Abstract
We report here an unusual presentation of peritoneal endometriosis with smooth muscle metaplasia as multiple protruding masses on the lateral pelvic wall. Smooth muscle metaplasia is a common finding in rectovaginal endometriosis, whereas in peritoneal endometriosis, smooth muscle metaplasia is uncommon and its nodular presentation on the pelvic wall is even rarer. To the best of our knowledge, this is the first case of nodular smooth muscle metaplasia occurring in peritoneal endometriosis. As observed in this case, when performing laparoscopic surgery in order to excise malignant tumors of intra-abdominal or pelvic organs, it can be difficult for surgeons to distinguish the metastatic tumors from benign nodular pelvic wall lesions, including endometriosis, based on the gross findings only. Therefore, an intraoperative frozen section biopsy of the pelvic wall nodules should be performed to evaluate the peritoneal involvement by malignant tumors. Moreover, this report implies that peritoneal endometriosis, as well as rectovaginal endometriosis, can clinically present as nodular lesions if obvious smooth muscle metaplasia is present. The pathological investigation of smooth muscle cells in peritoneal lesions can contribute not only to the precise diagnosis but also to the structure and function of smooth muscle cells and related cells involved in the histogenesis of peritoneal endometriosis.
Keywords: Endometriosis, peritoneum, pelvic wall, smooth muscle cells, metaplasia
Introduction
Smooth muscle metaplasia in ovarian endometriosis has been described in the literature [1,2]. However, little attention has been paid to smooth muscle metaplasia occurring in peritoneal endometriosis. In particular, endometriosis with smooth muscle metaplasia presenting as nodular masses in the pelvic cavity has not yet been reported. Herein, we describe an unusual presentation of peritoneal endometriosis with smooth muscle metaplasia as multiple lateral pelvic wall nodules in a 55-year-old woman. We also discuss the clinicopathological findings of smooth muscle metaplasia in peritoneal endometriosis and the immunohistochemical staining results.
Case presentation
A 55-year-old Korean woman was referred to the Department of Obstetrics and Gynecology at Samsung Medical Center (Seoul, Korea) because of a mass in the mid-sigmoid colon detected on colonoscopy. She had experienced intermittent episodes of recurrent pelvic pain. Her medical history included hypertension. She had taken antihypertensive medication for 10 years. There was no other significant medical history, no previous operation history, and no family history of malignancy including colorectal carcinoma. Colonoscopic examination revealed an encircling, lobulated mass with luminal narrowing and easy touch bleeding 32 cm above the anal verge. The colonoscopic biopsy sample revealed moderately-differentiated adenocarcinoma. The patient underwent laparoscopic anterior resection of the colon. Laparoscopically, the serosal surface of the small bowel and colon was smooth and glistening without associated adhesions or abnormal effusions. The uterus and bilateral ovaries were normal, and salpinges were patent. However, closer examination of the lateral pelvic wall revealed three nodular masses protruding from the surface. These polypoid nodules on the pelvic wall were excised and sent to the Department of Pathology for evaluating the presence of malignancy intraoperatively. Microscopic examination of frozen sections revealed glandular structures with bland nuclear morphology, embedded in fibrous stroma (Figure 1A). The absence of significant cytologic atypia, desmoplasia, or necrosis excluded the possibility of metastatic adenocarcinoma. The frozen section diagnosis was endometrial-type glands with fibrous stroma, suggestive of endometriosis, and no evidence of malignancy. The postoperative course was uneventful, and the patient left the hospital 5 days later.
Figure 1.
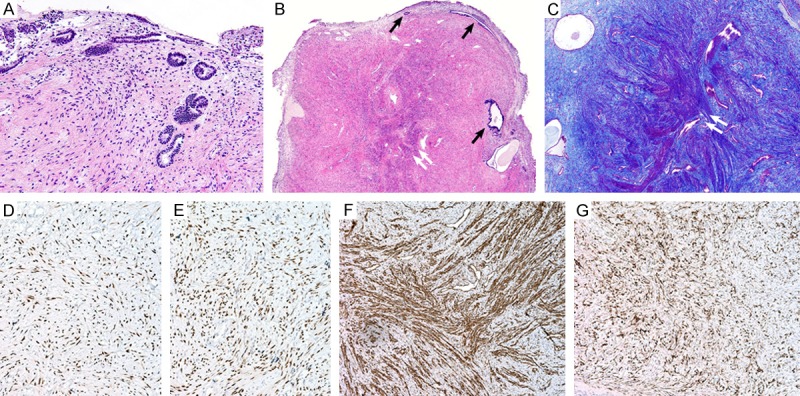
Histologic findings and immunohistochemical staining results of smooth muscle metaplasia occurring in peritoneal endometriotic foci. (A) The intraoperative frozen sections revealed scattered glandular structures within the fibrous stroma. (B) The permanent sections of the lateral pelvic wall nodules displayed endometrial-type glands (black arrows) embedded in the fibromuscular stroma. In the center of the nodules, smooth muscle cells arranged in discrete bundles and intersecting fascicles (white double arrow) were noted. (C) The muscle bundles (white double arrow) were highlighted purple red with Masson’s trichrome stain. Immunohistochemically, the endometrial-type glands and stroma, as well as the smooth muscle cells, were positive for (D) estrogen receptors and (E) progesterone receptors. (F) Additionally, the smooth muscle cells were positive for smooth muscle actin. (G) CD10 highlighted scattered endometrial stromal cells, which were difficult to detect with the conventional staining.
Pathologic findings
The permanent sections of the lateral pelvic wall nodules displayed irregular-shaped, endometrial-type glands scattered within the fibromuscular stroma. In particular, smooth muscle cells were arranged in discrete bundles and intersecting fascicles within the center of the nodules (Figure 1B). The muscle bundles were highlighted purple red in a fibrous background with Masson’s trichrome stain (Figure 1C).
Immunohistochemical staining of formalin-fixed, paraffin-embedded sections was performed. The endometrial-type glands and stroma, as well as the smooth muscle cells, were positive for estrogen receptors (Figure 1D) and progesterone receptors (Figure 1E). The smooth muscle cells were positive for smooth muscle actin and desmin (Figure 1F), but they were negative for inhibin-α and calretinin. Additionally, CD10 immunostaining highlighted scattered endometrial stromal cells, which were difficult to detect in the hematoxylin and eosin-stained slides (Figure 1G). Based on the microscopic findings and immunohistochemical staining results, the pelvic wall nodules were diagnosed as nodular smooth muscle metaplasia occurring in peritoneal endometriotic foci.
Discussion
It has been known that there are three distinct types of endometriotic lesions: ovarian, peritoneal, and rectovaginal [3]. The smooth muscle cells are frequent components of endometriosis of the rectovaginal pouch. In the fibromuscular tissue, aggregated smooth muscle cells are present within the fibrotic areas, and the muscle element is called smooth muscle metaplasia [4]. In fact, since the fibromuscular tissue rather than endometriotic tissue is the main component of the endometriotic nodule, rectovaginal endometriosis usually presents with a nodular lesion [4,5]. In contrast, grossly red, flame-like appearance of peritoneal endometriosis probably reflects the first stage of early implantation of endometrial tissue on the peritoneal surface [3,6]. Furthermore, since menstrual shedding induces an inflammatory reaction, the enclosed implant with presence of intraluminal debris becomes a black lesion. In this regard, presentation of peritoneal endometriosis as nodular masses protruding from the lateral pelvic wall is extremely unusual. Although the inflammatory process with subsequent fibrosis rarely devascularize the endometriotic foci, the remaining old collagen in the implant appears as plaques rather than nodular lesions [3]. As observed in this case, when performing laparoscopic surgery in order to excise malignant tumors of intra-abdominal or pelvic organs, it can be difficult for surgeons to distinguish the metastatic tumors from benign nodular pelvic wall lesions, including endometriosis, based on the gross findings only. Therefore, an intraoperative frozen section biopsy of the pelvic wall nodules should be performed to evaluate the peritoneal involvement by malignant tumors. Moreover, this report implies that peritoneal endometriosis, as well as rectovaginal endometriosis, can clinically present as nodular lesions if obvious smooth muscle metaplasia is present.
Histologically, typical peritoneal endometriosis is characterized by both epithelium and stroma of the endometrial type. In this case, however, several scattered endometrial-type glands were embedded in the fibromuscular stroma, and the proportion of stroma was much higher in comparison with that of glands. In conventional hematoxylin and eosin-stained slides, it was difficult to distinguish the endometrial-type stroma from the fibromuscular stroma. Furthermore, in the center of the nodules, completely bland smooth muscle cells arranged in short, regular intersecting fascicles were detected. The muscle bundles were immunoreactive for smooth muscle actin and desmin, and they stained purple red with Masson’s trichrome stain. Immunostaining for CD10 showed scattered endometrial stroma, probably fragmented by fibromuscular stromal tissue. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and endometrial stromal neoplasms [7,8]. The division and fragmentation of endometrial stromal cells, which were difficult to detect with the conventional staining, were easily confirmed with CD10 immunoreactivity. This indicates that CD10 immunostaining is useful in the diagnosis of endometriosis showing abundant fibrous and smooth muscle elements.
Regarding the presence of smooth muscle cells in peritoneal endometriosis, there may be two possibilities. Firstly, endometriotic foci involving smooth muscle are typically associated with striking proliferation of the smooth muscle [9-11]. Secondly, the endometriotic stroma may exhibit smooth muscle metaplasia, as has been demonstrated within the wall of ovarian endometriotic cysts [11,12]. In this case, the presence of smooth muscle in the endometrial stroma can be explained by the metaplastic theory. However, the reason for such metaplastic transformation still remains unclear. The other possibility is the presence of multipotent cells in the endometrial stroma that can differentiate into myofibroblasts and smooth muscle cells [13].
In rectovaginal endometriosis, fibromuscular nodules have been considered to be the major cause of pelvic pain and sexual dysfunction [4,5]. Our patient had experienced intermittent episodes of recurrent pelvic pain. The presence of smooth muscle coupled with fibrosis in the endometriotic foci raises the possibility of producing fibromuscular nodules related to the intermittent pelvic pain. Mechsner and colleagues [14] have shown that the epithelial cells and smooth muscle cells in peritoneal endometriotic foci express oxytocin receptors, in addition to the estrogen and progesterone receptors. It is likely that peritoneal smooth muscle contractions could stimulate peritoneal nociceptors leading to the generation of pelvic pain in patients with endometriosis [15]. It is also plausible that the inhibition of oxytocin receptors by specific inhibitors might be a useful approach for the treatment of endometriosis-related pelvic pain [14].
In summary, we report here an unusual presentation of peritoneal endometriosis with smooth muscle metaplasia as multiple protruding masses on the lateral pelvic wall. Smooth muscle metaplasia is a common finding in rectovaginal endometriosis, leading to the clinical presentation as a white nodule. In contrast, in peritoneal endometriosis, smooth muscle metaplasia is uncommon, and its nodular presentation on the lateral pelvic wall is even rarer. To the best of our knowledge, this is the first case of nodular smooth muscle metaplasia occurring in peritoneal endometriosis. The pathological investigation of smooth muscle cells in peritoneal lesions contributes not only to the precise diagnosis but also to the structure and function of smooth muscle cells and related cells involved in the histogenesis of peritoneal endometriosis.
Acknowledgements
This paper was supported by a grant of the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C3418).
Disclosure of conflict of interest
None.
References
- 1.Doss BJ, Wanek SM, Jacques SM, Qureshi F, Ramirez NC, Lawrence WD. Ovarian smooth muscle metaplasia: an uncommon and possibly underrecognized entity. Int J Gynecol Pathol. 1999;18:58–62. doi: 10.1097/00004347-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Fukunaga M. Smooth muscle metaplasia in ovarian endometriosis. Histopathology. 2000;36:348–352. doi: 10.1046/j.1365-2559.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- 3.Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68:585–596. doi: 10.1016/s0015-0282(97)00191-x. [DOI] [PubMed] [Google Scholar]
- 4.Itoga T, Matsumoto T, Takeuchi H, Yamasaki S, Sasahara N, Hoshi T, Kinoshita K. Fibrosis and smooth muscle metaplasia in rectovaginal endometriosis. Pathol Int. 2003;53:371–375. doi: 10.1046/j.1440-1827.2003.01483.x. [DOI] [PubMed] [Google Scholar]
- 5.Anaf V, Simon P, Fayt I, Noel J. Smooth muscles are frequent components of endometriotic lesions. Hum Reprod. 2000;15:767–771. doi: 10.1093/humrep/15.4.767. [DOI] [PubMed] [Google Scholar]
- 6.Nisolle M, Casanas-Roux F, Anaf V, Mine JM, Donnez J. Morphometric study of the stromal vascularization in peritoneal endometriosis. Fertil Steril. 1993;59:681–684. [PubMed] [Google Scholar]
- 7.McCluggage WG, Sumathi VP, Maxwell P. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms. Histopathology. 2001;39:273–278. doi: 10.1046/j.1365-2559.2001.01215.x. [DOI] [PubMed] [Google Scholar]
- 8.Yaziji H, Gown AM. Immunohistochemical analysis of gynecologic tumors. Int J Gynecol Pathol. 2001;20:64–78. doi: 10.1097/00004347-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Brosens IA. Is mild endometriosis a progressive disease? Hum Reprod. 1994;9:2209–2211. doi: 10.1093/oxfordjournals.humrep.a138422. [DOI] [PubMed] [Google Scholar]
- 10.Donnez J, Van Langendonckt A, Casanas-Roux F, Van Gossum JP, Pirard C, Jadoul P, Squifflet J, Smets M. Current thinking on the pathogenesis of endometriosis. Gynecol Obstet Invest. 2002;54(Suppl 1):52–58. doi: 10.1159/000066295. discussion 59-62. [DOI] [PubMed] [Google Scholar]
- 11.Witz CA. Pathogenesis of endometriosis. Gynecol Obstet Invest. 2002;53(Suppl 1):52–62. doi: 10.1159/000049425. [DOI] [PubMed] [Google Scholar]
- 12.Scully RE, Richardson GS, Barlow JF. The development of malignancy in endometriosis. Clin Obstet Gynecol. 1966;9:384–411. doi: 10.1097/00003081-196606000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Scully RE. Smooth-muscle differentiation in genital tract disorders. Arch Pathol Lab Med. 1981;105:505–507. [PubMed] [Google Scholar]
- 14.Mechsner S, Bartley J, Loddenkemper C, Salomon DS, Starzinski-Powitz A, Ebert AD. Oxytocin receptor expression in smooth muscle cells of peritoneal endometriotic lesions and ovarian endometriotic cysts. Fertil Steril. 2005;83(Suppl 1):1220–1231. doi: 10.1016/j.fertnstert.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 15.Odagiri K, Konno R, Fujiwara H, Netsu S, Yang C, Suzuki M. Smooth muscle metaplasia and innervation in interstitium of endometriotic lesions related to pain. Fertil Steril. 2009;92:1525–1531. doi: 10.1016/j.fertnstert.2008.08.101. [DOI] [PubMed] [Google Scholar]


