIII segment and dissection of two enlarged lymph nodes. One month after the operation, pre-chemotherapy abdominal computed tomography (CT) showed retroperitoneal enlarged lymph nodes, considered as local recurrence. Therefore, 3 cycles of oxaliplatin and tegafur gimeracil oteracil potassium capsule and 3 cycles of paclitaxel and cisplatin were offered, and post-chemotherapy abdominal CT revealed disease remained stable. The patient has been alive for 6 months since performance of surgery. Our report suggests that even locally advanced lymphoepithelioma-like hepatocellular carcinoma may have a good prognosis and operation and postoperative chemotherapy may benefit the patient.
Keywords: Hepatocellular carcinoma, lymphoepithelioma-like carcinoma, hepatectomy
Introduction
Lymphoepithelioma-like carcinoma (LELC) is a tumor composed of large undifferentiated epithelial cells with intense lymphoid stroma, which widely exists in nasopharynx, esophagus, stomach, lungs, and several other organs [1-5]. But the tumor is rarely reported in the liver, especially for hepatocellular LELC, according to the PubMed database, only 18 cases were reported from 2000 to date [6-12]. Lymphoepithelioma-like carcinoma hepatocellular carcinoma has been recognized as a variant of hepatocellular carcinoma by the World Health Organization [13]. Herein, we report a locally advanced case of lymphoepithelioma-like hepatocellular carcinoma which characterized that an enlarged metastatic lymph node in retroperitoneum is larger than the primary lesion in size.
Case report
A 50-year-old male with chronic hepatitis B virus (HBV) infection and type 2 diabetes mellitus complained of a mass in the liver when taking regular medical examination. The lab test showed HBsAg (+), HBsAb (-), HBeAg (-), HBeAb (+), HBcAb (+), HCV-Ab (-), HBV-DNA (-), moderate increasing AFP: 31.93 ng/ml (normal range: 0-7 ng/ml), and normal CA199:10.51 U/ml (in normal range), as well as normal liver function without elevated liver enzymes and normal blood coagulation capacity.
Magnetic resonance imaging (MRI) revealed liver cirrhosis and a 2.7×2.2 cm tumor in the anterior segment of right lobe of liver, hypointense on T1-weighted images, hyperintense on T2-weighted images, limited diffusion on diffusion weighted imaging (DWI), enhancement in the arterial phase, decreased enhancement in the venous phase and delayed phase. Multiple enlarged lymph nodes were showed in retroperitoneum, and the biggest one was about 5.2×3.4 cm in size (Figure 1). Ultrasound revealed a hypoechoic nodule in hepatic dome, sized in 1.9×2.5 cm, and a hypoechoic lymph node in retroperitoneum, sized in 4.2×2.9 cm, without explicit signal of blood stream. After discussion by multidisciplinary team (MDT), the patient underwent endoscopy and colonoscopy, and malignant lesion in stomach or colorectum was excluded. Percutaneous ultrasound-guided biopsy was performed for the liver lesion and enlarged lymph node, the pathology of which showed no cancer in the liver lesion but existing cancer cells in the lymph node. To assure the diagnosis, the patient received 18F-FDG PET-CT for whole body, which revealed increasing radioactive uptake lesion in the VIII segment of liver, considered as primary liver cancer, and increasing radioactive uptake lymph nodes in portal, retropancreatic, and retroperitoneal regions. After second discussion by multidisciplinary team, liver tumor and enlarged lymph nodes resection was recommended. Written informed consent was obtained from the patient. The patient underwent hepatectomy of VIII segment and two enlarged lymph nodes resection on May 23, 2014. Intraoperative findings: the liver was dark red in slightly decreased size with diffuse micronodular sclerosis. The tumor was in the segment VIII of right lobe, in the size about 3.5 cm×3 cm (Figure 2A). The removal specimen showed in Figure 2B. One enlarged lymph node was behind duodenal ligament, next to the portal vein, about 4.5 cm×3 cm(Figure 2C). Another was on the upper margin of pancreas, next to common hepatic artery and abdominal aorta, about 5.5 cm×4 cm (Figure 2D).
Figure 1.
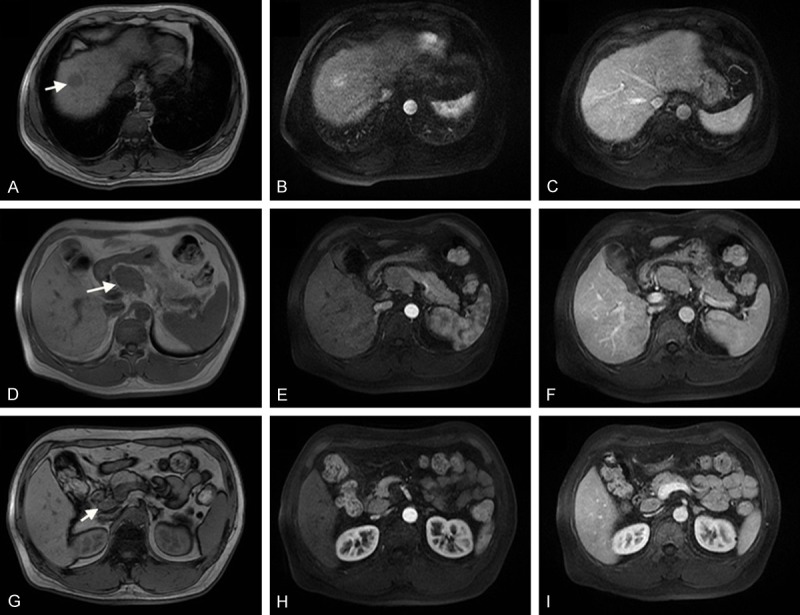
(A-C) Preoperative MRI showing a 2.7×2.2 cm tumor in segment VIII, hypointense on T1-weighted images (A), enhancement in arterial phase (B), hyperattenuation on venous phase (C). (D-F) An enlarged lymph node was on the upper margin of pancreas, next to common hepatic artery and abdominal aorta, about 5.2 cm×3.4 cm. (G-I) An enlarged lymph node behind duodenal ligament, next to the portal vein.
Figure 2.
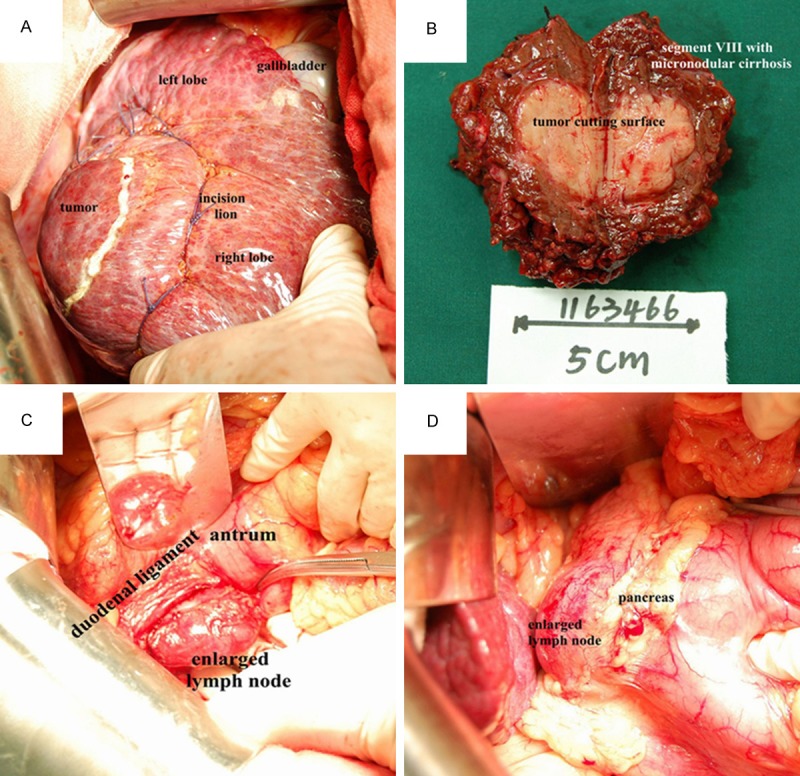
A. The tumor was in the segment VIII, in the size about 3.5 cm×3 cm. B. The removal specimen: a 3.5×3.5×3 cm circumscribed grey-white soft tumor mass 0.8 cm from the nearest resection margin. Adjacent liver tissue was micronodular cirrhosis. C. An enlarged lymph node was behind duodenal ligament, next to the portal vein, about 4.5 cm×3 cm. D. Another enlarged lymph node was on the upper margin of pancreas, next to common hepatic artery and abdominal aorta, about 5.5 cm×4 cm.
Macroscopically, a 10×8.5×7 cm segment of the liver was resected. There was a 3.5×3.5×3 cm circumscribed grey-white soft tumor mass 0.8 cm from the nearest resection margin. Adjacent liver tissue was micronodular cirrhosis (Figure 2B). Microscopically, the tumor was composed of poorly differentiated hepatocellular carcinoma with lymphoid intratumoral infiltrate. There was no liver capsular invasion or microvascular invasion. And the margin of liver incision was negative (Figure 3A). The two resected lymph nodes were infiltrated with hepatocellular cancer cells in center (Figure 3B). Immunochemistry analysis shows AE1/AE3 (2+) (Figure 3C), AFP (1+), CK18 (2+), CK19 (2+), CK20 (1+), CK7 (-), and EBER (-) (Figure 3D). The pathological diagnosis is hepatocellular LELC staged pT1N1M0 IVA.
Figure 3.
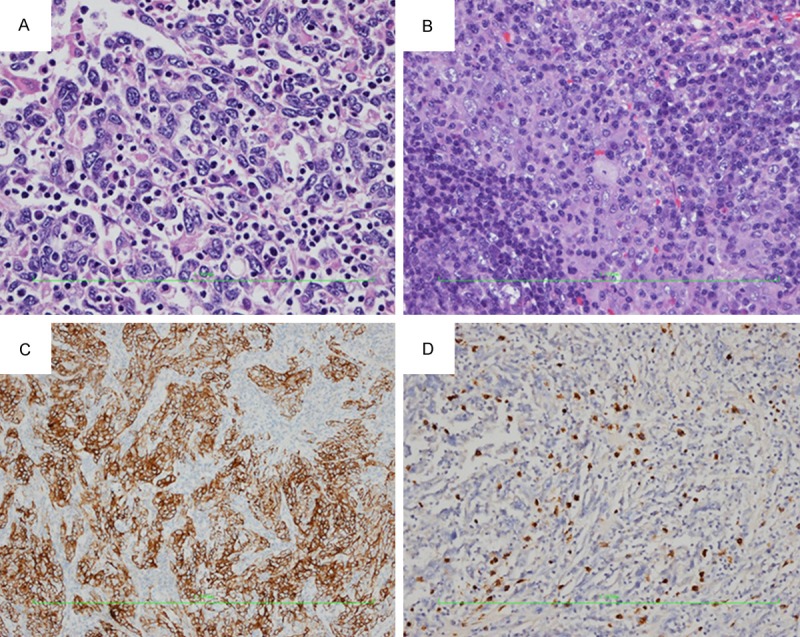
A. Proliferation of atypical large cells, characterized by an eosinophilic cytoplasm, with large nuclei and prominent nucleoli. Epithelial cells were surrounded by a dense lymphoid stroma, extending inside the tumor (HE, ×400). B. Metastatic lymph node with poorly differentiated hepatocellular carcinoma in center (HE, ×200). C. Immunohistochemical staining for AE1/AE3 is (2+) positive in hepatocellular LELC (IHC, ×100). D. Immunohistochemical staining for EBER is negative in epithelial cells of hepatocellular LELC (IHC, ×100).
The patient recovered smoothly from operation. Postoperative chemotherapy is recommended, considering the patients with retroperitoneal lymph nodes metastasis. One month after the operation, pre-chemotherapy abdominal CT showed retroperitoneal enlarged lymph nodes, considered as local recurrence. Therefore, 3 cycles of oxaliplatin and tegafur gimeracil oteracil potassium capsule and 3 cycles of paclitaxel and cisplatin were offered, and post-chemotherapy abdominal CT revealed disease remained stable. The patient has been alive for 6 months since performance of surgery.
Discussion
Clinicopathological data of all the 19 cases including the 18 case reported from 2000 to date and this case was shown in Table 1. There were 11 men and 8 women, with a mean age of 60.4 years (range, 39-79 years). 4 patients are the yellow race of East Asia, 15 patients are white from Europe and the United States. A total of 36.84% (7/19) of patients had hepatitis B or/and C virus infection and liver cirrhosis of varying severity.
Table 1.
Clinical features of all study patients with lymphoepithelioma-like hepatocellular carcinoma
| Case (ref) | Age (year) | sex | race | EBV | HBV | HCV | liver cirrhosis | tumor number | tumor size (cm) | surgery | preo/postoperative therapy | recur rence | survival | OS (month) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 [6] | 50 | M | W | N | P | P | Yes | 1 | 4.0 | LT | Yes | No | AWND | 120 |
| 2 [6] | 54 | M | W | N | N | N | No | Multi | 2.0 | LT | No | Yes | DD | 92.4 |
| 3 [6] | 59 | M | W | N | P | P | Yes | Multi | 5.0 | LT | Yes | No | AWND | 96 |
| 4 [6] | 45 | M | W | N | N | P | Yes | 1 | 2.0 | LT | Yes | No | AWND | 56.4 |
| 5 [6] | 64 | M | W | N | N | N | Yes | M | 4.0 | LT | Yes | No | AWND | 36 |
| 6 [7] | 39 | F | W | P | N | P | Yes | 1 | 1.0 | LT | Yes | Yes | DD | 5 |
| 7[8] | 56 | M | Y | N | N | P | Yes | 1 | 3.0 | LR | Yes | Yes | DD | 21 |
| 8 [9] | 47 | F | W | N | N | N | No | 1 | 2.2 | LR | No | Yes | AWD | 15 |
| 9 [10] | 57 | M | Y | N | P | N | Yes | 1 | 2.7 | LR | No | No | AWND | 50 |
| 10 [11] | 79 | M | Y | U | N | N | No | 1 | 4.2 | LR | Yes | Yes | AWD | 20 |
| 11 [12] | 74 | F | W | N | N | N | No | Multi | 6.5 | LR+LND | Yes | Yes | DD | 24 |
| 12 [12] | 65 | M | W | N | N | N | No | 1 | 4.8 | LR | Yes | No | DUC | 60 |
| 13 [12] | 65 | F | W | N | N | N | No | 1 | 1.3 | LR | Yes | Yes | AWD | 108 |
| 14 [12] | 70 | F | W | N | N | N | No | 1 | 2.7 | LR | Yes | No | AWND | 72 |
| 15 [12] | 61 | F | W | N | N | N | No | Multi | 9.5 | LR | Yes | No | AWND | 4 |
| 16 [12] | 78 | M | W | N | N | N | No | 1 | 10.5 | LR | Yes | Yes | DUC | 48 |
| 17 [12] | 78 | F | W | N | N | N | No | 1 | 6 | LR | Yes | No | AWND | 24 |
| 18 [12] | 57 | F | W | N | N | N | No | 1 | 13 | LR | Yes | No | DUC | 1 |
| 19 | 50 | M | Y | N | P | N | Yes | 1 | 3 | LR+LND | Yes | No | AWD | 6 |
M, male; F, female; W, white from Europe and the United States; Y, yellow from East Asia; N, negative; P, positive; U, unknown; Multi, Multifocal; LT, Liver transplantation; LR, Liver resection; LND, resection of the swollen lymph node; AWND, alive with no disease; AWD, alive with disease; DD, died disease; DUC, died of unrelated cause.
The risk factor for hepatocellular LELC is unknown yet. Among the 19 patients, 7 were HBV and/or HCV positive including 2 HBV positive, 2 HBV and HCV positive and 3 HCV positive. The association with EBV established in other organs has not yet been conclusively shown in hepatocellular LELC. Only 1 case in the 19 cases showed EBV positive. EBER was negative and EBV-RNA was not amplified for this case, which indicated the tumor might not be associated with EBV. While, lymphoepithelioma-like cholangiocarcinoma seems to relate with EBV, because 14/20 reported cases are positive for EBV in lymphoepithelioma-like cholangiocarcinoma patients [12].
The clinical presentation of hepatocellular LELC is usually nonspecific and insufficient to establish diagnosis. Most patients complained of mass found in the liver during regular health examination. Some have acute abdominal pain, localized in the right upper abdomen [15], and others have symptoms like chronic cholangitis [9,11].
Diagnosis is mainly based on pathological character, commonly which is poorly differentiated or undifferentiated large apparent atypical tumor cells, polygonal with nucleus in the center, easy to see karyokinesis and abundant eosinophilic cytoplasm. Lots of mature lymphocytes are infiltrated in the tumor stroma. Epithelial cells are often various CK (+) in immunochemistry analysis, while hepatic cells are Hep par (+) and AFP (+). And in cholangiocarcinoma cells are often CK7 (+) and CK19 (+), as they are biliary-type cytokeratins. When it is a mixed LELC in the liver, including hepatic cells and bile duct cells, it shows CK (+) and Hep (+) [14]. And other hepatocellular LELC also shows CK (+) [15]. It seems that various CK can be positive in any kind of LELC in the liver, which needs further studies. In our case, since it is AFP (+), we can diagnose the tumor as hepatocellular LELC. The mean tumor size was 4.6 cm (range, 1.0-13.0 cm). 5 patients had multifocal tumors, and the other 14 patients had single tumor. There are 5 cases with vascular invasion, 4 cases with local lymph node metastasis in this series.
All of the 19 hepatocellular LELC patients received resection, among which 6 underwent liver transplantation, the other 13 underwent hepatectomy. And 13 cases received preoperative and/or postoperative adjuvant therapy. The therapeutic effect of adjuvant therapy is not unclear because of the rarity for this disease, which needs further study.
Though the tumor is rare, the prognosis seems to be better than the conventional hepatocellular carcinoma, which has a reported 5-year survival rate of 47% to 53%, and the 2-year tumor recurrence rate is as high as 55% [16]. Among all the 19 patients, 4 patients died of disease progression, 3 died of unrelated cause, 8 alive with no recurrence or metastasis, 4 alive with disease recurrence. The overall survival time range from is 1 month to 120 months, with the median time of 36 months.
There are certain limitations in our survey. First, the case that we reported only was followed up for 6 months, so the long-term survival and the long-term efficacy could not be adequately evaluated. Second, because of the other 18 cases data from the other 7 research teams of different countries spanning for 14 years, statistical analysis is difficult to perform. Third, due to the rarity of Hepatocellular LELC, it is hard to carry out multi-center clinical research including large sample.
Conclusion
Hepatocellular LELC is differentiated from hepatocellular carcinoma mainly in pathological character, the former being infiltrated by lots of mature lymphocytes. Though they are similar in etiology, clinical presentation and radiology, they are totally different tumors. Hepatocellular LELC has better prognosis than hepatocellular carcinoma. Due to the limited numbers of cases, we still needs further and more detailed studies.
Disclosure of conflict of interest
None.
References
- 1.Applebaum EL, Mantravadi P, Haas R. Lymphoepithelioma of the nasopharynx. Laryngoscope. 1982;92:510–514. doi: 10.1288/00005537-198205000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Mori M, Watanabe M, Tanaka S, Mimori K, Kuwano H, Sugimachi K. Epstein-Barr virus-associated carcinomas of the esophagus and stomach. Arch Pathol Lab Med. 1994;118:998–1001. [PubMed] [Google Scholar]
- 3.Pittaluga S, Wong MP, Chung LP, Loke SL. Clonal Epstein-Barr virus in lymphoepithelioma-like carcinoma of the lung. Am J Surg Pathol. 1993;17:678–682. doi: 10.1097/00000478-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Lee SA, Kim H, Cho EY, Kim J. Lymphoepithelioma-like carcinoma in the trachea: report of a case. Surg Today. 2007;37:584–586. doi: 10.1007/s00595-006-3467-3. [DOI] [PubMed] [Google Scholar]
- 5.Dinney CP, Ro JY, Babaian RJ, Johnson DE. Lymphoepithelioma of the bladder: a clinicopathological study of 3 cases. J Urol. 1993;149:840–841. doi: 10.1016/s0022-5347(17)36228-6. [DOI] [PubMed] [Google Scholar]
- 6.Emile JF, Adam R, Sebagh M, Marchadier E, Falissard B, Dussaix E, Bismuth H, Reynès M. Hepatocellular carcinoma with lymphoid stroma: a tumor with good prognosis after liver transplantation. Histopathology. 2000;37:523–529. doi: 10.1046/j.1365-2559.2000.00952.x. [DOI] [PubMed] [Google Scholar]
- 7.Si MW, Thorson JA, Lauwers GY, DalCin P, Furman J. Hepatocellular lymphoepithelioma-like carcinoma associated with Epstein-Barr virus: a hitherto unrecognized entity. Diagn Mol Pathol. 2004;13:183–189. doi: 10.1097/01.pas.0000124336.90615.8d. [DOI] [PubMed] [Google Scholar]
- 8.Chen CJ, Jeng LB, Huang SF. Lymphoepithelioma-like hepatocellular carcinoma. Chang Gung Med J. 2007;30:172–177. [PubMed] [Google Scholar]
- 9.Nemolato S, Fanni D, Naccarato AG, Ravarino A, Bevilacqua G, Faa G. Lymphoepithelioma-like hepatocellular carcinoma: a case report and a review of the literature. World J Gastroenterol. 2008;14:4694–4696. doi: 10.3748/wjg.14.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park HS, Jang KY, Kim YK, Cho BH, Moon WS. Hepatocellular carcinoma with massive lymphoid infiltration: a regressing phenomenon? Pathol Res Pract. 2009;205:648–652. doi: 10.1016/j.prp.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Shinoda M, Kadota Y, Tsujikawa H, Masugi Y, Itano O, Ueno A, Mihara K, Hibi T, Abe Y, Yagi H, Kitago M, Kawachi S, Tanimoto A, Sakamoto M, Tanabe M, Kitagawa Y. Lymphoepithelioma-like hepatocellular carcinoma: a case report and a review of the literature. World J Surg Oncol. 2013;11:97. doi: 10.1186/1477-7819-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel KR, Liu TC, Vaccharajani N, Chapman WC, Brunt EM. Characterization of inflammatory (lymphoepithelioma-like) hepatocellular carcinoma: a study of 8 cases. Arch Pathol Lab Med. 2014;138:1193–1202. doi: 10.5858/arpa.2013-0371-OA. [DOI] [PubMed] [Google Scholar]
- 13.Bosman FT, Carneiro F, Hruban RH. WHO Classification of Tumours. 3rd edition. Lyon, France: IARC Press; 2010. [Google Scholar]
- 14.Chan AW, Tong JH, Sung MY, Lai PB, To KF. Epstein-Barr virus-associated lymphoepithelioma-like cholangiocarcinoma: a rare variant of intrahepatic cholangiocarcinoma with favourable outcome. Histopathology. 2014;65:674–683. doi: 10.1111/his.12455. [DOI] [PubMed] [Google Scholar]
- 15.Li GQ, Hou J, Tan YS. Hepatocellular lymphoepithelioma-like carcinoma: a report of 3 cases and review literatures. J Clin Exp Pathol. 2007;30:172–177. [Google Scholar]
- 16.Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer. 2014;120:2824–2838. doi: 10.1002/cncr.28730. [DOI] [PubMed] [Google Scholar]


