Abstract
To investigate the impact of different surgical margin and recurrence-free survival in patients with hepatocellular carcinoma (HCC). The data of 601 patients who underwent curative hepatectomy for HCC between January 1997 and December 2009 were analyzed. Milan group and exceeding Milan group were divided according to the Milan Criteria. Each of them was divided into 3 groups: group A (surgical margin ≤ 1 mm), group B (1 mm < surgical margin ≤ 9 mm) and group C (surgical margin ≥ 10 mm). The relationship between surgical margin and recurrence-free survival in different groups was analyzed. In Milan group recurrence-free survival of group C was more than group B and group B more than group A (P < 0.05). And in the exceeding Milan group recurrence-free surgical of group B was more than group A. There were no statistic differences within groups of B and C. Enlarging surgical margin may increase recurrence-free survival in HCC under Milan criteria.1mm in cases of exceeding Milan criteria may be regarded as the suitable surgical margin for operation of HCC.
Keywords: Surgical margin, hepatocellular carcinoma, recurrence-free survival, prognosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common and most malignant tumors, the incidence rate ranks the fifth in the malignant tumor in the world and the mortality rate ranked the third. The incidence and prevalence of HCC are highest in Southeast Asia and West Africa, but are rising in developed countries. Both surgical resection and liver transplantation are potentially curative treatments for resectable HCC. With the surgical technology gradually improvement and peri-operation period of the treatment of perfect, safety of liver resection and the success rate is improved, but the recurrence rate is still very high, and there are some reports showed that the cumulative recurrence rate of 5 years was 75%~100% [1,2]. Generally, surgeons think that enough margin is the premise of radical operation. What the margin should be defined and different operation margins with different tumor stages are still unclear. There is no uniform standard, and the impact factors of the postoperative recurrence are also controversial. The primary purpose of this study was to investigate the impact of different surgical margin and recurrence-free survival and investigate different influence factors of early recurrence after resection of HCC, this paper retrospectively analyzed the HCC radical resection of clinical pathology data and results.
Materials and methods
Patients
681 cases with liver resection for HCC from January 1997 to December 2009 in the Affiliated Hospital of Qingdao University Medical College were analyzed, 10 cases died within 30 days after surgery (1.46%), 42 cases were lost (6.2%) and non R0 resection in 24 cases. A total of 601 cases with R0 resection entered retrospectively, including 503 male cases and 98 female cases. The average age was 54.1 years (14~82 years old). HBsAg was positive in 520 patients (86.5%), anti-HCV was positive in 9 patients (1.5%), HbsAg and anti-HCV were positive in 5 cases (0.5%), negative serum hepatitis virus in 67 cases (11.1%).
Follow-up
In the first 3 months after operation, 1 time every month, liver function, serum alpha fetoprotein, abdominal B ultrasound and chest X ray were checked. Patients will be rechecked if they are suspected recurrent upper abdominal CT, lung CT and (or) hepatic arterial lipiodol angiography. Postoperative recurrence was diagnosed and confirmed as tumor by imaging examination. The follow-up was ended on December 31, 2011 or death and the median follow-up was 33 months (2~167.7 months).
601 patients were classified into Milan group and exceeding Milan group according to the international standard of Milan into Milan eligible group, and beyond the Milan criteria group (referred to as exceeding Milan group), were subgrouped according to sizes of specimen from the operation. The Milan group was divided into three groups: group A, margin ≤ 1 mm, 36 cases; group B, 1 mm < margin ≤ 9 mm, 129 cases; group C, margin ≥ 10 mm, 131 cases. With the method of exceeding Milan group was divided into A group, 72 cases; group B, 136 cases; group C, 97 cases; group D (B+C group), 233 cases. Comparison of different operation margin of DFS after HCC.
Statistics
Statistical analysis was performed using SPSS18.0 version. Kaplan-Meier method and Log rank test analysis were used for disease-free survival. P < 0.05 was considered significant differences statistically.
Results
Analysis of clinical and pathological data of different operation margin group
In different surgical margin cutting edge group, intraoperative hemorrhage (P = 0.002), blood transfusion (P = 0.001) had significant difference. With the increase of operation margin, bleeding rate (> 1000 ml) was by 12.7% in group A, 17.4% in group B and 27.8% in group C. Transfusion rate was 28.9% in group A, 31.7% in group B and 49.1% in group C, suggesting that along with the increase of operation margin, the possibility of bleeding, blood transfusion during operation increased. There were no significant differences in other clinical pathological data (Table 1). There was statistically significant difference between Milan group and exceeding Milan group (P = 0.000). It was suggested that they were two different sample groups, and Milan group of DFS was significantly prolonged (Figure 1).
Table 1.
Clinical pathological data in the groups with different operation margins
| Factors | ≤ 1 mm | 2~9 mm | ≥ 10 mm | χ 2 | P |
|---|---|---|---|---|---|
|
| |||||
| 108 | 265 | 228 | |||
| Age (>60 years old) | 32 (29.6%) | 76 (28.7%) | 55 (24.1%) | 1706 | 0.426 |
| Gender (Male) | 87 (80.6%) | 230 (86.8%) | 186 (816%) | 3391 | 0184 |
| Preoperation TACE | 19 (17.6%) | 31 (11.7%) | 27 (11.8%) | 2.696 | 0.260 |
| Child-Pugh Grade B | 3 (2.8%) | 16 (6.0%) | 16 (7.0%) | 2.442 | 0.295 |
| Serum AFP>400 ng/ml | 59 (55.7%) | 141 (55.1%) | 123 (55.9%) | 0.034 | 0.983 |
| Patients with cirrhosis | 100 (92.6%) | 236 (89.1%) | 201 (88.2%) | 1.558 | 0.459 |
| Accompanied by portal hypertension | 19 (17.6%) | 47 (17.7%) | 27 (11.8%) | 3.706 | 0.157 |
| Anatomical resection | 25 (23.1%) | 57 (21.5%) | 65 (28.5%) | 3.372 | 0.185 |
| Intraoperative hepatic inflow occlusion | 56 (51.9%) | 157 (59.2%) | 108 (47.4%) | 7.076 | 0.059 |
| operative hemorrhage > 1000 ml | 13 (12.1%) | 46 (17.4%) | 65 (28.7%) | 12.915 | 0.002 |
| Red blood cell transfusion | 31 (28.9%) | 84 (31.7%) | 111 (49.1%) | 14.188 | 0.001 |
| Regional lymph node metastasis | 5 (4.6%) | 5 (1.9%) | 4 (1.8%) | 3.071 | 0.215 |
| Tumor invasion to liver capsule | 77 (71.3%) | 186(70.2%) | 157 (68.9%) | 0.228 | 0.892 |
| With satellite nodules | 14 (13.0%) | 27 (10.2%) | 26 (11.4%) | 0.62 | 0.733 |
| Intrahepatic recurrence | 59 (78.7%) | 131 (49.4%) | 111 (48.7%) | 7.636 | 0.106 |
| Microscopic tumor thrombus | 17 (39.5%) | 23 (34.8%) | 16 (34.8%) | 0.299 | 0.861 |
| ALB | 21 (19.4%) | 34 (12.9%) | 27 (11.9%) | 3.77 | 0.152 |
| ALT | 33 (30.6%) | 63 (23.8%) | 62 (27.2%) | 1.976 | 0.372 |
| CRP | 42 (52.5%) | 89 (58.2%) | 54 (58.7%) | 0.853 | 0.653 |
Figure 1.
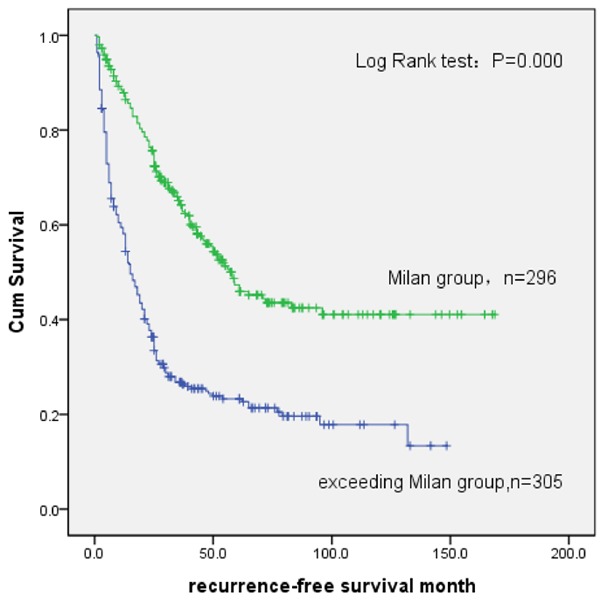
Comparison of DFS between the Milan group and the exceeding Milan group.
Comparison of DFS in Milan and exceeding Milan groups
To investigate the tumor free survival of HCC, different margin between the groups of DFS was compared, and there was significant difference (P = 0.010) between group A and B (P = 0.041), C and B (P = 0.020) in Milan group, as shown in Figure 2. The results showed that with the increase of margin, DFS increased accordingly. As shown in Figure 3, different margins of the groups compared, and DFS was with significant difference (P = 0.010) between group B and A (P = 0.008), group C and A (P = 0.045), group D and A (P = 0.04) in exceeding Milan group. It indicated that there was statistically difference for disease-free survival time. Tips for operation margin 1mm is bounded, group C to B P = 0.490, which suggested that DFS did not prolong when expanding the surgical margin.
Figure 2.
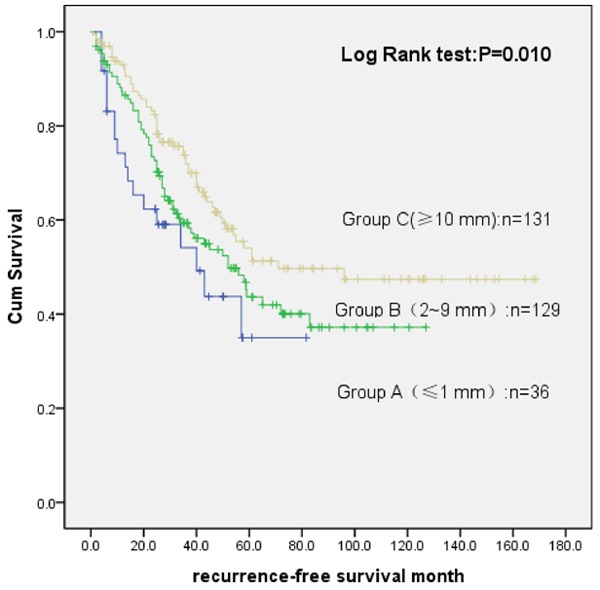
Survival analysis of different surgical margin in the Milan group.
Figure 3.
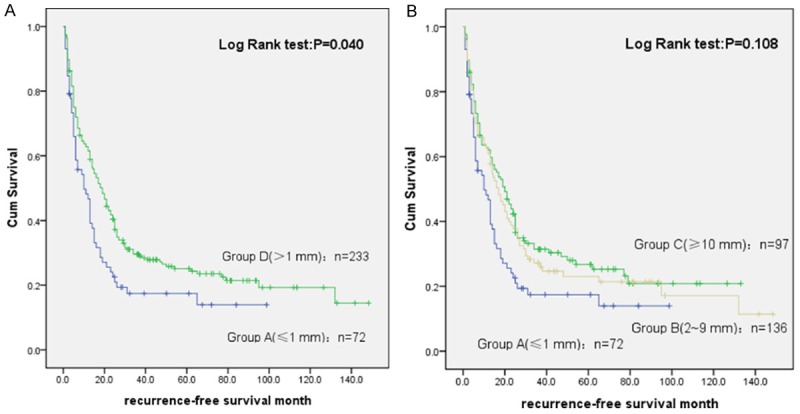
Tumor free survival analysis between different surgical margins in the exceeding Milan group. A: Comparison between group A and D; B: Comparison between group A-C.
Discussion
Hepatocellular carcinoma, a certain amount of surrounding nontumorous liver tissue is resected to prevent recurrences caused by microsatellite nodules and/or cancer embolus around the main tumor. To date, some studies have shown that a wide resection margin is an independent prognostic factor for recurrence and survival [3-6]. Chau et al. considered that margin (< 1 cm) is related to tumor recurrence of primary tumor in liver, put forward to enlarging the margin to ensure the negative margin and can reduce the probability of tumor recurrence to a certain extent [7]. Shi et al found that For macroscopically solitary HCC, a resection margin aiming grossly at 2 cm efficaciously and safely decreased postoperative recurrence rate and improved survival outcomes when compared with a gross resection margin aiming at 1 cm, especially for HCC ≤ 2 cm [8]. Lee et al. reported there was no statistically difference in tumor free survival rate after radical resection with different margins [9]. More research results indicated that there no clear relationship between margin size and postoperative recurrence of hepatocellular carcinoma, but it can increase the operation complications [10-12].
In this study, 601 cases of HCC patients with HCC radical resection were followed up continuously and set up the groups according to whether they met the Milan criteria in order to reduce bias as much as possible to achieve a homogeneous. There was significant difference between two groups of DFS after operation (P = 0.000), suggesting that two groups of samples were from different sources. To study the relationship between the different the cutting edge of operation in the two groups respectively and DFS, trying to explore the appropriate operation margin in different stage of HCC.
In our study, in the Milan group, with the increase of operation margin (from 1 mm to 2~9 mm to 10 mm above), DFS longer correspondingly; in the exceeding Milan group, DFS in operation margin with > 1 mm was higher than that with margin ≤ 1 mm group. When the cutting edge was more than 1 mm, with the increase of cutting margin, there was no increase in DFS. The reason may be: HCC in the early period, tumor invasion and metastasis is poor, the appropriate expansion of margin could increase radical and improve prognosis; while in the later period of HCC, there might be occult in microvessels or invasion or distant metastasis in the liver and DFS was not increased with margin expansion, however which reduced the rest liver, increased operation risks of liver failure and bleeding. Our research found that the likelihood of bleeding and blood transfusion increased during operation with the expansion of operation margin. The bleeding and blood transfusion were the risk factors for early recurrence of HCC in exceeding Milan group, which suggested that excessive pursuit of operation margin may reduce DFS in exceeding Milan group. Thus selection of liver cancer radical resection margin cannot lump together, and we should select different surgical margins of HCC according to the tumor staging.
The causes of recurrence after HCC are complicated, including factors of tumor itself, such as the number, size of tumor, tumor thrombus in the portal vein, vascular invasion, satellite nodules, pathological stage, differentiation, cancer cell types, and also including the host inflammatory state. The study found that early recurrence of HCC was related to tumor capsule and solitary in the two groups. The tumor became more malignant and more aggressive when HCC had incomplete capsule and indicated there were more possibility of recurrence and poor prognosis [8-10]. It was also found that intraoperative blood loss, blood transfusion, anatomic resection were the short-term risk factors of HCC recurrence in the ultra Milan group. The possible reasons were bleeding/blood transfusion peri operation period reflected the liver trauma made by operation that affected the liver inflammation after operation, then influence liver cancer recurrence, at the same time, blood transfusion peri-operation period may inhibit the immune anti-tumor ability. Postoperative inflammation of HCC can cause liver cell damage, leading to liver cell regeneration in the liver, increasing the mutation and the opportunities of HCC recurrence, which is the root of multi center of postoperative recurrence of hepatocellular carcinoma. Anatomical resection is more in line with the principle of radical surgery, and it can cut off the tumor and liver micro metastases at the same time, also can reduce the loss and liver for liver cancer cell operation caused by the extrusion of tumor dissemination and metastasis [11,12]. This study suggests that protection liver, reducing bleeding, blood transfusion, the anatomical resection in peri-operation period could reduce early recurrence the ultra Milan standard HCC.
A document titled “Evidence-based clinical guidelines for the diagnosis and treatment of HCC”, which was edited by the Japan Society of Hepatology, stated that “a minimum surgical margin is sufficient for hepatectomy” [13-15]. However, this idea has not been accepted worldwide, and the basic evidence does not support this recommendation sufficiently because the studies had relatively small samples or heterogeneous patient populations, which confounded the analysis of postoperative outcomes. In patients with a normal liver and tolerable liver function, a wider resection margin is an acceptable and feasible treatment strategy. However, liver resection for HCC in cirrhotic patients with a hepatoviral infection must strike a balance between resecting the tumor and preserving remaining liver function. In patients with hepatoviral infection, multicentric carcinogenesis and background liver dysfunction make this problem more difficult. A wider resection margin may control the local recurrence and potential intrahepatic metastasis caused by a residual microsatellite lesion. However, at the same time, a large-volume hepatectomy may diminish the remaining liver function and become an obstacle for treating recurrent HCC, and it could decrease the overall survival rate [16-18]. Moreover, even if hepatectomy with a wider resection margin controlled the recurrence caused by microsatellite lesions, multicentric recurrence may occur in the remaining part of the liver, which has been damaged due to hepatoviral infection.
In conclusion, the results of this study showed that there were different prognosis of HCC in different stages, DFS may be increased when the operation margin of HCC increasing under the standard of Milan. In this group of patients, tumor capsule, solitary, HP and preoperative GGT are the independent risk factors of early recurrence after operation. In the groups of beyond the Milan criteria, the cutting edge of 1 mm may be more appropriate in operation and tumor capsule and solitary lesion are the independent risk factors of early recurrence after operation. This study is a single center study, and results need multi-center study to further confirm.
Disclosure of conflict of interest
None.
References
- 1.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Chiu JH, Wu CW, P’eng FK. Prognostic significance of surgical margin in hepatocellular carcinoma resection: an analysis of 165 Child’s A patients. J Surg Oncol. 1997;66:122–126. doi: 10.1002/(sici)1096-9098(199710)66:2<122::aid-jso9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Tang YH, Wen TF, Chen X. Resection margin in hepatectomy for hepatocellular carcinoma: a systematic review. Hepatogastroenterology. 2012;59:1393–7. doi: 10.5754/hge10600. [DOI] [PubMed] [Google Scholar]
- 4.Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: A critical reappraisal. Ann Surg. 2000;231:544–51. doi: 10.1097/00000658-200004000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torzilli G, Donadon M, Montorsi M. The surgical margin in liver resection for shepatocellular carcinoma: a real problem or not? Ann Surg. 2007;246:690–1. doi: 10.1097/SLA.0b013e318156e286. [DOI] [PubMed] [Google Scholar]
- 6.Lise M, Bacchetti S, Da Pian P, Nitti D, Pilati PL, Pigato P. Prognostic factors affecting long term outcome after liver resection for hepatocellular carcinoma: results in a series of 100 Italian patients. Cancer. 1998;82:1028–36. doi: 10.1002/(sici)1097-0142(19980315)82:6<1028::aid-cncr4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 7.Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Chiu JH, Wu CW, P’eng FK. Prognostic significance of surgical margin in hepatocellular carcinoma resection: an analysis of 165 Childs’ A patients. J Surg Oncol. 1997;66:122–6. doi: 10.1002/(sici)1096-9098(199710)66:2<122::aid-jso9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, Lau WY, Li JQ. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245:36–43. doi: 10.1097/01.sla.0000231758.07868.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KT, Wang SN, Su RW, Chen HY, Shi HY, Ker CG, Chiu HC. Is wider surgical margin justified for better clinical outcomes in patients with resectable hepatocellular carcinoma? J Formos Med Assoc. 2012;111:160–170. doi: 10.1016/j.jfma.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Zhou YM, Yang JM, Li B, Yin ZF, Xu F, Wang B, Xu W, Kan T. Risk factors for early recurrence of small hepatocellular carcinoma after curative resection. Hepatobiliary Pancreat Dis Int. 2010;9:33–37. [PubMed] [Google Scholar]
- 11.Regimbeau JM, Abdalla EK, Vauthey JN, Lauwers GY, Durand F, Nagorney DM, Ikai I, Yamaoka Y, Belghiti J. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: results of a multicenter study. J Surg Oncol. 2004;85:36–41. doi: 10.1002/jso.10284. [DOI] [PubMed] [Google Scholar]
- 12.Nara S, Shimada K, Sakamoto Y, Esaki M, Kishi Y, Kosuge T, Ojima H. Prognostic impact of marginal resection for patients with solitary hepatocellular carcinoma: Evidence from 570 hepatectomies. Surgery. 2012;151:526–36. doi: 10.1016/j.surg.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Martone T, Gillio-Tos A, De Marco L, Fiano V, Maule M, Cavalot A, Garzaro M, Merletti F, Cortesina G. Association between hyperrnethylated tumor and paired surgical margins in head and neck squamous cell carcinomas. Clin Cancer Res. 2007;13:5089–5094. doi: 10.1158/1078-0432.CCR-07-0119. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic Impact of Anatomic Resection For Hepatocellular Carcinoma. Ann Surg. 2005;242:252. doi: 10.1097/01.sla.0000171307.37401.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho YB, Lee KU, Lee HW. Anatomic versus nonanatomic recection for small single hepatocellular. Hepatogastronerology. 2007;54:1766. [PubMed] [Google Scholar]
- 16.Sasaki K, Matsuda M, Ohkura Y, Kawamura Y, Hashimoto M, Ikeda K, Kumada H, Watanabe G. Minimum resection margin should be based on tumor size in hepatectomy for hepatocellular carcinoma in hepatoviral infection patients. Hepatol Res. 2013;43:1295–30. doi: 10.1111/hepr.12079. [DOI] [PubMed] [Google Scholar]
- 17.Nanashima A, Sumida Y, Abo T, Nagasaki T, Tobinaga S, Fukuoka H, Takeshita H, Hidaka S, Tanaka K, Sawai T, Yasutake T, Nagayasu T. Comparison of survival between anatomic and non-anatomic liver resection in patients with hepatocellular carcinoma: significance of surgical margin in non-anatomic resection. Acta Chir Belg. 2008;108:532–7. [PubMed] [Google Scholar]
- 18.Tralhão JG, Kayal S, Dagher I, Sanhueza M, Vons C, Franco D. Resection of hepatocellular carcinoma: the effect of surgical margin and blood transfusion on long-term survival. Analysis of 209 consecutive patients. Hepatogastroenterology. 2007;54:1200–6. [PubMed] [Google Scholar]


