Abstract
Follistatin-like 5 (FSTL5), a member of the follistatin family of genes, encodes a secretory glycoprotein. Previous study revealed that it might play a suppressive role in hepatocellular carcinoma (HCC). However, its clinical significances, biological functions and molecular mechanisms in HCC development are poorly understood. To gain insight to the functions of FSTL5 in HCC, We examined FSTL5 expression pattern in 117 HCC tissue samples. The results of immunohistochemical staining analysis showed that FSTL5 is more commonly down-regulated in HCC compared to adjacent tissues and further clinicopathological analysis showed that its expression level is closely correlated with tumor size, TNM stage, local infiltration and patient prognosis. Both gain function assays and recombinant human FSTL5 protein treatment assays in vitro revealed that over-expressing FSTL5 could inhibit the abilities of cancer cell proliferation and survival. Further, we found that those effects on HCC growth and survival are associated with Wnt/β-catenin signaling. Taken together, all of our results validate that FSTL5 plays a suppressive role in HCC and suggest that down-regulated FSTL5 could elevate abilities of growth and survival of HCC cells by activation of Wnt/β-catenin signaling.
Keywords: Follistatin-like 5, hepatocellular carcinoma, cell proliferation, apoptosis, Wnt/β-catenin signaling
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignant neoplasm and the third most frequent cause of cancer death [1], while the rank even reached up to the second in china [2]. There are many risk factors such as viral infection, aflatoxin, alcohol have been identified could contribute to HCC [3]. Despite the developments in the detection and management of HCC, patients with HCC remain suffered a bad life for current therapies. Thus, there remains further research for this malignant tumor.
Both genetic alterations [4] and tumor specific microenvironment [5] contribute to the progressive biological behaviors of HCC cells and then result in different prognosis of HCC patients. There are many oncogenic and anti-oncogenic genes have been identified by different approaches during the past decades. In a previous publication, Zender et al. [6] combined short hairpin RNAs (shRNAs) and a mosaic mouse model functionally identified and validated 12 anti-oncogenic genes which had not been reported in cancer and Follistatin-like 5 (FSTL5), as a suppressive gene was found in paper.
Follistatin-like 5 (FSTL5), a member of the follistatin family of genes, encodes a secretory glycoprotein [7]. Previous studies revealed that FSTL5 is a marker of poor prognosis in non-WNT/Non-SHH medulloblastoma [8]. Recently, Tomoyuki Ma. et al. reported that FSTL5 might play a role in maintaining odor perception in adult mouse [9]. While the clinicopathological significance and its biological functions and associated mechanisms in HCC have not reported to date. In current study, we analyzed the significance of clinicopathologic of FSTL5 in 117 HCC samples and found it is correlated to tumor size, TNM stage and local infiltration. Further investigated for its biological functions reveal that FSTL5 could inhibit cell proliferation and promote cell apoptosis. To gain insight into the mechanisms, we found FSTL5 inhibits Wnt/β-catenin signaling. Taken together, our studies enlarge the knowledge of the understanding of FSTL5 in HCC, and give clues for further study to fully elucidate the role of FSTL5 in malignant tumor.
Materials and methods
Patients and specimens
All 117 samples were collected in Department of Hepatobiliary Surgery, the First Affiliated Hospital of Bengbu Medical College. All these samples were constructed into tissue microarray (TMA). The median age of this cohort of patients was 50 years (range 17-65 years). All procedures were performed in accordance with the China Ethical Review Committee.
Immunohistochemical staining and analysis
All 117 samples of HCC on a TMA slice were subjected to Immunohistochemical staining. All stained and analyzed methods were according to a previous report [10].
Cell culture
The HCC cell lines SK-Hep1, HepG2 were purchased from American type culture collection (ATCC), SMMC-7721 and MHCC-LM3 was purchased from Cell Bank of the Chinese Academy of Sciences. Cells were cultured in Dulbecco’s Dodified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin at 37°C in a humidified incubator under 5% CO2 condition.
Quantitative real-time PCR
Total RNA were extracted from indicated HCC cell lines using Trizol reagent (Takara, Dalian, China) and reversely transcribed using PrimeScript RT-PCR kit (Takara) according to the manufacturer’s instruction. Quantitative real-time PCR analyses were performed with SYBR Premix Ex Taq (Takara) on VIIA7 (Applied Biosystems Inc., USA). Primers for this study as follows: GAPDH, forward, 5’-GGAGCGAGATCCCTCCAAAAT-3’; reverse, 5’-GGCTGTTGTCATACTTCTCATGG-3’. FSTL5, forward, 5’-TGAAGTGCACAGAGCTGCTT-3’; reverse, 5’-AGCATATTTTTCATCTTGCTGTATTC-3’. The relative expression of FSTL5 was analyzed by the comparative cycle threshold method (ΔΔCT method), which was normalized to GAPDH.
Western blotting
Total protein were extracted using RIPA lysis buffer (P0013B, beyotime, China) followed the protocol and 30-50 μg of those proteins were separated by reduced SDS-PAGE, and transferred onto nitrocellulose membrane then the membrane was blocked in TBS buffer containing 5% BSA (sangon, China) for 1 hour. These membranes were incubated with primary antibodies for FSTL5 (1:1000, protein tech, USA), β-catenin (1:1000, Cell Signaling Technology, USA), p-β catenin (1:1000, Cell Signaling Technology, USA), cleaved PARP (1:1000, Cell Signaling Technology, USA), GSK3β (1:1000, Cell Signaling Technology, USA), p-GSK3β (1:1000, Cell Signaling Technology, USA), GAPDH (1:5000, Cell Signaling Technology, USA) overnight, and then followed by horseradish peroxidase (HRP)-linked secondary antibody (Cell signaling, USA). ImmobilonTM Western Chemiluminscent HRP Substrate kit (Millipore Corporation, Germany) was used for detection.
Over-expressing FSTL5 in HCC cell line
The expression vector containing the open reading frame of FSTL5 was purchased from Genecopoeia (Guangzhou, China). 50×104 SK-Hep1 cells seeded in 6-well plate were transfected with 2 μg over-pressing vector using Lipofectamine Reagent (Invitrogen, USA). After 48 h incubation, stably transfected cells were selected by administration of 2 μg/ml puromycin in DMEM for two weeks. The puromycin-resistant colonies were isolated by a limited dilution approach. They were expanded and then maintained in regular growth medium containing 2 μg/ml puromycin.
Recombinant human FSTL5 treatment
Cells were seeded into a 96-well plate at 4×103 cells per well with 100 μl and 1 ml conditional medium with 5% FBS (v/v) and 0, 10 and 50 nM Recombinant human FSTL5 (rFSTL5) (Proteintech, USA) as indicated for following cell viability assays. For apoptosis assays, cells were cultured with DMEM didn’t containing any FBS but supplemented with 0, 10 and 50 nM rFSTL5 for 48 hours. For luciferase reporter assays, Recombinant FSTL5 or vehicle control was added 24 hours after transfection, and luciferase activity was determined.
Cell viability assay
Cells were seeded into a 96-well plate at 4×103 cells per well with 100 ul complete medium and cultured at 37°C. The cell viability was quantified by addition 10 ul of cell counting kit (CCK8, Dojindo, Japan). After 1.5 hours incubation, the plates were monitored by Power Wave XS microplate reader (BIO-TEK) at an absorbance 450 nm.
Cell apoptosis assay
20×104 cells per well were cultured in 6-well plates with serum starvation for 48 hours. Then cells were harvested in complete DMEM medium and centrifuged at 1000 rpm for 5 minutes. Each of those cell lines was washed with pre-cooled PBS and stained with propidium iodide and Annexin V-FITC (BD Pharmingen, USA) following the manufacturer’s instructions. Then Flow Cytometry (FCM, BD Pharmingen) were subjected to analyze cell apoptosis.
Luciferase reporter assay
Stable over-expressing FSTL5 SK-Hep1 cells were seeded in 96-well plates and transfected with mixture of 100 ng TCF/catenin reporter plasmid (Wnt/β-catenin signaling) and 10 ng Renilla following the recommended protocol for the Lipofectamine 2000 transfection system. SMMC-7721 cells were treated with rFSTL5 protein at a concentration of 50 nM. After 48 hours of incubation, firefly and Renilla luciferase activities were measured using the dual-luciferase reporter assay system (Promega, Madison, WI) from the cell lysates.
Statistical analysis
Data were presented as the means ± SD. Correlation of FSTL5 expression with clinicopathologic parameters was evaluated by chi-square test. The student’s t-test was used for comparison between groups. P < 0.05 is considered to be statistically significant.
Results
The expression of FSTL5 is down-regulated in HCC and closely related to poor patient prognosis
To compare the expression of FSTL5 in HCC tissues and their adjacent tissues, we detect the levels of FSTL5 in 117 HCC samples and their paired adjacent tissues by immunochemical staining. Stronger FSTL5 staining was detected in the adjacent tissues than HCC tissues (Figure 1A). To further investigate the clinical significance of FSTL5 in HCC, we analyzed the correlations between the FSTL5 expression status and clinicopathological characteristics of 117 HCC samples. Those samples were divided into two groups: the high expression group and low expression group. The results indicated that the expression levels of FSTL5 in the HCC tissues was closely associated with tumor size (P = 0.007), TNM stage (P = 0.012) and local infiltration (P = 0.041) (Table 1). We then examined the correlation between FSTL5 level and patient prognosis and found that patients with strong FSTL5 expression had higher rates of overall survival (P = 0.05) than patient with none or weak FSTL5 expression (Figure 1B). Taken together, these data strongly indicate that FSTL5 might play a suppressive role in HCC.
Figure 1.
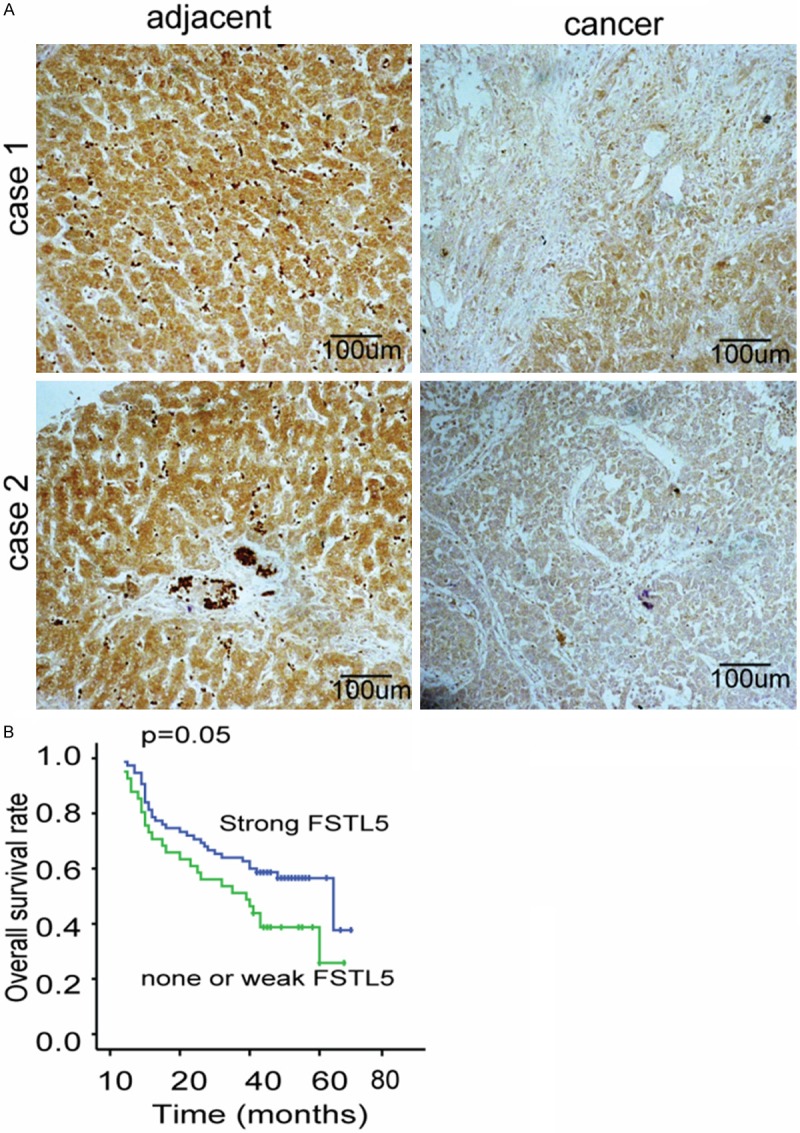
FSTL5 is significantly down-regulated in HCC compared with adjacent tissues and is closely related to the prognosis of HCC. A. Representative pictures demonstrate FSTL5 is mildly expressed in HCC tissues while is significantly elevated in HCC. (Original magnification: 200×. Scale bars, 100 μm). B. Kaplan-Meier analysis of overall survival between FSTL5 negative or slightly positive patients and highly positives patients show a significant survival disadvantage expression in FSTL5 high expression group (P = 0.05).
Table 1.
Correlation between FSTL5 and key clinicopathological parameters
| Variable | NRSN2 (n = 117) | |||
|---|---|---|---|---|
|
| ||||
| Low | High | P value | ||
| Age | ≤ 50 years | 40 | 19 | 0.915 |
| > 50 years | 36 | 22 | ||
| Gender | Female | 9 | 4 | 0.732 |
| Male | 67 | 37 | ||
| Liver Cirrhosis | Yes | 61 | 34 | 0.725 |
| No | 15 | 7 | ||
| Tumor size | ≤ 5 cm | 44 | 13 | 0.007* |
| > 5 cm | 32 | 28 | ||
| Local infilitration | Yes | 35 | 6 | 0.041* |
| No | 72 | 3 | ||
| TNM stage | I | 23 | 21 | 0.012* |
| II | 24 | 4 | ||
| III-IV | 29 | 16 | ||
p < 0.05.
Over-expressing FSTL5 inhibits HCC cell proliferation, promotes cell apoptosis
The significance of FSTL5 in HCC clinical pathology reminds us to explore the cellular functions of FSTL5 in HCC cell lines. Firstly, we detected the expression level of FSTL5 in HCC cell lines, and found that FSTL5 was relative weakly expressed in SK-Hep1 cell lines (Figure 2). Then we over-expressing FSTL5 in the cell line and the FSTL5 levels were significantly increased (Figure 3A). We first examined the effect of over-expressing FSTL5 on HCC cell viability. The results showed that over-expressing FSTL5 inhibits the cell proliferation significantly in SK-Hep1cells by Cell Counting Kit-8 (CCK8) assay (Figure 3B). To further explore the cellular functions about cell survival, we also performed the cell apoptosis assays. The results showed us that elevated the level of FSTL5 could promote cell apoptosis when deprived serum (Figure 3C).
Figure 2.
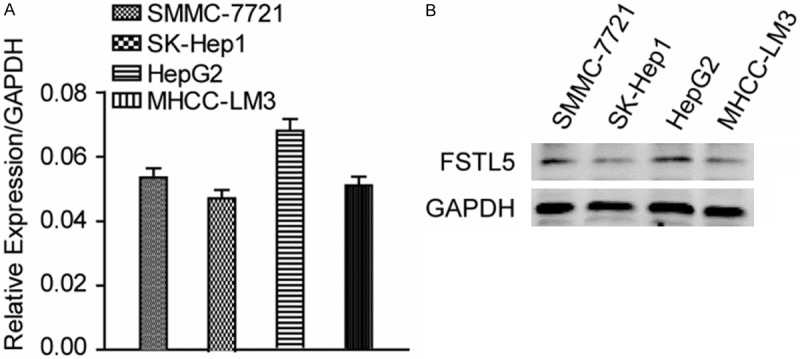
The levels of FSTL5 in HCC cell lines. A. Relative mRNA expression of FSTL5 in four HCC cell lines, GAPDH as the reference gene. B. FSTL5 was detected in four HCC cell lines. GAPDH was used as a loading control.
Figure 3.
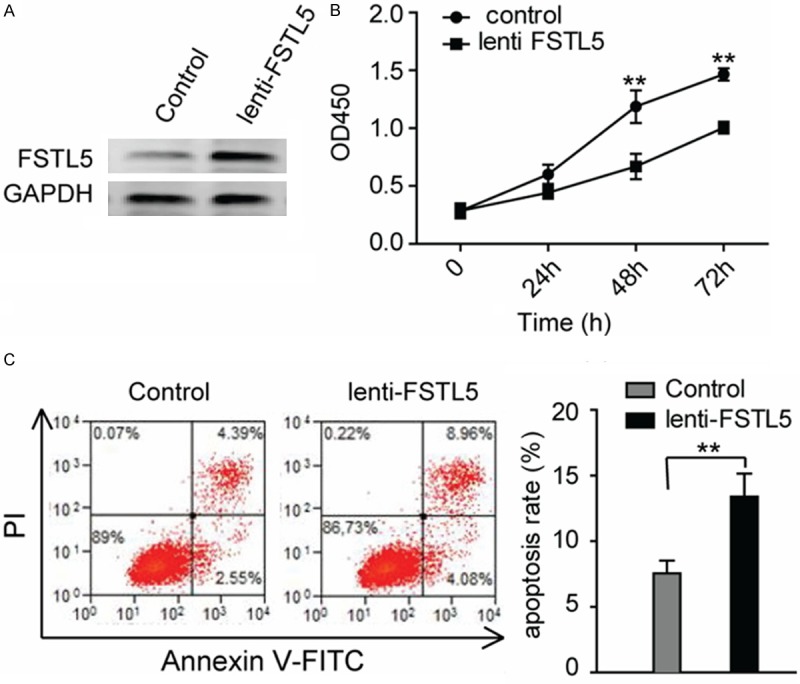
Over-expressing FSTL5 inhibits cancer cell proliferation and survival. A. FSTL5 levels were detected in SK-Hep1 cells after over-expressing. B. Cell viability analysis of SK-Hep1 cells when FSTL5 heightened, as measured by CCK-8 assay. (Values are mean ± SD. **P < 0.01). C. Cell apoptosis was analyzed by Annexin-V/ PI staining and flow cytometric analysis. (**P < 0.01), Statistic data shown in right panel are means ± SD of apoptotic cell rates. Data are representative of three to five independent experiments.
Recombinant human FSTL5 protein inhibits HCC cell proliferation, promotes apoptosis
Considering the effects in HCC cell line when the over-expression of FSTL5, we used the rFSTL5 protein to confirm the cellular functions of FSTL5. Convincingly, we found that when treated with rFSTL5, the cellular viability of SMMC-7721 decreased (Figure 4A) and the number of apoptotic cells increased after starving cells by depriving serum (Figure 4B).
Figure 4.
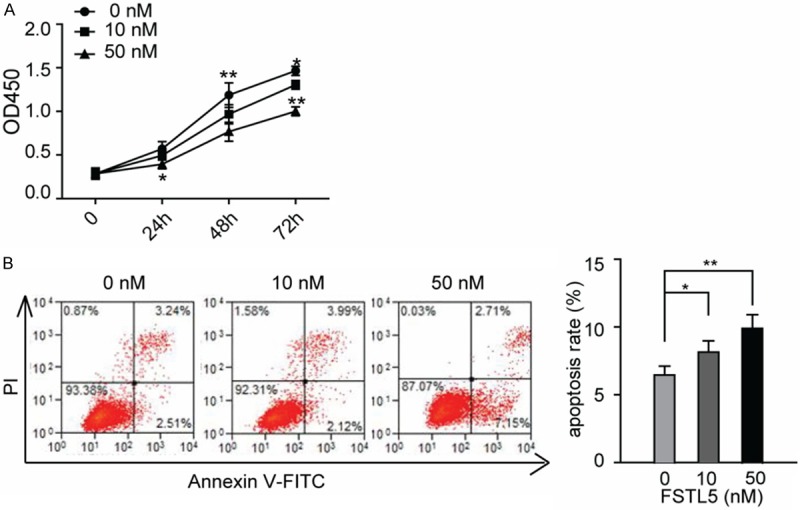
Recombinant human FSTL5 protein inhibits cancer cell viability and survival in a dose-dependent manner. A. CCK-8 cell viability analysis was determined in SMMC-7721 cells when rFSTL5treatment. (Values are mean ± SD. *P < 0.05, **P < 0.01). B. Representative images of flow cytometric analysis of SMMC-7721 cell apoptosis when treated with indicated concentration of rFSTL5 protein. Statistical analysis are shown in right panel (**P < 0.01). Data are representative of three independent experiments.
FSTL5 inhibits Wnt/β-catenin signaling pathway in HCC cell line
Considering the decreased cell viabilities and the anti-apoptosis effect after FSTL5 over-expressing and rFSTL5 treatment, we examined the change of Wnt/β-catenin pathway, which has been reported associated with HCC progression. Stable SK-Hep1 cells transfected with a Wnt/β-catenin reporter plasmid (TCF/catenin plasmid) and negative control. As shown in Figure 5A, the Wnt/β-catenin pathway was inhibited after over-expressing FSTL5. We further confirmed the inhibitory effect of FSTL5 on Wnt/β-catenin signaling by western blot assay (Figure 5B). The level of phosphorylated β-catenin and GSK3βwas increased, which indicates the degradation of β-catenin. Meanwhile, the cleaved PARP was significantly increased after FSTL5 over-expressing, which plays as an apoptosis marker. Convincingly, those results were confirmed by rFSTL5 protein treatment in SMMC-7721 cells, as shown in Figure 5C and 5D.
Figure 5.
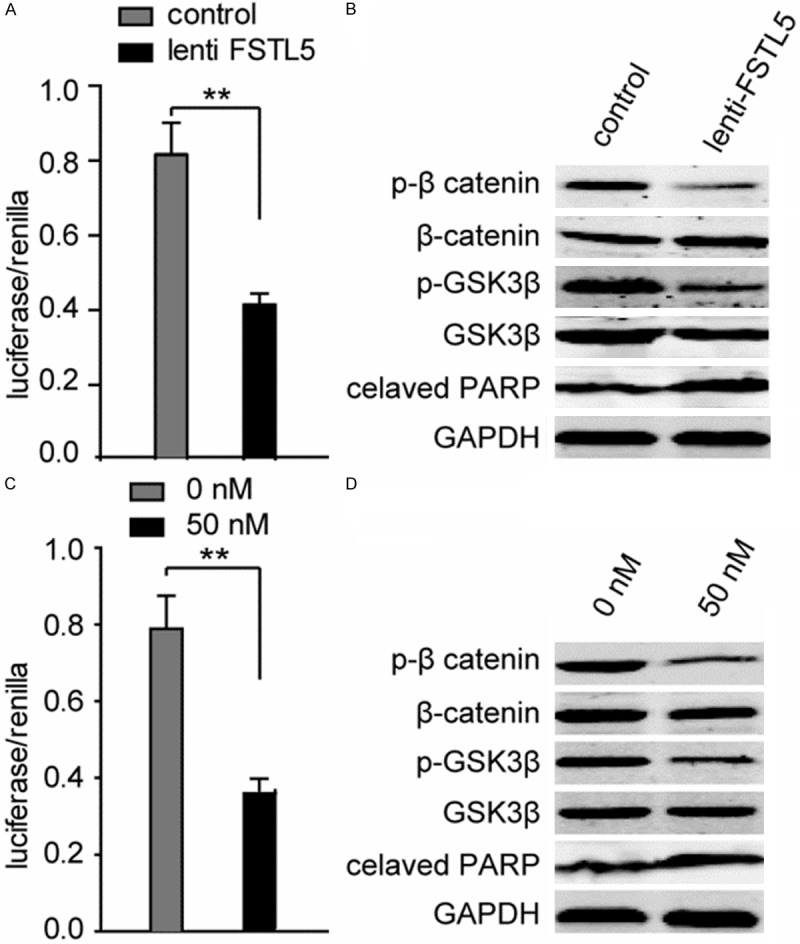
FSTL5 inhibits Wnt/β-catenin signaling. A. Representative dual-luciferase reporter assay showed that over-expressing FSTL5 inhibits Wnt/β-catenin signaling. B. The expression of phosphorylation of β-catenin, GSK3β and cleaved PARP were examined after elevated the level of FSTL5 in SK-Hep1 cells. C. Representative dual-luciferase reporter assay showed that rFSTL5 protein inhibited Wnt/β-catenin signaling in SMMC-7721 cells (**P < 0.01). D. The expression of phosphorylation of β-catenin, GSK3β and cleaved PARP were examined after treated SMMC-7721 cells with indicated concentration of rFSTL5 protein (**P < 0.01). Data are representative of three independent experiments.
Discussion
This is the first study focus on the biological functions and associated mechanisms of FSTL5 in HCC. FSTL5 is a poorly studied gene which had been identified as a tumor suppressive gene in a Genome-wide shRNAs screen experiment [6]. However the cellular functions of FSTL5 haven’t been reported in tumor to date. Our group investigates the clinical significance of FSTL5 in HCC tissues and found that patients with higher FSTL5 expression more commonly indicate a relatively better prognosis and FSTL5 was closely correlated to tumor size and TNM stage, this clinicopathologic clues inspired us to explore the cellular functions and associated mechanisms in HCC.
We over-expressed FSTL5 in HCC cell line SK-Hep1 which relative weakly expressed this protein, and then employed these stable cells to conduct the cellular function assays in vitro. The findings in stable cells tell us that FSTL5 plays an inhibitory role in HCC for it could inhibit cancer cell proliferation and promote malignant tumor cells to apoptosis. These inhibitory effects were confirmed by recombinant human FSTL5 protein treatment in cellular function assays in another HCC cell line SMMC-7721.
FSTL5, a member of the follistatin family of genes, encodes a secretory glycoprotein [7]. Previous studies revealed that other members of this family including FSTL1 and FSTL3 play an essential role in development, homeostasis, and congenital disorders [11,12]. However, the biological functions of FSTL5 are poorly understood, not mention to the associated mechanisms. In a recent paper, Marc et al. reported that FSTL5 is a marker of poor prognosis in non-WNT/Non-SHH medulloblastoma [8] and FSTL5 transcripts were most up-regulated in Group C and Group D tumors with unfavorable prognosis, whereas WNT medulloblastomas showed marked down-regulation [13], these results remind us to explore the association between FSTL5 and Wnt signaling in HCC. As we known Aberrant activation of the Wnt/β-catenin pathway has been implicated in tumor of multiple tissues including the brain, breast, colon, skin, and the liver [14] and 20% to 90% of HCCs display β-catenin activation [15,16]. In current study, we performed luciferase reporter assays and found that Wnt/β-catenin signaling is inhibited when FSTL5 over-expressing or rFSTL5 treatment. These results suggest that FSTL5 could inhibit the activation of Wnt/β-catenin signaling to inhibit cell proliferation and promote cell apoptosis in bad growth environment.
As we known, the niche of a cancer cell plays important roles in cancer development. A major component of the niche is the extracellular matrix (ECM) [17]. Here we report an ECM protein FSTL5 plays a suppressive role in HCC through inhibits Wnt/β-catenin signaling, while unfortunately, it was down-regulated in HCC. However, the mechanism of why FSTL5 decreased in HCC still remains to be elucidated. As we known, epigenetic alterations which including hypermethylation of the CpG islands in promoter regions, histone deacetylation, and microRNA alterations play an essential role in cancer progression [18-20], while experiments should be performed in further study.
In conclusion, FSTL5, a secretory glycoprotein was down-regulated in HCC and it exhibits tumor suppressive role during HCC progression and as a secretory glycoprotein, it might be an agent for HCC treatment.
Acknowledgements
This work was supported by Bengbu medical college PhD research start-up funds.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El–Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 5.Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol. 2011;21:35–43. doi: 10.1016/j.semcancer.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zender L, Xue W, Zuber J, Semighini CP, Krasnitz A, Ma B, Zender P, Kubicka S, Luk JM, Schirmacher P, McCombie WR, Wigler M, Hicks J, Hannon GJ, Powers S, Lowe SW. An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell. 2008;135:852–864. doi: 10.1016/j.cell.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuda T, Kai N, Sakuma C, Kobayashi K, Koga H, Yaginuma H. Laser capture microdissection and cDNA array analysis for identification of mouse KIAA/FLJ genes differentially expressed in the embryonic dorsal spinal cord. Brain Res. 2009;1249:61–67. doi: 10.1016/j.brainres.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Remke M, Hielscher T, Korshunov A, Northcott PA, Bender S, Kool M, Westermann F, Benner A, Cin H, Ryzhova M. FSTL5 is a marker of poor prognosis in non-WNT/non-SHH medulloblastoma. J. Clin. Oncol. 2011;29:3852–61. doi: 10.1200/JCO.2011.36.2798. [DOI] [PubMed] [Google Scholar]
- 9.Masuda T, Sakuma C, Nagaoka A, Yamagishi T, Ueda S, Nagase T, Yaginuma H. Follistatin-like 5 is expressed in restricted areas of the adult mouse brain: Implications for its function in the olfactory system. Congenit Anom (Kyoto) 2014;54:63–66. doi: 10.1111/cga.12022. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, Zhang P, Shi B, Zhou M, Jiang H, Zhang H, Pan X, Gao H, Sun H, Li Z. Galectin-1 overexpression promotes progression and chemoresistance to cisplatin in epithelial ovarian cancer. Cell Death Dis. 2014;5:e991. doi: 10.1038/cddis.2013.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel K, Connolly D, Amthor H, Nose K, Cooke J. Cloning and early dorsal axial expression of Flik, a chick follistatin-related gene: evidence for involvement in dorsalization/neural induction. Dev Biol. 1996;178:327–342. doi: 10.1006/dbio.1996.0222. [DOI] [PubMed] [Google Scholar]
- 12.Towers P, Patel K, Withington S, Isaac A, Cooke J. Flik, a Chick Follistatin-Related Gene, Functions in Gastrular Dorsalisation/Neural Induction and in Subsequent Maintenance of Midline Sonic Hedgehog Signalling. Dev Biol. 1999;214:298–317. doi: 10.1006/dbio.1999.9421. [DOI] [PubMed] [Google Scholar]
- 13.Remke M, Hielscher T, Korshunov A, Northcott PA, Bender S, Kool M, Benner A, Ryzhova M, Sturm D, Witt H. FSTL5 improves prognostic subclassification of medulloblastoma. Cancer Res. 2011;71:3447–3447. [Google Scholar]
- 14.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 15.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyoshi Y, Iwao K, Nagasawa Y, Aihara T, Sasaki Y, Imaoka S, Murata M, Shimano T, Nakamura Y. Activation of the beta-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res. 1998;58:2524–2527. [PubMed] [Google Scholar]
- 17.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 19.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 20.Fabbri M, Calin GA. Epigenetics and miRNAs in human cancer. Adv Genet. 2010;70:87–99. doi: 10.1016/B978-0-12-380866-0.60004-6. [DOI] [PubMed] [Google Scholar]


