Abstract
Background
Postoperative delirium is common in patients recovering from cardiac surgery. Tight glucose control has been shown to reduce mortality and morbidity. We therefore sought to determine the effect of tight intraoperative glucose control using a hyper-insulinemic normoglycemic clamp approach on postoperative delirium in patients undergoing cardiac surgery.
Methods
We enrolled 198 adult patients having cardiac surgery in this randomized, double-blinded single-center trial. Patients were randomly assigned to either tight intraoperative glucose control with a hyperinsulinemic-normoglycemic clamp (target blood glucose: 80–110 mg/dL) or standard therapy (conventional insulin administration with blood glucose target < 150 mg/dL). Delirium was assessed using a comprehensive delirium battery. We considered patients to have experienced postoperative delirium when Confusion Assessment Method testing was positive at any assessment. A positive Confusion Assessment Method test was defined by the presence of features 1 (acute onset and fluctuating course) and 2 (inattention), and either 3 (disorganized thinking) or 4 (altered consciousness).
Results
Patients randomized to tight glucose control were more likely to be diagnosed as being delirious than those assigned to routine glucose control (26/93 vs. 15/105; Relative Risk (RR), 95% CI: 1.89, 1.06–3.37; P = 0.03), after adjusting for preoperative usage of calcium channel blocker and American Society of Anesthesiologist (ASA) physical status. Delirium severity, among patients with delirium, was comparable with each glucose management strategy.
Conclusions
Intraoperative hyperinsulinemic-normoglycemia augments the risk of delirium after cardiac surgery, but not its severity.
INTRODUCTION
Postoperative delirium is common in patients recovering from cardiac surgery. It is a disturbing complication that is associated with prolonged duration of hospitalization, increased costs, mortality, and long-term neurocognitive impairment.1–8 The causes of postoperative delirium remain unclear, but are thought to include pain, sleep deprivation, anesthetic and narcotic effects, concomitant medications, surgical stress, and the inflammatory response to surgery.2,9–19
Hyperglycemia is both a consequence and a cause of perioperative inflammation. The surgical stress response and concomitant inflammation augment perioperative blood glucose concentrations.20 Hyperglycemia is thus particularly common in patients having cardiac surgery. “Stress hyperglycemia” is exacerbated by factors specific to cardiopulmonary bypass including heparin administration, hypothermia, and administration of glucose-containing cardioplegic solutions.21–23 Other factors that worsen hyperglycemia during cardiac surgery include increased renal absorption of glucose, increased substrate availability in the form of lactate, and decreased exogenous insulin activity.24 Hyperglycemia itself also induces inflammation and expression of proinflammatory cytokines.25 Consequently, tight perioperative glucose control with intensive insulin therapy decreases perioperative inflammation.26,27 Insulin per se also decreases concentrations of proinflammatory cytokines, adhesion molecules, chemokines, acute phase proteins, and C-reactive protein.26
It thus seems likely that tight glucose control and reduced inflammation will also diminish the risk of postoperative delirium. Consistent with this theory, a retrospective analysis suggests an association between hyperglycemia and delirium in patients having abdominal surgery.28 To the extent that tight glucose control reduces the risk of delirium, using a hyper-insulinemic normoglycemic clamp may be especially helpful since insulin per se is anti-inflammatory. We therefore tested the primary hypothesis that tight glucose control using a hyper-insulinemic normoglycemic clamp approach decreases the incidence of postoperative delirium as assessed by the Confusion Assessment Method (CAM) in patients recovering from cardiac surgery. Secondarily, we tested the hypothesis that normoglycemic clamp reduces the severity of delirium as assessed by the Memorial Delirium Assessment Score (MDAS).
MATERIAL AND METHODS
With Cleveland Clinic Institutional Review Board approval, 203 consenting adults having cardiac surgery with cardio-pulmonary bypass between March 2008 and January 2010 were randomly assigned to tight intraoperative glucose control with a hyper-insulinemic normoglycemic clamp or standard therapy. Randomization was based on a computer-generated sequence in random-sized permuted blocks of 4–16 patients. Assignments were stratified by procedure (coronary artery bypass, valve, or both) and history of diabetes (none vs. any of the following: diet-controlled, type I or type II diabetes)). Allocation was concealed until just before surgery by a web-based system. These patients were part of a large multi-center study evaluating the effect of tight glucose control on postoperative morbidity and mortality in cardiac surgical patients (ClinicalTrials.gov identifier: NCT00524472).
Patients randomized to tight intraoperative glucose control received a hyperinsulinemic-normoglycemic clamp. A constant infusion of insulin (5 mU/kg/min) was given with a concomitant variable infusion of dextrose 20% titrated to maintain blood glucose concentrations 80–110 mg/dL. The standard therapy group received standard insulin infusion as per the Cleveland Clinic insulin treatment protocol, which targets blood glucose concentrations <150 mg/dL.
We recorded blood glucose concentrations preoperatively, prior to anesthetic induction, beginning of cardiopulmonary bypass, end of cardiopulmonary bypass, after weaning from cardiopulmonary bypass, arrival in the Intensive Care Unit (ICU) and 12, 24, and 48 hours after arrival in ICU. In addition, frequent blood glucose concentrations were evaluated during the intraoperative period (every 5–10 minutes in the treatment group; every 30–60 minutes in the control group) and every 30–60 min during the first two hours of ICU stay.
The designated treatment with either hyperinsulinemic-normoglycemic clamp or standard therapy began after induction of anesthesia and continued until sternal closure. Thereafter, the hyperinsulinemic normoglycemic clamp infusion was reduced to 1 mU/kg/min. Upon arrival in the ICU, both groups received standard glucose control therapy as per our ICU protocol which aimed for blood glucose concentrations between 80 and 150 mg/dL on day of surgery, and between 80 and 120 mg/dL on subsequent postoperative days.
We attempted delirium assessments the evening of surgery and then twice daily (morning and evening) for five postoperative days while patients remained hospitalized (a maximum of 11 assessments per patient). Reasons for non-assessments included patient refusal, and when patients were intubated and sedated or ventilated or having a procedure. Delirium was assessed using a comprehensive delirium battery consisting of Richmond Agitation and Sedation Scale (RASS), the Confusion Assessment Method (CAM), Memorial Delirium Assessment Scale and Digit Span. In previous studies utilizing this delirium battery trained research personnel was nearly as accurate in identifying delirium as psychiatrists using DSM-criteria.29
Delirium was assessed by trained investigators to who were blinded to intraoperative glucose management. Each investigator underwent a series of mock assessments and required certification before performing assessments on study patients. Monthly meetings and feedback sessions with case discussions ensured quality and consistency of the assessments. We considered patients to have experienced postoperative delirium when CAM testing was positive at any assessment.30 CAM evaluates four features: 1) acute onset and fluctuating course, 2) inattention, 3) disorganized thinking, and, 4) altered consciousness. A positive CAM test was defined by the presence of features 1 and 2, and either 3 or 4.
Sedation level was evaluated in every patient at the beginning of an assessment using the Richmond Agitation and Sedation Scale (RASS).31 Patients with a RASS score of −4 or −5 were excluded from further evaluations. The digit span test evaluates working memory and consists of repeating back a series of numbers in the correct order.32,33 Patients were further evaluated with the Memorial Delirium Assessment Scale (MDAS), which measures 10 items related to the severity of delirium. Disturbances in arousal, level of consciousness, memory, attention, orientation and disturbances of thinking are rated on a four point scale (0–3).34,35
Statistical Analysis
The two randomized groups were compared for balance on demographics and baseline characteristics using standard summary statistics and the absolute standardized difference (ASD), defined as the absolute difference in means or proportions divided by the pooled standard deviation. Any variable with an ASD greater than 0.2 was considered to be imbalanced.
The hyper-insulinemic normoglycemic clamp group and standard therapy group were compared on postoperative delirium using Chi-square test and generalized regression model with log link with adjustment for any imbalanced covariables. The RR was estimated along with the confidence interval. We also conducted a sensitivity analysis to evaluate the treatment effect on postoperative delirium, where we assumed all missing CAM assessments (due to refusal) were assigned to be positive delirium.
Secondly, we evaluated the difference between the two randomized groups on the average digit span score and average and maximum Memorial Delirium Assessment Scores (a total of 3 analyses), using the independent student t test and the Wilcoxon rank-sum test as appropriate. The significance criterion for the three secondary analyses was P < 0.017 (i.e., 0.05/3, Bonferroni).
This is a sub study of a prospective, randomized, double-blinded single-center trial. All the available data were used to examine the focused aims and hypotheses. SAS software version 9.3 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
RESULTS
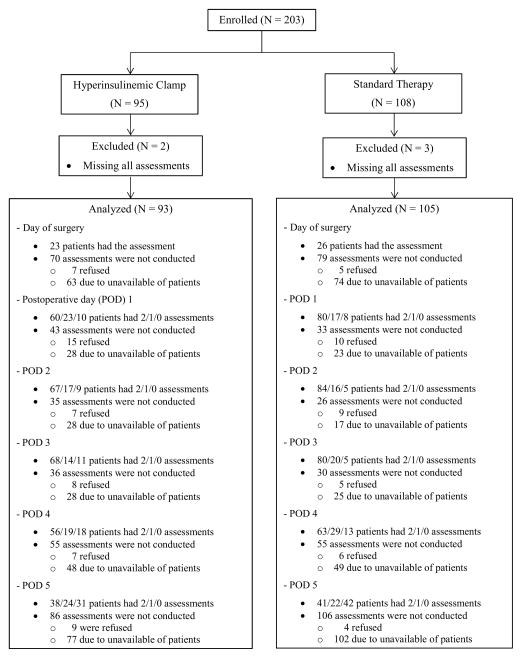
Among 203 participating patients, 5 patients did not have any postoperative CAM assessments and were excluded; we thus included a total of 198 patients each with at least one postoperative CAM assessment (Fig. 1). On average, patients in each group had 8 ± 2 CAM assessments of the 11 possible measurements. Overall, 19% of the patients had assessments twice daily for 5 days, 28%, 26%, 14%, and 10% patients had assessments twice daily for 4 to 1 day, respectively. Among patients who were available for assessment (in hospital, awake, and unintubated), 94% of the evaluations were completed.
Figure 1.
Trial diagram. Patients missed Confusion Assessment Method (CAM) assessments for the following reasons were considered as missing due to unavailability, including out of the unit, medical reasons (such as, severe pain, shortness of breath, and etc.), asleep, ventilated, intubated, and discharge. POD = postoperative day.
Demographics and baseline variables were well balanced between the hyperinsulinemic normoglycemic clamp and the standard therapy groups (ASD < 0.2, except for preoperative usage of calcium channel blocker and American Society of Anesthesiologist (ASA) physical status, Table 1). Thus, we adjusted for these two variables when we compared the two randomized groups on the postoperative delirium. Intraoperative transfusions, fluids, opioids, medications (except insulin), total clamp and bypass times, and duration of surgery were also similar in each group (Table 2).
Table 1.
Summary of demographics and baselines characteristics (N = 198)
| Hyperinsulinemic Clamp N = 93 |
Standard Therapy N = 105 |
ASD * | |
|---|---|---|---|
| Variable | |||
| Gender, Male - % | 71 | 73 | 0.05 |
| Age - yrs | 65 ± 15 | 66 ± 12 | 0.01 |
| Body mass index - kg/m2 | 28 [25, 31] | 28 [25, 33] | 0.06 |
| Medical History - % | |||
| Chronic obstructive pulmonary disease | 19 | 12 | 0.19 |
| Pulmonary hypertension | 26 | 33 | 0.17 |
| Stroke | 8 | 6 | 0.07 |
| Hypertension | 72 | 71 | 0.01 |
| Diabetes status | 0.10 | ||
| No diabetes | 67 | 68 | |
| Diet controlled | 4 | 3 | |
| Oral medication controlled | 20 | 19 | |
| Insulin controlled | 9 | 10 | |
| Congestive heart failure | 28 | 30 | 0.06 |
| Myocardial infraction | 22 | 20 | 0.04 |
| Dialysis | 1 | 0 | 0.15 |
| Cardiac surgery | 32 | 26 | 0.14 |
| Peripheral vascular disease | 12 | 8 | 0.14 |
| Smoking | 56 | 52 | 0.07 |
| Atrial fibrillation | 25 | 19 | 0.14 |
| Ventricular tachycardia | 1 | 1 | 0.01 |
| Preoperative medication - % | |||
| ACE inhibitor† | 30 | 29 | 0.03 |
| Antiarrhythmics | 10 | 16 | 0.19 |
| Beta Blockers | 42 | 46 | 0.08 |
| Calcium Blockers | 12 | 24 | 0.32 |
| Cox-2 Inhibitor | 3 | 1 | 0.16 |
| Statins | 47 | 55 | 0.16 |
| Steroid | 2 | 6 | 0.18 |
| Anti-Diabetic Drugs | |||
| Sulfonylureas and Meglitinides | 11 | 9 | 0.07 |
| Biguanides (metformin) | 13 | 10 | 0.11 |
| Thiazolidinediones | 5 | 4 | 0.08 |
| Insulin | 13 | 10 | 0.11 |
| CCF severity score | 5 ± 3 | 4 ± 3 | 0.12 |
| ASA physical status - % | 0.26 | ||
| II | 1 | 0 | |
| III | 20 | 18 | |
| IV | 77 | 82 | |
| V | 2 | 0 | |
| Procedure - % | 0.14 | ||
| CABG (No valve) + other | 24 | 21 | |
| CABG + Valve (and anything else) | 28 | 24 | |
| Valve (No CABG) + other | 48 | 55 | |
ACE = Angiotensin-converting enzyme; ASA = American Society of Anesthesiologists; ASD = Absolute standardized difference; CABG = Coronary Artery Bypass Graft; CCF = Cleveland Clinic Foundation
Statistics are presented as percentage, mean ± SD or median [inter-quartiles].
Absolute standardized difference: absolute difference in means or proportions divided by the pooled standard deviation; 0.2, 05, and 0.8 suggest small, medium, and large difference.
Including angiotensin receptor blockers
Table 2.
Summary of intraoperative characteristics (N = 198).
| Variable | Hyperinsulinemic Clamp (N = 93) | Standard Therapy (N = 105) | ASD * |
|---|---|---|---|
| Total clamp time, minutes | 73 [60, 100] | 67 [55, 85] | 0.23 |
| Total bypass time, minutes | 94 [80, 122] | 89 [69, 112] | 0.24 |
| Duration of surgery, minutes | 376 [319, 449] | 342 [303, 412] | 0.30 |
| Etomidate, mg | 16 [0, 20] | 18 [10, 20] | 0.29 |
| Fentanyl, mg | 1.0 [0.8, 1.0] | 1.0 [0.8, 1.0] | 0.25 |
| Midazolam, mg | 0 [0, 0] | 0 [0, 0] | 0 |
| Phenylephrine, mg | 0.3 [0.1, 0.8] | 0.3 [0.1, 0.7] | 0.09 |
| Nitroglycerin, mg | 1.3 [0.4, 3.8] | 2.3 [0.7, 5.9] | 0.30 |
| Epinephrine, mg | 0 [0, 0.3] | 0 [0, 0.1] | 0.20 |
| Norepinephrine, mg | 0 [0, 0.1] | 0 [0, 0] | 0.13 |
| Phenylephrine, mg | 0.3 [0.1, 0.8] | 0.3 [0.1, 0.7] | 0.10 |
| Insulin bolus, units | 24 [8, 64] | 6 [4, 11] | 1.04 |
| Insulin infusion, units | 94 [68, 116] | 11 [7, 17] | 3.11 |
| Crystalloid, L | 3.0 ± 1.1 | 3.0 ± 1.0 | 0.02 |
| Colloid, L | 0 [0, 0.5] | 0.3 [0, 0.5] | 0.09 |
| Red blood cell, units | 0 [0, 1] | 0 [0, 0] | 0.28 |
| Platelets, units | 0 [0, 0] | 0 [0, 0] | 0.17 |
| Fresh frozen plasma, units | 0 [0, 0] | 0 [0, 0] | 0.18 |
ASD = Absolute standardized difference
Absolute standardized difference: absolute difference in means or proportions divided by the pooled standard deviation; 0.2, 05, and 0.8 suggest small, medium, and large difference.
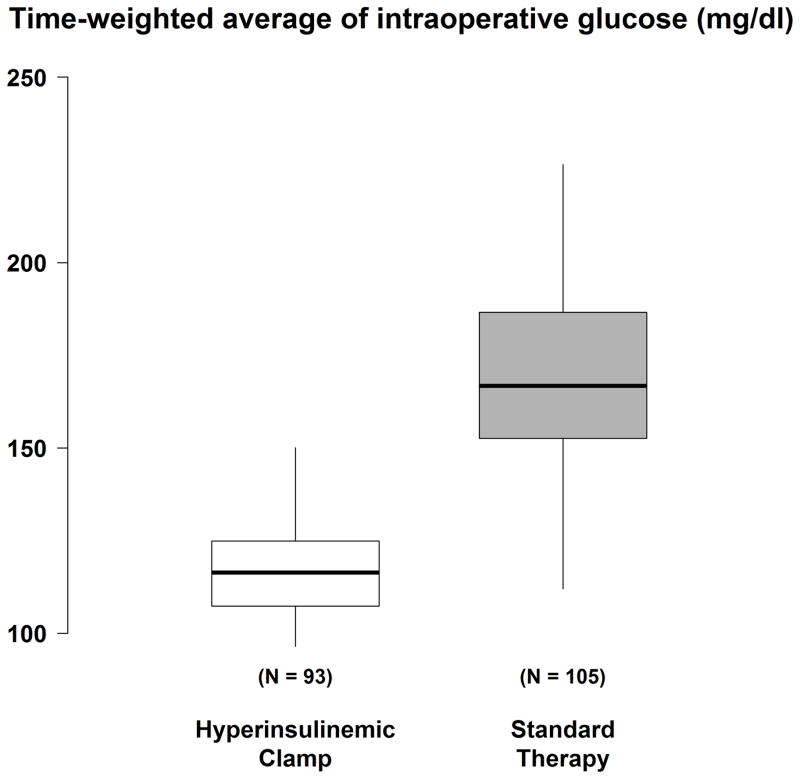
The median intraoperative insulin dose was 126 [interquartile range: 94, 184] units for patients in the hyperinsulinemic normoglycemic clamp group and 18 [11, 27] units for patients in the standard therapy group. There were three patients in the standard therapy group who did not require insulin. Time-weighted average intraoperative glucose was 119 ± 18 mg/dl in the hyperinsulinemic-normoglycemic clamp group and 171 ± 29 mg/dl in the standard therapy group (Fig. 2). No glucose assessments showed hypoglycemia (i.e., < 40 mg/dl).
Figure 2.
Time-weighted average (TWA) glucose concentration in patients assigned the hyperinsulinemic-normoglycemic clamp and to routine glucose management. Results presented as boxplots: the first quartile, median, and third quartile comprise the boxes; whiskers extend to the most extreme observations within 1.5 times the interquartile range of the first and third quartiles, respectively.
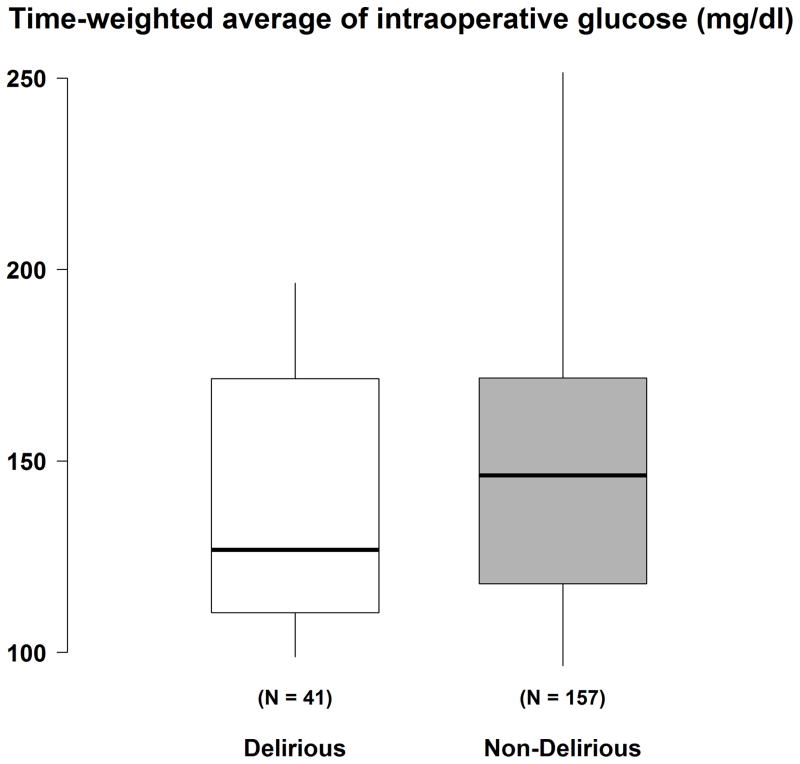
The average RASS was comparable in the clamp (-0.22 ± 0.4) and routine (-0.22 ± 0.5) glucose management groups (P > 0.99). Twenty-one percent (41 / 198) patients screened positive for delirium on at least one assessment. Patients randomized to tight glucose control thus had a higher probability of being diagnosed as delirious than those assigned to standard glucose control. The incidence of delirium was 28% (26 / 93) in hyperinsulinemic-normoglycemic clamp group and 14% (15 / 105) in the standard therapy group (unadjusted RR was 1.96 (95% CI: 1.11–3.46), P = 0.02). After adjusting for preoperative usage of calcium channel blockers and ASA physical status (ASD > 0.20), the estimated RR was 1.89 (95% CI: 1.06–3.37), P = 0.03. Time-weighted average intraoperative glucose concentrations were 138 ± 31 mg/dl in the delirious patients and 149 ± 37 mg/dl in those without delirium (P = 0.14, Fig. 3). For a 10-mg/dl decrease in minimum of intraoperative glucose, the estimated odds ratio of experiencing delirium was 1.15 (0.99, 1.34) (P = 0.06, post-hoc analysis). For information purpose, baseline characteristics were summarized for delirious patients and those without delirium separately (Appendix 1).
Figure 3.
Time-weighted average (TWA) glucose concentration in delirious (both hyperinsulinemic-normoglycemic clamp and routine glucose management) and non-delirious (both hyperinsulinemic-normoglycemic clamp and routine glucose management) patients. Results presented as boxplots: the first quartile, median, and third quartile comprise the boxes; whiskers extend to the most extreme observations within 1.5 times the interquartile range of the first and third quartiles, respectively.
Appendix 1.
Summary of demographics and baselines characteristics by delirium (N = 198)
| Variable | Delirium N = 41 |
No-Delirium N = 157 |
P-value | ASD * |
|---|---|---|---|---|
| Age - yrs | 73.0 ± 9.5 | 63.3 ± 13.6 | <0.001 | 0.83 |
| Gender, Male - % | 66 | 74 | 0.31 | 0.18 |
| Body mass index - kg/m2 | 28.7 [24.7, 31.9] | 27.6 [24.8, 31.4] | 0.86 | 0.03 |
| Medical History - % | ||||
| Chronic obstructive pulmonary disease | 17 | 15 | 0.78 | 0.05 |
| Pulmonary hypertension | 39 | 27 | 0.15 | 0.25 |
| Stroke | 10 | 6 | 0.48 | 0.15 |
| Hypertension | 73 | 71 | 0.82 | 0.04 |
| Diabetes status | 0.08 | 0.49 | ||
| No diabetes | 0 | 4 | ||
| Diet controlled | 32 | 17 | ||
| Oral medication controlled | 12 | 9 | ||
| Insulin controlled | 56 | 70 | ||
| Congestive heart failure | 44 | 25 | 0.02 | 0.39 |
| Myocardial infraction | 27 | 19 | 0.28 | 0.18 |
| Dialysis | 0 | 1 | 0.99 | -0.11 |
| Cardiac surgery | 39 | 26 | 0.10 | 0.28 |
| Peripheral vascular disease | 15 | 8 | 0.24 | 0.20 |
| Smoking | 56 | 54 | 0.77 | 0.05 |
| Atrial fibrillation | 34 | 18 | 0.03 | 0.36 |
| Ventricular tachycardia | 0 | 1 | 0.99 | -0.16 |
| Preoperative medication - % | ||||
| ACE inhibitor | 34 | 28 | 0.44 | 0.13 |
| Antiarrhythmics | 17 | 12 | 0.40 | 0.14 |
| Beta Blockers | 44 | 44 | 0.99 | -0.00 |
| Calcium Blockers | 12 | 20 | 0.26 | -0.21 |
| Cox-2 Inhibitor | 5 | 1 | 0.19 | 0.21 |
| Statins | 59 | 50 | 0.31 | 0.18 |
| Steroid | 10 | 3 | 0.06 | 0.30 |
| Anti-Diabetic Drugs | ||||
| Sulfonylureas and Meglitinides | 10 | 10 | 0.99 | 0.01 |
| Biguanides (metformin) | 12 | 11 | 0.78 | 0.04 |
| Thiazolidinediones | 2 | 5 | 0.69 | -0.14 |
| Insulin | 15 | 10 | 0.41 | 0.13 |
| CCF severity score | 5.6 ± 3.2 | 4.3 ± 3.1 | 0.03 | 0.40 |
| ASA physical status - % | 0.04 | 0.40 | ||
| II | 0 | 1 | ||
| III | 24 | 17 | ||
| IV | 71 | 82 | ||
| V | 5 | 0 | ||
| Procedure - % | 0.13 | 0.36 | ||
| CABG (No valve) + other | 24 | 22 | ||
| CABG + Valve (and anything else) | 39 | 55 | ||
| Valve (No CABG) + other | 37 | 23 | ||
ACE = Angiotensin-converting enzyme; ASA = American Society of Anesthesiologists; ASD = Absolute standardized difference; CABG = Coronary Artery Bypass Graft; CCF = Cleveland Clinic Foundation
Statistics are presented as percentage, mean ± SD or median [inter-quartiles].
P-values were derived from t-test or Wilcoxon rank sum test for continuous variables and Pearson’s chi-square test or Fisher’s exact test for categorical variables. All tests were two-sided.
Absolute standardized difference: absolute difference in means or proportions divided by the pooled standard deviation; 0.2, 05, and 0.8 suggest small, medium, and large difference.
Five percent of planned CAM assessments could not be done in the hyperinsulinemic-normoglycemic clamp patients because they refused evaluation; 3% of the assessments in the standard therapy group could not be completed for the same reason (Table 3). Refusal may have been non-random as delirium per se often reduces cooperation. We therefore performed a sensitivity analysis in which all missing (due to refusal) CAM assessments were assigned to be positive. Under this assumption, the incidence of delirium in the hyperinsulinemic-normoglycemic clamp group (49%, 46 / 93) was again higher than in the standard therapy groups (32%, 34 / 105). These results were consistent with our original analysis indicating that the hyperinsulinemic-normoglycemic clamp causes delirium (RR, 95% CI: 1.60, 1.13–2.27; P = 0.01), after adjusting for preoperative usage of calcium channel blockers and ASA status.
Table 3.
Summary of CAM assessments (N = 198)
| CAM assessment | Hyperinsulinemic Clamp N = 93 |
Standard Therapy N = 105 |
|---|---|---|
| Number of planned CAM assessments* | 1023 | 1155 |
| Overall CAM assessment summary – N (%†) | ||
| Evaluated cooperatively | 579 (57) | 747 (65) |
| Refused but evaluated | 119 (12) | 79 (7) |
| Refused and not evaluated | 53 (5) | 39 (3) |
| Not evaluated due to reasons ‡ other than refusing | 272 (27) | 290 (25) |
| Within patient CAM assessment summary | ||
| % refused and not evaluated assessments # | 7.5 ± 13.4 | 4.9 ± 10.8 |
| Any refused and not evaluated – N (%) | 35 (38) | 24 (23) |
CAM = Confusion Assessment Method
Statistics are presented as number (percentage) or mean ± SD.
11 assessments planned per patient
Out of total number of planned CAM assessments
Patients who were non-available for the delirium assessment were due to one of the following reasons: intubation, sleep, ventilation, other procedures, or hospital discharge.
Within each patient, number of refused and not evaluated assessments out of the total number of assessments in which patient were available to participate.
Additionally we compared the incidence of delirium in patients who refused one or more delirium assessment (34%, 20 / 59) versus patients who never refused any assessment (including patients missed evaluations due to unavailability) (15%, 21 / 139). Patients refusing a delirium assessment were 2.2 times (95% CI: 1.3, 3.8) more likely to develop delirium at a later point in time (P = 0.003, univariably). We found that the average digit span was lower (worse) in hyperinsulinemic-normoglycemic clamp group (4.2 ± 0.7) than in the standard therapy group (4.5 ± 0.6). The estimated mean difference in the average digit span score was −0.32 (98.3% CI: −0.57, −0.08; P = 0.002), after adjusting for usage of calcium channel blocker.
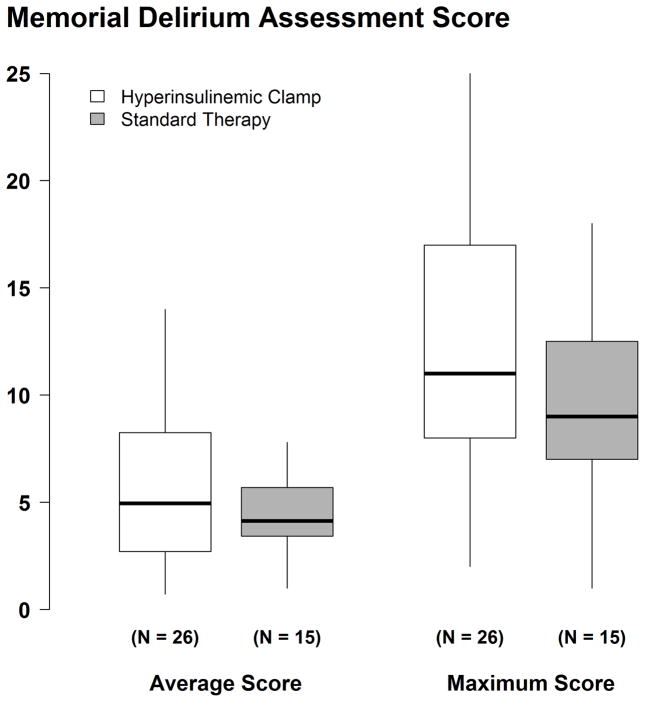
Among the 41 delirious patients, median MDAS scores did not differ significantly in patients assigned to the hyperinsulinemic-normoglycemic clamp (4.9 [2.7, 8.3]) than for patients assigned to standard glucose control [4.1 (3.3, 6.2), P = 0.43]. The estimated median difference in the average MDAS score was 0.6 (98.3% CI: −1.6, 3.4). Likewise, maximum MDAS score was not different in the hyper-insulinemic normoglycemic group [11 (8, 17)] than in the standard therapy group [9 (7, 13)] with an estimated median difference of 2 (98.3% CI: −2, 7), P = 0.29 (Fig. 4).
Figure 4.
Average and maximum Memorial Delirium Assessment Score (MDAS) score in all assessments of 26 delirious patients assigned the hyperinsulinemic-normoglucemic clamp and in 15 delirious patients assigned to routine glucose management. Results presented as boxplots: the first quartile, median, and third quartile comprise the boxes; whiskers extend to the most extreme observations within 1.5 times the interquartile range of the first and third quartiles, respectively.
DISCUSSION
The pathophysiology of delirium is multifactorial and remains poorly understood. However, delirium appears to result from reversible impairment of cerebral oxidative metabolism and multiple neurotransmitter abnormalities. Surgical stress upregulates sympathetic tone and downregulates parasympathetic tone, impairing cholinergic function and thus contributing to delirium. Another theory is that cytokine activation and alteration of growth factors are causes of postoperative delirium.36
Hyperglycemia is both a response to inflammation and is itself inflammatory, whereas insulin is anti-inflammatory.37,38 We thus expected tight glucose control with a hyper-insulinemic strategy to reduce the risk of postoperative delirium. In distinct contrast to our hypothesis, we found that tight intraoperative glucose control using hyperinsulinemic normoglycemic clamp significantly increased the incidence of delirium in patients recovering from cardiac surgery.
While tight intraoperative glucose control using a hyperinsulinemic normoglycemic clamp increased the incidence of delirium, it did not alter its severity. (Digit span was significantly reduced in the clamp patients, the difference was not clinically important.) Maximum MDAS scores were elevated in both glucose management groups, but did not differ significantly between the groups, with the maximum scores in the delirious patients being about 10 points. Typically a score of 13 has been associated with delirium diagnosis. Our maximum MDAS scores are slightly lower, but show a great variability. This variability in combination with low overall average MDAS scores (Fig. 4) could indicate the fluctuating nature of delirium or the effects of medical interventions to improve delirium symptoms.
Observational studies report that hyperglycemia worsens delirium risk.28,39,40 The difficulty with these analyses is that hyperglycemia is a response to inflammation, as well as potentially causing inflammation. Patients with hyperglycemia are thus likely to have baseline inflammatory conditions and have experienced greater surgical stress — both of which likely provoke delirium independent of glycemic status. It is thus difficult to convincingly attribute delirium to hyperglycemia in observational studies because of potential confounding. Randomization eliminates selection bias and confounding, thus providing more reliable results. Our randomized results show just the opposite effect: tight glucose control worsened delirium risk.
Our results are based on controlling glucose with a hyper-insulinemic strategy which we expected to enhance protection since insulin is anti-inflammatory. While it remains possible that worsened delirium was specific to our hyper-insulinemic strategy, it seems unlikely that insulin per se caused delirium. Consistent with this theory, there was no association between insulin concentration and delirium in patients having hip surgery.41 However, we did not measure insulin concentrations in our patients and therefore cannot directly address this issue.
A consequence of tight glucose control is the occasional episode of severe hypoglycemia (i.e., ≤40 mg/dl), although none was observed in our patients. There is thus little reason to believe that severe hypoglycemia contributed to postoperative delirium. In contrast, it is worth considering that hyperglycemia is the normal physiological response to stress — and cardiac surgery is an enormous stress. In fact, blood glucose concentrations in the routine management group averaged about 170 mg/dl which is consistent with the Society of Thoracic Surgeons recommendation to keep glucose <180 mg/dl adult cardiac surgery.42 American Diabetes Association43 and the American Association of Clinical Endocrinologists44 recommendations are similar. It may be that various organs benefit from generous glucose availability during periods of stress, and that the brain is among them.
A difficulty with delirium assessments is that they are facilitated by a degree of patient cooperation. (Cooperation is more important for hypoactive delirium than for hyperactive delirium which is usually readily apparent.) A small fraction of the patients refused one or more assessments. The concern is that refusal may be non-random in that patients who suspect a cognitive problem may avoid confirmatory testing. Consistent with this theory, patients who refused a delirium assessment were twice as likely to subsequently have a positive CAM test. However, several sensitivity analyses to evaluate attrition bias did not materially alter the results which is unsurprising since attrition was small and comparable in each group (3% in the routine management patients and 5% in the clamp patients).
Our study was restricted to patients recovering from cardiac surgery, a particularly stressful procedure and one that is frequently accompanied by delirium.45–50 The extent to which our results generalize to other populations remains to be determined. We included both diabetic and non-diabetic patients, but did not attempt a sub-analysis because only about 30 patients in each group were diabetic. A final and important limitation of our study is that it is relatively small. Thus while the incidence of delirium was significantly reduced in patients assigned to hyperinsulinemic-normoglycemic clamp management, this result is fragile and should be confirmed in a much larger trial.
In summary, our present results suggest that delirium incidence is higher in patients treated with intraoperative insulin therapy administered to achieve tight glucose control. These findings provide basis for re-evaluation of beneficial effects of tight intraoperative control in cardiac surgical patients on outcome with specific aim to assess delirium.
Acknowledgments
Funding: This research was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number HL093065 (Andra E. Duncan) and the Department of Outcomes Research, Cleveland Clinic, Cleveland, Ohio, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure
The authors declare no competing interests.
References
- 1.Balas MC, Happ MB, Yang W, Chelluri L, Richmond T. Outcomes Associated With Delirium in Older Patients in Surgical ICUs. Chest. 2009;135:18–25. doi: 10.1378/chest.08-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–62. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 3.Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13:234–42. doi: 10.1046/j.1525-1497.1998.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162:457–63. doi: 10.1001/archinte.162.4.457. [DOI] [PubMed] [Google Scholar]
- 5.Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, Truman B, Bernard GR, Dittus RS, Ely EW. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–62. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 6.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173–8. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 7.Rudolph JL, Marcantonio ER, Culley DJ, Silverstein JH, Rasmussen LS, Crosby GJ, Inouye SK. Delirium is associated with early postoperative cognitive dysfunction. Anaesthesia. 2008;63:941–7. doi: 10.1111/j.1365-2044.2008.05523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomason JW, Ely EW. Delirium in the intensive care unit is bad: what is the confusion? Crit Care Med. 2004;32:2352–4. doi: 10.1097/01.ccm.0000146140.60441.25. [DOI] [PubMed] [Google Scholar]
- 9.Bowman AM. Sleep satisfaction, perceived pain and acute confusion in elderly clients undergoing orthopaedic procedures. J Adv Nurs. 1997;26:550–64. doi: 10.1046/j.1365-2648.1997.t01-16-00999.x. [DOI] [PubMed] [Google Scholar]
- 10.Duggleby W, Lander J. Cognitive status and postoperative pain: older adults. J Pain Symptom Manage. 1994;9:19–27. doi: 10.1016/0885-3924(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 11.Eikelenboom P, Hoogendijk WJ, Jonker C, van Tilburg W. Immunological mechanisms and the spectrum of psychiatric syndromes in Alzheimer’s disease. J Psychiatr Res. 2002;36:269–80. doi: 10.1016/s0022-3956(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 12.Fong HK, Sands LP, Leung JM. The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review. Anesth Analg. 2006;102:1255–66. doi: 10.1213/01.ane.0000198602.29716.53. [DOI] [PubMed] [Google Scholar]
- 13.Johns MW, Large AA, Masterton JP, Dudley HA. Sleep and delirium after open heart surgery. Br J Surg. 1974;61:377–81. doi: 10.1002/bjs.1800610513. [DOI] [PubMed] [Google Scholar]
- 14.Lynch EP, Lazor MA, Gellis JE, Orav J, Goldman L, Marcantonio ER. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86:781–5. doi: 10.1097/00000539-199804000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Morrison RS, Magaziner J, Gilbert M, Koval KJ, McLaughlin MA, Orosz G, Strauss E, Siu AL. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76–81. doi: 10.1093/gerona/58.1.m76. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen LS, O’Brien JT, Silverstein JH, Johnson TW, Siersma VD, Canet J, Jolles J, Hanning CD, Kuipers HM, Abildstrom H, Papaioannou A, Raeder J, Yli-Hankala A, Sneyd JR, Munoz L, Moller JT. Is peri-operative cortisol secretion related to post-operative cognitive dysfunction? Acta Anaesthesiol Scand. 2005;49:1225–31. doi: 10.1111/j.1399-6576.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 17.Shigeta H, Yasui A, Nimura Y, Machida N, Kageyama M, Miura M, Menjo M, Ikeda K. Postoperative delirium and melatonin levels in elderly patients. Am J Surg. 2001;182:449–54. doi: 10.1016/s0002-9610(01)00761-9. [DOI] [PubMed] [Google Scholar]
- 18.Stratone A, Stratone C, Chiruta R, Topoliceanu F. Normal and pathologic implication of cytokines. Rev Med Chir Soc Med Nat Iasi. 2001;105:657–61. [PubMed] [Google Scholar]
- 19.Walzer TA, Herrmann M. Neuropsychological and psychopathologic changes following cardiac surgical procedures. Fortschr Neurol Psychiatr. 1998;66:68–83. doi: 10.1055/s-2007-995241. [DOI] [PubMed] [Google Scholar]
- 20.Thorell A, Nygren J, Ljungqvist O. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care. 1999;2:69–78. doi: 10.1097/00075197-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Lee KU, Lee HK, Koh CS, Min HK. Artificial induction of intravascular lipolysis by lipid-heparin infusion leads to insulin resistance in man. Diabetologia. 1988;31:285–90. doi: 10.1007/BF00277409. [DOI] [PubMed] [Google Scholar]
- 22.Lehot JJ, Piriz H, Villard J, Cohen R, Guidollet J. Glucose homeostasis. Comparison between hypothermic and normothermic cardiopulmonary bypass. Chest. 1992;102:106–11. doi: 10.1378/chest.102.1.106. [DOI] [PubMed] [Google Scholar]
- 23.Werb MR, Zinman B, Teasdale SJ, Goldman BS, Scully HE, Marliss EB. Hormonal and metabolic responses during coronary artery bypass surgery: role of infused glucose. Journal of Clinical Endocrinology & Metabolism. 1989;69:1010–8. doi: 10.1210/jcem-69-5-1010. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho G, Moore A, Qizilbash B, Lachapelle K, Schricker T. Maintenance of normoglycemia during cardiac surgery. Anesth Analg. 2004;99:319–24. doi: 10.1213/01.ANE.0000121769.62638.EB. [DOI] [PubMed] [Google Scholar]
- 25.Lefebvre PJ, Scheen AJ. The postprandial state and risk of cardiovascular disease. Diabet Med. 1998;15 (Suppl 4):S63–8. doi: 10.1002/(sici)1096-9136(1998120)15:4+<s63::aid-dia737>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Hansen TK, Thiel S, Wouters PJ, Christiansen JS, Van den Berghe G. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab. 2003;88:1082–8. doi: 10.1210/jc.2002-021478. [DOI] [PubMed] [Google Scholar]
- 27.Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, Hansen TK, Van den Berghe G. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest. 2005;115:2277–86. doi: 10.1172/JCI25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganai S, Lee KF, Merrill A, Lee MH, Bellantonio S, Brennan M, Lindenauer P. Adverse outcomes of geriatric patients undergoing abdominal surgery who are at high risk for delirium. Arch Surg. 2007;142:1072–8. doi: 10.1001/archsurg.142.11.1072. [DOI] [PubMed] [Google Scholar]
- 29.Simon SE, Bergmann MA, Jones RN, Murphy KM, Orav EJ, Marcantonio ER. Reliability of a structured assessment for nonclinicians to detect delirium among new admissions to postacute care. J Am Med Dir Assoc. 2006;7:412–5. doi: 10.1016/j.jamda.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Wong CL, Holroyd-Leduc J, Simel DL, Straus SE. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304:779–86. doi: 10.1001/jama.2010.1182. [DOI] [PubMed] [Google Scholar]
- 31.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 32.Pompei P, Foreman M, Cassel CK, Alessi C, Cox D. Detecting delirium among hospitalized older patients. Arch Intern Med. 1995;155:301–7. [PubMed] [Google Scholar]
- 33.Wechsler D. WAIS-III: Administration and scoring manual. 3. San Antonio, TX: Harcourt Brace; 1997. [Google Scholar]
- 34.Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. J Pain Symptom Manage. 1997;13:128–37. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 35.Roth-Roemer S, Fann J, Syrjala K. The importance of recognizing and measuring delirium. J Pain Symptom Manage. 1997;13:125–7. doi: 10.1016/s0885-3924(96)00315-6. [DOI] [PubMed] [Google Scholar]
- 36.Adamis D, Treloar A, Martin FC, Gregson N, Hamilton G, Macdonald AJ. APOE and cytokines as biological markers for recovery of prevalent delirium in elderly medical inpatients. Int J Geriatr Psychiatry. 2007;22:688–94. doi: 10.1002/gps.1732. [DOI] [PubMed] [Google Scholar]
- 37.Dandona P, Chaudhuri A, Mohanty P, Ghanim H. Anti-inflammatory effects of insulin. Curr Opin Clin Nutr Metab Care. 2007;10:511–7. doi: 10.1097/MCO.0b013e3281e38774. [DOI] [PubMed] [Google Scholar]
- 38.Hyun E, Ramachandran R, Hollenberg MD, Vergnolle N. Mechanisms behind the anti-inflammatory actions of insulin. Crit Rev Immunol. 2011;31:307–40. doi: 10.1615/critrevimmunol.v31.i4.30. [DOI] [PubMed] [Google Scholar]
- 39.Heymann A, Sander M, Krahne D, Deja M, Weber-Carstens S, MacGuill M, Kastrup M, Wernecke KD, Nachtigall I, Spies CD. Hyperactive delirium and blood glucose control in critically ill patients. J Int Med Res. 2007;35:666–77. doi: 10.1177/147323000703500511. [DOI] [PubMed] [Google Scholar]
- 40.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, Williams BA, Schrader LM, Rizza RA, McMahon MM. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. 2005;80:862–6. doi: 10.4065/80.7.862. [DOI] [PubMed] [Google Scholar]
- 41.Bisschop PH, de Rooij SE, Zwinderman AH, van Oosten HE, van Munster BC. Cortisol, insulin, and glucose and the risk of delirium in older adults with hip fracture. J Am Geriatr Soc. 2011;59:1692–6. doi: 10.1111/j.1532-5415.2011.03575.x. [DOI] [PubMed] [Google Scholar]
- 42.Lazar HL, McDonnell M, Chipkin SR, Furnary AP, Engelman RM, Sadhu AR, Bridges CR, Haan CK, Svedjeholm R, Taegtmeyer H, Shemin RJ Society of Thoracic Surgeons Blood Glucose Guideline Task F. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87:663–9. doi: 10.1016/j.athoracsur.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 43.American Diabetes A. Standards of medical care in diabetes--2008. Diabetes Care. 2008;31 (Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 44.Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, Hellman R, Jellinger PS, Jovanovic LG, Levy P, Mechanick JI, Zangeneh F Force ADMCPGT. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13 (Suppl 1):1–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 45.Blachy PH, Starr A. Post-Cardiotomy Delirum. Am J Psychiatry. 1964;121:371–5. doi: 10.1176/ajp.121.4.371. [DOI] [PubMed] [Google Scholar]
- 46.Crosby G, Culley DJ. Anesthesia, the aging brain, and the surgical patient. Can J Anaesth. 2003;50:R1–R5. [Google Scholar]
- 47.Egerton N, Kay JH. Psychological Disturbances Associated with Open Heart Surgery. Br J Psychiatry. 1964;110:433–9. doi: 10.1192/bjp.110.466.433. [DOI] [PubMed] [Google Scholar]
- 48.Koster S, Oosterveld FG, Hensens AG, Wijma A, van der Palen J. Delirium after cardiac surgery and predictive validity of a risk checklist. Ann Thorac Surg. 2008;86:1883–7. doi: 10.1016/j.athoracsur.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 49.Rudolph JL, Jones RN, Levkoff SE, Rockett C, Inouye SK, Sellke FW, Khuri SF, Lipsitz LA, Ramlawi B, Levitsky S, Marcantonio ER. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–36. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan MC, Felde A, Kuskowski M, Ward H, Kelly RF, Adabag AS, Dysken M. Incidence and predictors of post-cardiotomy delirium. Am J Geriatr Psychiatry. 2008;16:575–83. doi: 10.1097/JGP.0b013e318172b418. [DOI] [PubMed] [Google Scholar]