Abstract
Endometrial stromal sarcoma (ESS) is the second most common malignant uterine mesenchymal tumor. It affects women primarily in the perimenopausal age group. ESSs are morphologically heterogeneous. The distinction between uterine smooth muscle tumors such as cellular leiomyoma and myxoid leiomyosarcoma and low-grade ESS can be problematic when stromal sarcomas show prominent smooth muscle differentiation and abundant myxoid stroma, respectively. We herein present a rare case of fibromyxoid variant of ESS, which was misdiagnosed as hydropic leiomyoma on intraoperative frozen section examination. Grossly, the uterine mass consisted of intracavitary and intramural portions. The intracavitary portion with extensive hydropic degeneration mimicked a hydropic leiomyoma. In contrast, the intramural portion displayed an obvious tongue-like myometrial invasion. Histologically, the tumor consisted of both cellular (20%) and myxoid (80%) areas. In the cellular areas, oval to spindle-shaped tumor cells with bland nuclear features were found to surround concentrically a rich vascular network of arterioles, a characteristic of ESS. In addition, two relatively well-circumscribed nodular lesions showing atypical bizarre nuclei were identified in the myxoid area. Immunohistochemically, the tumor cells were diffusely and strongly positive for CD10. The present case indicates a wide morphological spectrum of ESS. Fibromyxoid variant of ESS should be considered in the differential diagnosis of intracavitary and/or intramural uterine mesenchymal tumors with myxoid differentiation. It is important to avoid confusion between fibromyxoid ESS and myxoid leiomyosarcoma because of the differences in their clinical course, treatment, and prognosis.
Keywords: Endometrial stromal sarcoma, fibromyxoid variant, atypical bizarre nuclei
Introduction
Endometrial stromal sarcoma (ESS) is a malignant tumor consisting of tumor cells that resemble endometrial stromal cells seen in proliferative-phase endometrium [1,2]. Permeative, infiltrative growth into the myometrium and the presence of vascular invasion are the main characteristics of ESS [3]. In the case of low-grade ESS, tumor cells show relatively uniform and oval to fusiform nuclei surrounding a delicate network of arterioles, which resembles the endometrial spiral arterioles. Most ESSs show classical low-grade histologic appearance similar to that mentioned above, but some of them may resemble other uterine mesenchymal tumors since they are morphologically heterogeneous. For example, it can be difficult to distinguish ESS from cellular leiomyoma when low-grade ESS shows prominent smooth muscle or fibroblastic differentiation [4,5]. In such cases, it is important to confirm the characteristic features of ESS, including an irregular tongue-like myoinvasion, vascular invasion, and tumor cells whirling around the spiral arterioles. Furthermore, ESS can exhibit sex cord-like differentiation, mimicking a sex cord-stromal cell tumor of the ovary. Rhabdoid, epithelioid, or clear cell changes, as well as adipocytic and skeletal muscle differentiation, have also been reported in ESSs [1].
Fibromyxoid variant of ESS is a rare type of uterine mesenchymal tumor. Several authors have reported that the ESSs show myxoid or fibromyxoid changes [6-10], but their biological or clinical behavior still remains to be clarified. We herein present an extremely rare case of the fibromyxoid variant of ESS with atypical bizarre nuclei. To the best of our knowledge, only one case of fibromyxoid ESS with bizarre nuclei has been reported [10]. We describe histopathological findings of the rare variant of ESS and the results of the immunohistochemical study.
Clinical presentation
A 53-year-old premenopausal Korean woman (gravida 2, para 2) was referred to the Department of Obstetrics and Gynecology at Samsung Medical Center (Seoul, South Korea). Pelvic examination indicated an enlarged uterus consistent with a pregnancy of 12 weeks’ gestation. Transvaginal ultrasonography revealed multiple uterine masses. Their irregular contours and degenerative changes raised the suspicion of sarcoma. Pelvic magnetic resonance imaging (MRI) scan was performed to clarify the existence of malignancy and to determine the therapeutic strategy. MRI scan revealed a uterine mass, which occupied both the endometrial cavity and the myometrium (Figure 1A). The mass was well-enhanced, with high signal intensity on the T2-weighted image. The mass seemed to be a hypervascular, infiltrative uterine mesenchymal tumor rather than a benign leiomyoma. Invasion into surrounding organs or pelvic blood vessels was not observed. Bilateral ovaries were atrophic without a tumorous lesion. No evidence of peritoneal seeding or lymph node metastasis was observed. The uterine cervix was also free of tumor. Based on the imaging findings, the differential diagnosis of the uterine mass included leiomyosarcoma, endometrial stromal sarcoma, and intravenous leiomyomatosis confined to the uterus. The serum levels of CA-125 and CA 19-9 were within their normal limits. Total abdominal hysterectomy was performed, and the specimen was sent to the Department of Pathology. Macroscopic examination for frozen section examination revealed an intracavitary protruding mass with myxoid and gelatinous appearance (Figure 1B). Microscopic examination of the mass revealed a uterine mesenchymal tumor showing extensive hydropic change. The hypocellular tumor tissue displayed an edematous stroma and oval to spindle-shaped nuclei with mild cytologic atypia and rare mitotic figures, suggestive of hydropic leiomyoma (Figure 1C). The operation included total abdominal hysterectomy, bilateral salpingo-oophorectomy, and partial omentectomy. The postoperative course was uneventful, and the patient left the hospital 3 days later.
Figure 1.
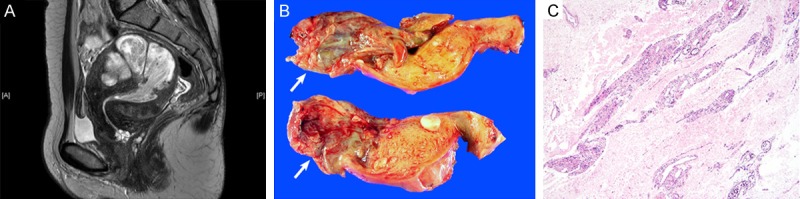
Imaging and intraoperative frozen section findings. A. Sagittal T2-weighted MRI scan revealed an infiltrative, hypervascular tumor that occupied the endometrial cavity and myometrium. B. Macroscopically, the intracavitary mass (white arrows) appeared to undergo extensive cystic and hydropic degeneration, resulting in a thin, membranous appearance, without definite evidence of a solid lesion. C. Intraoperative frozen section revealed an edematous stroma, suggesting extensive hydropic degeneration in a leiomyomatous nodule. The frozen section diagnosis was hydropic leiomyoma.
Pathologic findings
After formalin fixation, the intracavitary mass appeared as a shrunken, thin cystic wall without a solid tumor component, due to extensive cystic degeneration (Figure 2A). Serial sectioning of the myometrium revealed an intramural portion of the mass, which was not detected during the frozen section examination. A tongue-like projection with gelatinous appearance and cystic change was noted in the intramural portion. Based on the macroscopic viewpoint, the tumor consisted of intracavitary and intramural portions. The intracavitary portion showed an extensive degenerative change, mimicking myxoid or hydropic leiomyoma; in contrast, the intramural portion showed a tongue-like myometrial invasion, a characteristic of ESS.
Figure 2.
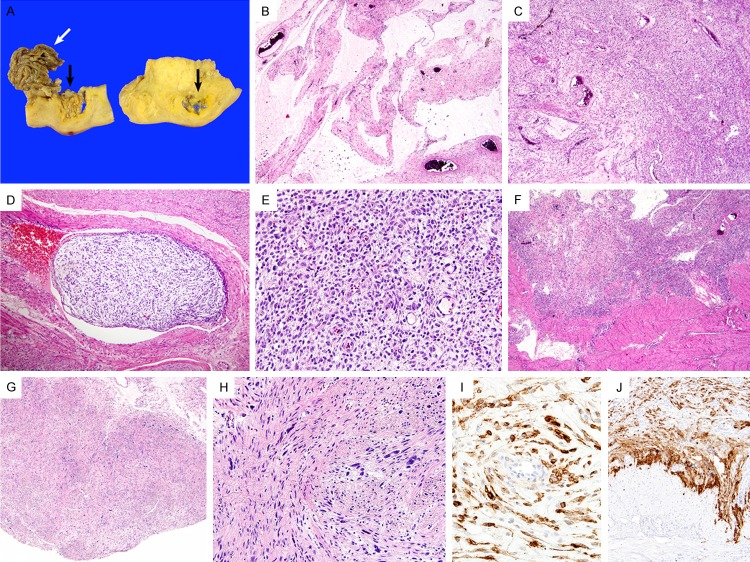
Pathologic findings. (A) Macroscopically, the intracavitary portion (white arrows) appeared as a shrunken cystic wall after formalin fixation. Serial sectioning of the myometrium revealed an intramural mass, which was not identified during the frozen section examination. The intramural portion (black arrows) displayed a tongue-like projection into the myometrium and underwent cystic and myxoid changes. (B) Similar to the frozen section slide, the permanent section of the intracavitary portion showed hypocellular tumor tissue with abundant myxoid matrix. (C) In contrast, the intramural portion exhibited higher cellularity. (D) Vascular invasion was present. (E) Small arterioles, resembling endometrial spiral arterioles, were surrounded by tumor cells. (F) At the invasive tumor front, tumor tissue infiltrated into the myometrium. These findings were typical of ESS. (G) In the myxoid area, two nodular lesions showing atypical bizarre nuclei were detected. (H) Scattered atypical cells displayed markedly pleomorphic, hyperchromatic nuclei. (I, J) Immunohistochemically, the tumor cells in the (I) fibromyxoid stroma and at the (J) invasive front were diffusely and strongly positive for CD10.
On microscopic examination, abundant myxoid or fibromyxoid matrix of the tumor was observed in both the intracavitary and intramural portions. Hypocellular lesions with a prominent myxoid stroma (myxoid areas; Figure 2B) accounted for approximately 80% of the entire tumor, while scattered hypercellular lesions with occasional fibromyxoid stroma (cellular areas; Figure 2C) accounted for the remaining 20% of the tumor. Foci of vascular invasion were obviously identified (Figure 2D). In the cellular areas, numerous, small, thin-walled blood vessels resembling spiral arterioles and surrounding tumor cells were clearly observed (Figure 2E). The majority of tumor cells had oval to spindle-shaped hyperchromatic nuclei with mild to moderate pleomorphism. The invasive tumor front exhibited much higher cellularity and obvious infiltration into the myometrium (Figure 2F). The mitotic rate was 3-4 and 0-1/10 high power fields in the cellular and myxoid areas, respectively. In addition, in the myxoid area, there were two relatively well-circumscribed nodular lesions (Figure 2G), showing scattered atypical bizarre cells with markedly pleomorphic, hyperchromatic nuclei (Figure 2H). No necrosis was identified.
Immunohistochemical staining was performed. The tumor cells of myxoid areas and invasive front were diffusely (more than 95%) and strongly positive for CD10 (Figure 2I, 2J), estrogen receptor (ER), and progesterone receptor (PR). The MIB-1 (Ki-67) labelling index was 5-10% in the cellular areas and 1-5% in the myxoid areas. In contrast, the tumor cells were negative for h-caldesmon, smooth muscle actin, pan-cytokeratin (CK), epithelial membrane antigen (EMA), S-100, and CD34. The bizarre cells were also positive for CD10, ER, and PR, but they were negative for h-caldesmon, smooth muscle actin, pan-CK, EMA, S-100, and CD34, demonstrating the same immunophenotype as that of the majority of tumor cells.
Discussion
ESS can present as either an intracavitary or intramural mass. The intramural ESS often shows nodular or diffuse myometrial permeation with worm-like plugs of tumor tissue in the myometrial vessels. It can be accompanied by a cystic change, but it is rarely prominent. In the present case, we had difficulty in determining the uterine tumor as ESS due to the abundant myxoid matrix, which is rarely observed in ESS. Firstly, the gross appearance of the intracavitary mass mimicked a hydropic leiomyoma. Secondly, not examining the intramural portion thoroughly and only examining the intracavitary portion without being aware of the infiltrative nature of the tumor resulted in misdiagnosis on the intraoperative frozen section examination. Thirdly, microscopic examination revealed that the intracavitary mass showed rather bland nuclear features similar to those of a benign smooth muscle tumor, in a background of edematous, myxoid matrix. Nevertheless, hydropic leiomyoma does not exhibit myometrial invasion; in other words, it does not show infiltrative growth. In addition, although the tumor was accompanied by an extensive degenerative change, an overt endometrial stromal differentiation suggests ESS. We observed that the tumor cells resembling endometrial stromal cells were found to surround small arterioles in both the cellular and myxoid areas. Interestingly, this finding was evident at the invasive tumor front. Immunopositivity for CD10, ER, and PR, together with negativity for h-caldesmon, also contributed to determining the tumor as ESS.
Myxoid leiomyosarcoma is an important differential diagnosis, which should not be confused with ESS. It is a rare variant of leiomyosarcoma, and consists of infiltrative tumor tissue that is not accompanied by significant cytologic atypia. It has a low mitotic activity and shows an abundant myxoid matrix [11,12]. Previous studies have reported that focal and weak staining for CD10 can be observed in myxoid leiomyosarcoma [11]. Therefore, it can be difficult to distinguish between fibromyxoid ESS and myxoid leiomyosarcoma solely based on the immunohistochemical staining. The two most important factors for differentiation between the two entities are as follows: firstly, the tumor cells with a whirling pattern, which surround prominent vascularization, are not conspicuous in myxoid leiomyosarcoma [11,13]. Secondly, the immunostaining result for a sensitive and specific marker for smooth muscle differentiation, h-caldesmon, is negative in the case of endometrial stromal tumors including ESS. However, the result for h-caldesmon is positive in leiomyosarcoma [14].
Details of the clinical behavior of fibromyxoid ESS have not been documented because of its rarity. Yilmaz and colleagues [9] reported 12 cases of ESS, with fibromyxoid features in 7 cases and smooth muscle differentiation in 5 cases, and all patients with these neoplasms were alive with metastasis at 6-20 years. The authors stated that the presence of even focal endometrial stromal differentiation in an invasive uterine mesenchymal lesion with a predominant smooth muscle, fibroblastic, or fibromyxoid phenotype should permit classification as low-grade ESS. Based on the previous cases reported in the literature [6,8-10], it is likely that there are no differences in the survival time for patients who have ESS with unusual histological features and those with typical low-grade ESS. Nevertheless, accumulation of cases with fibromyxoid ESS is necessary to elucidate the clinical outcome.
The pathogenetic mechanism and clinical implication of bizarre nuclei in ESS also remain unknown. To the best of our knowledge, only one case showing the presence of atypical cells with bizarre nuclei in myxoid ESS was reported. Kibar and colleagues [10] described that atypical bizarre cells with pleomorphic, hyperchromatic nuclei and large prominent nucleoli were observed in the endometrial curettage specimen. In the present case, there were two well-circumscribed nodular lesions showing scattered bizarre nuclei in the myxoid areas. The immunophenotype of these cells was the same as that of ESS cells. Since it accounts for less than 1% of the entire tumor volume, it is less likely to different clinical behavior from myxoid ESS showing no bizarre nuclei. No recurrence was detected in the previous case [10]. However, only two cases have been described so far, and further investigation on the clinical meaning of presence of atypical bizarre nuclei in myxoid or fibromyxoid ESS is necessary.
In summary, we presented an extremely rare case of fibromyxoid ESS with atypical bizarre nuclei. Such an unusual variant of ESS may cause diagnostic challenges, especially during an intraoperative frozen section diagnosis. It may be mistaken for myxoid leiomyosarcoma, hydropic or myxoid leiomyoma, or other mesenchymal tumors of the uterus that show a myxoid appearance. Clinicopathological and immunohistochemical features as well as the presence of overt endometrial stromal differentiation are helpful in the differential diagnosis.
Acknowledgements
This paper was supported by a grant of the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C3418).
Disclosure of conflict of interest
None.
References
- 1.Oliva E, Loening T, Carcangiu ML, Longacre TA, Carinelli SG, Nucci MR, Ip P, Prat J, Zaloudek CJ. Mesenchymal tumours. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO Classification of Tumours of Female Reproductive Organs. Lyon: International Agency for Research on Cancer; 2014. p. 135. [Google Scholar]
- 2.Jun SY, Ha H, Park IA, Kim KR. Uterine low grade endometrial stromal sarcoma presented as extrauterine masses. Korean J Pathol. 2002;36:262–265. [Google Scholar]
- 3.Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol. 1990;14:415–438. doi: 10.1097/00000478-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18:535–558. [PubMed] [Google Scholar]
- 5.Oliva E, Clement PB, Young RH, Scully RE. Mixed endometrial stromal and smooth muscle tumors of the uterus: a clinicopathologic study of 15 cases. Am J Surg Pathol. 1998;22:997–1005. doi: 10.1097/00000478-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Kasashima S, Kobayashi M, Yamada M, Oda Y. Myxoid endometrial stromal sarcoma of the uterus. Pathol Int. 2003;53:637–641. doi: 10.1046/j.1440-1827.2003.01525.x. [DOI] [PubMed] [Google Scholar]
- 7.Oliva E, Young RH, Clement PB, Scully RE. Myxoid and fibrous endometrial stromal tumors of the uterus: a report of 10 cases. Int J Gynecol Pathol. 1999;18:310–319. doi: 10.1097/00004347-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Stadsvold JL, Molpus KL, Baker JJ, Michael K, Remmenga SW. Conservative management of a myxoid endometrial stromal sarcoma in a 16-year-old nulliparous woman. Gynecol Oncol. 2005;99:243–245. doi: 10.1016/j.ygyno.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz A, Rush DS, Soslow RA. Endometrial stromal sarcomas with unusual histologic features: a report of 24 primary and metastatic tumors emphasizing fibroblastic and smooth muscle differentiation. Am J Surg Pathol. 2002;26:1142–1150. doi: 10.1097/00000478-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kibar Y, Aydin A, Deniz H, Balat O, Cebesoy B, Al-Nafussi A. A rare case of low-grade endometrial stromal sarcoma with myxoid differentiation and atypical bizarre cells. Eur J Gynaecol Oncol. 2008;29:397–398. [PubMed] [Google Scholar]
- 11.Fraga M, Prieto O, Garcia-Caballero T, Beiras A, Forteza J. Myxoid leiomyosarcoma of the uterine cervix. Histopathology. 1994;25:381–384. doi: 10.1111/j.1365-2559.1994.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 12.Mittal K, Popiolek D, Demopoulos RI. Uterine myxoid leiomyosarcoma within a leiomyoma. Hum Pathol. 2000;31:398–400. doi: 10.1016/s0046-8177(00)80258-0. [DOI] [PubMed] [Google Scholar]
- 13.Toki N, Kashimura M, Hasegawa T, Fukuoka K, Kawagoe T, Sugihara K, Koyama C, Hisaoka M. Myxoid leiomyosarcoma of the uterus. Report of a case with cytologic findings. Acta Cytol. 2000;44:415–419. doi: 10.1159/000328489. [DOI] [PubMed] [Google Scholar]
- 14.Rush DS, Tan J, Baergen RN, Soslow RA. h-Caldesmon, a novel smooth muscle-specific antibody, distinguishes between cellular leiomyoma and endometrial stromal sarcoma. Am J Surg Pathol. 2001;25:253–258. doi: 10.1097/00000478-200102000-00014. [DOI] [PubMed] [Google Scholar]