Abstract
Defective glucagon secretion during hypoglycemia after islet transplantation has been reported in animals and humans with type 1 diabetes. To ascertain whether this is true of islets from non-diabetic humans, subjects with autoislet transplantation in the intrahepatic site only (TP/IAT-H) or in intrahepatic plus non-hepatic (TP/IAT-H+NH) sites were studied. Glucagon responses were examined during stepped hypoglycemic clamps. Glucagon and symptom responses during hypoglycemia were virtually absent in subjects who received islets in the hepatic site only (glucagon increment over baseline = 1 +/− 6, pg/ml, mean +/− SE, n = 9, p = ns; symptom score = 1 +/− 1, p=ns). When islets were transplanted in both intrahepatic + non-hepatic sites, glucagon and symptom responses were not significantly different than Control Subjects (TP/IAT-H+NH: glucagon increment = 54 +/− 14, n = 5; symptom score = 7 +/− 3; Control glucagon increment = 67 +/− 15, n = 5; symptom score = 8 +/− 1). In contrast, glucagon responses to intravenous arginine were present in TP/IAT-H recipients (TP/IAT: glucagon response = 37 +/− 8, n=7).). Transplantation of a portion of the islets into a non-hepatic site should be seriously considered in TP/IAT to avoid post-transplant abnormalities in glucagon and symptom responses to hypoglycemia.
Keywords: Autoislets, defective glucagon secretion
Increasing consideration is being given to TP/IAT as a treatment for chronic, painful pancreatitis(1–3). This disease is characterized by frequent bouts of severe abdominal pain, loose stools, malabsorption, weight loss, and poor quality of life. Narcotic usage and frequent hospitalization for pain management and interventional gastro-intestinal procedures are common. Courses lasting as long as a decade are not unusual and are often punctuated by pancreatic duct stenting and partial or total pancreatectomy. Diabetes mellitus frequently arises as scarring of the acinar portions of the pancreas involves pancreatic islets. Partial pancreatic resection accelerates onset of diabetes, which is inevitable after total pancreatectomy without islet transplant.
In an earlier study of TP/IAT subjects we made the incidental observation of absent glucagon responses during insulin tolerance tests (4), a result that was also observed in type 1 diabetic recipients of intrahepatic alloislets (5). This loss of glucagon responsiveness is important because hypoglycemia in non-diabetic humans is physiologically counter-regulated by glucagon secretion from pancreatic islet α-cells that release glucagon into the hepatic portal vein to stimulate hepatic glycogenolysis and thereby correct low systemically circulating blood glucose levels (6, 7). This sequence of events is dependent on cross-talk between β-cells and α-cells. During normoglycemia and hyperglycemia, insulin secreted from β-cells into the peri-islet circulation tonically suppresses glucagon secretion from downstream alpha cells. When systemic arterial glucose levels fall below normal, β-cells cease releasing insulin, which signals α-cells to secrete glucagon. When β-cells are severely dysfunctional or absent, the islet fails to provide this “switch-off” signal and, consequently, there is little or no glucagon response to hypoglycemia.
We now report studies designed to ascertain whether inclusion of a non-hepatic site in the TP/IAT procedure might obviate the loss of alpha cell function during hypoglycemia that is present when the hepatic site only is used.
STUDY DESIGN AND METHODS
Participants
14 patients with chronic, unrelentingly painful pancreatitis underwent pancreatectomy and intrahepatic islet autotransplantation at the University of Minnesota (n=13) and Massachusetts General Hospital (n=1). Five of these patients also had islets placed in non-hepatic sites. Subjects with a recent history of being able to maintain normal or nearly normal levels of fasting blood glucose (<126 mg/dl) and HbA1c (<6.5%) using no insulin (n=12) or only minimal doses of long-acting insulin (n=2) were recruited for the study. Subjects were 6.0 +/− 1.8 (range = 1–21) years post-TP/IAT at the time of study (Table 1). The recipients came from many different sites in the United States and traveled to Seattle or Minneapolis for metabolic studies. They were lodged in a nearby hotel, fasted from 10:00 pm, and came to study units at the Pacific Northwest Diabetes Research Institute or the University of Minnesota at 8:00 am the next morning for review of consent information provided during earlier telephone conversations. Consent forms witnessed by medical personnel were then signed. Study protocols were approved by the Western Institutional Review Board (IRB) and the University of Minnesota IRB. The participants were placed at bed rest and indwelling percutaneous cannulas were placed in both antecubital veins for infusions and blood draws through 3-way stopcocks attached to infusions of ½ normal saline to maintain patency of the cannulas. 10 subjects were studied in Seattle and 4 were studied in Minneapolis. Seven healthy control subjects were recruited from and studied in Seattle. All hormone assays were performed in Seattle.
Table 1.
Clinical profile of 14 chronic pancreatitis islet recipients who had undergone pancreatectomy and intrahepatic islet autotransplantation. Data shown as mean +/− SE.
| Gender, female | 84% |
| Etiology | 6 Idiopathic |
| 4 Sphincture of Oddi Disease | |
| 3 Cystic Fibrosis | |
| 1 Pancreas Divisum | |
| 1 Alcohol | |
| Yrs. of abdominal pain pre-TP/IAT | 4.8 +/− 1.2 yr |
| Yrs. of narcotic use pre-TP/IAT | 1.6 +/− 0.4 yr |
| Weight loss pre-TP/IAT | 20 +/− 6 lb |
| Number of stent procedures pre-TP/IAT | 1.4 +/− 0.3 |
| Yrs elapsed between TP/IAT and the current metabolic studies | 6.0 +/− 1.8 yr |
| Age at time of the current studies | 34 +/− 3 yr |
| BMI at time of the current studies | 26.2 +/− 1.3 Kg/m2 |
| Fasting plasma glucose at time of the current studies | 99 +/− 5 mg/dl |
| Blood HbA1c level at time of the current studies | 5.7 +/− 0.2 (5.1–6.5) % |
| Normal Controls (n = 6) | |
| Gender female: | 67% |
| Age: | 28 +/− 3 yr |
| BMI: | 28 +/− 1 Kg/m2 |
| FPG: | 100 +/− 3 mg/dl |
| HbA1c: | 5.1 +/− 0.2 % |
Surgical Procedure
The procedure of total pancreatectomy and islet autotransplant has been described in detail elsewhere (3). Briefly, in our recipients, total pancreatectomy, splenectomy, and partial duodenectomy were performed with small bowel re-anastomosis by duodenoduodenostomy or Roux-en-Y procedure (Table 2). The resected pancreas was distended by intraductal infusion of a collagenase solution and mechanically digested using the semi-automated method of Ricordi to release pancreatic islet tissue (Table 3). Pancreatic islets were infused into the portal venous circulation with monitoring of portal pressure. When elevated portal pressure prevented safe infusion of all islets, the remaining islets were dispersed in the intraperitoneal cavity (n=3) or bowel/stomach subserosal space (n=2).
Table 2.
Surgical procedures for TP/IAT in hepatic (n=9) and hepatic + 2nd site (n=5) recipients.
| Hepatic | Hepatic + 2nd site | ||
|---|---|---|---|
| Pylorus preserved | 6 | 4 | |
| Extent of duodenal resection | |||
| None (nearly total pancreatectomy) | 1 | 1 | |
| Partial | 5 | 3 | |
| Complete | 3 | 1 | |
| Reanastomosis | |||
| None (duodenum spared) | 1 | 1 | |
| Duodenoduodenostomy | 1 | 2 | |
| Duodenojejunostomy | 4 | 1 | |
| Gastrojejunostomy | 3 | 1 | |
Some recipients underwent duodenectomy with or without pylorus resection performed as part of other surgeries prior to TPIAT. Table above reflects the patient’s final anatomy subsequent to TPIAT
Table 3.
Islet isolation in hepatic only and hepatic + non-hepatic transplant groups.
| Hepatic (n=9) | Non-hepatic + 2nd site (n=5) | ||
|---|---|---|---|
| Patient age, yrs | 34.9 (±2.5) | 16.8 (±1.4) | |
| Total islet number | 406,289 (±75,808) | 571,480 (±62,235) | |
| Hepatic islet number | 406,289 (±75,808) | 395,678 (±60,948) | |
| Non-hepatic islet number | 175,802 (±23,904) | ||
| Total islet equivalents | 378,242 (±50,893) | 451,299 (±38,605) | |
| Hepatic islet equiv. | 378,242 (±50,893) | 313,695 (±32,997) | |
| Non-hepatic islet equiv. | 137,604 (±18,731) | ||
| Tissue volume (mL) | 14.8 (±4.1) | 25.6 (±3.8) | |
| Purity, % | 48 ±8 | 12 ±4 | |
| Cobe used | |||
| Yes | 4 | 1 | |
| Unknown | 2 | ||
Methods
Glucagon responses to hypoglycemia
Stepped hyperinsulinemic clamps with variable glucose infusion rates were used to achieve 45 minute glucose plateaus at 70, 60, and 50 mg/dl, as previously described (5). Blood samples were drawn at −10, −5, and 0 minutes before the infusions and every 5 minutes thereafter. Insulin and glucose were infused through separate ports into one intravenous line and blood samples were drawn from the other. The insulin infusion was set at a constant rate of 2 mU/min/Kg body weight and the variable rate glucose infusion was performed with a 20% glucose solution. Glucagon responses were calculated by subtracting the mean of the three baseline values observed prior to the clamp from the glucagon levels observed at the 70, 60 and 50 mg/dl glucose nadirs during the clamp. Assays for glucose, C-peptide, and glucagon were performed as previously described (5). Symptom responses to hypoglycemia were scored according to Hoeldtke et al (8).
Glucagon and insulin responses to arginine
Arginine-induced glucagon secretion was determined by giving a 5 gm arginine IV pulse. Blood samples were drawn at −10, −5, and 0 minutes before and at 2, 3, 4, 5, 7 minutes after a pulse of arginine. Acute glucagon (AGRarg) and acute insulin (AIRarg) responses to the arginine pulse were calculated as the mean of the three highest arginine-stimulated glucagon and insulin values observed at 2–5 min. after the arginine pulse minus the mean of the three baseline values. Samples were analyzed for concentrations of glucose, insulin, and glucagon as previously reported (5).
Continuous glucose monitoring
Continuous glucose monitoring (CGM) was performed in a subset of subjects, all insulin independent, for three to seven days using either a Dexcom Seven (DexCom, n=2) or iPro (Medtronic, n=3) sensor. Mean glucose, duration of time with glucose <70 mg/dL, and minimum glucose values were obtained from the CGM.
Statistics
Data were expressed as mean +/− standard error. Intergroup comparisons were made using Student t-tests, Mann-Whitney tests, or ANOVA, where appropriate. Correlation coefficients were calculated by Pearson’s Product Moment.
RESULTS
Demographics
Demographic data are provided on Table 1; surgical and islet isolation details are provided in Tables 2 and 3. Levels of fasting plasma glucose and HbA1c at the time of these studies were 99 +/− 5 mg/dl and 5.7 +/− 0.2 (range = 5.1–6.5) %, respectively. 84% of the TP/IAT subjects gave a positive history of post-transplant hypoglycemia (Table 1). Five non-insulin treated TP/IAT-H patients were studied in their usual daily routine using CGM for a mean of 5.6 +/− 0.7 days. While average glucose was normal (107 +/− 6 mg/dl), TP/IAT subjects exhibited frequent hypoglycemic excursions < 70 mg/dl. All subjects had excursions < 70 mg/dl with a mean of 15.2 +/− 3.5 % of the monitored time spent at a glucose level <70 mg/dl and a mean nadir of 42 +/− 6 mg/dl (Table 4).
Table 4.
History of hypoglycemia after TP/IAT.
| Recipients experiencing hypoglycemic episodes post-transplant | 11/14 |
| Daily, 46% | |
| Weekly, 18% | |
| Monthly, 18% | |
| Rarely, 18% | |
| No. recipients using bedtime long-acting insulin | 2/11 |
| No. recipient associations with hypoglycemia | 3 after meals only |
| 3 after exercise only | |
| 5 after both meals and exercise | |
| Lowest home-recorded capillary blood glucose level | 37 +/− 4 mg/dl |
| Continuous glucose monitoring (n=5), nadir, using no insulin | 42 +/− 6 mg/ml |
| Continuous glucose monitoring (n=5), % time <70 mg/dl | 15.2 +/− 3.5% |
Glucagon responses to hypoglycemia and to intravenous arginine
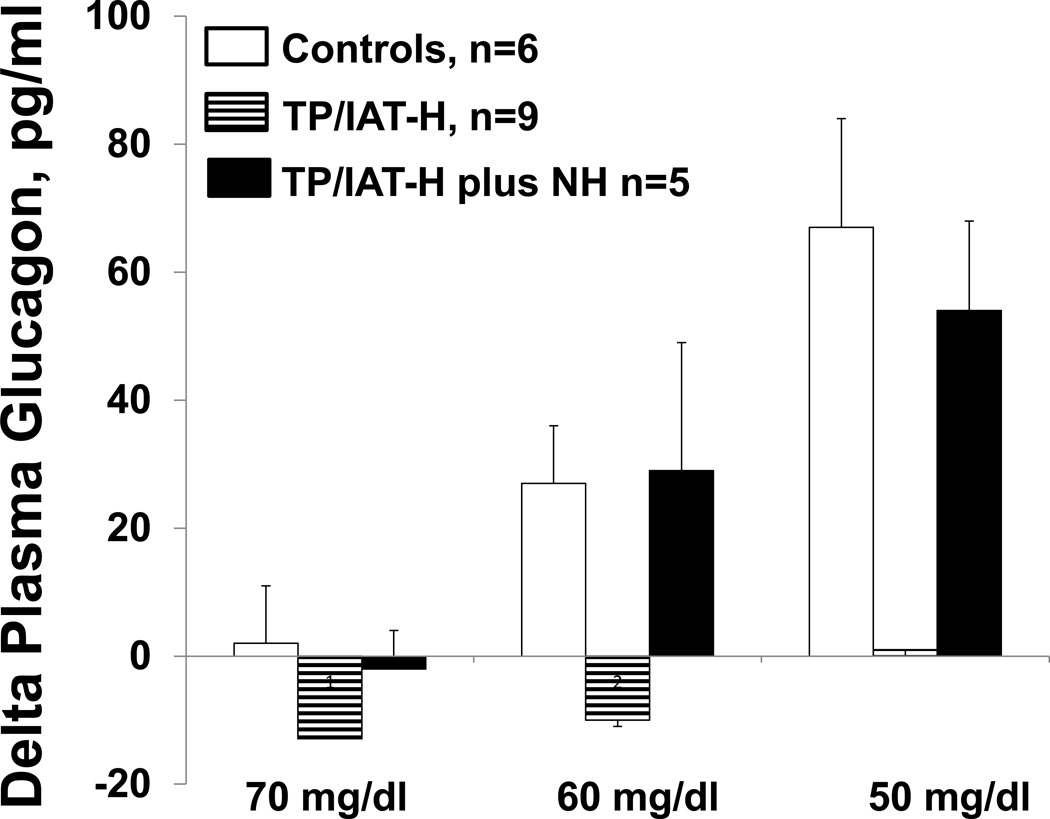
The group of 9 recipients with only intrahepatic transplanted islets (TP/IAT-H) had markedly reduced glucagon responses during the hypoglycemic clamp (Fig 1). Their values at a plasma glucose level of 50 mg/dl were statistically less than controls (TP/IAT-H = 1 +/− 6, n=9; Control = 67 +/− 15, pg/ml; increase from baseline, p<0.01). The subgroup of 5 subjects who had received a combination of intrahepatic and non-hepatic islets (TP/IAT-H + NH) had increases in glucagon levels (54 +/− 14 pg/ml) during hypoglycemia (plasma glucose of 50 mg/dl) that were significantly greater than the TP/IAT-H group (p<0.001) and not significantly different from the control group.
Figure 1. Comparison of glucagon responses at plasma glucose levels of 70, 60, and 50 mg/dl during stepped hypoglycemic, hyperinsulinemic clamps.
Glucagon responses in the control group occurred by the time glucose levels reached 60 mg/dl and increased further by the time levels of 50 mg/dl were reached. In contrast, the TP/IAT-H group failed to have significant glucagon responses. However, glucagon responses were present in a subset of 5 recipients who had islets transplanted both in intrahepatic and non-hepatic (TP/IAT-H + NH) sites. See Results for statistics.
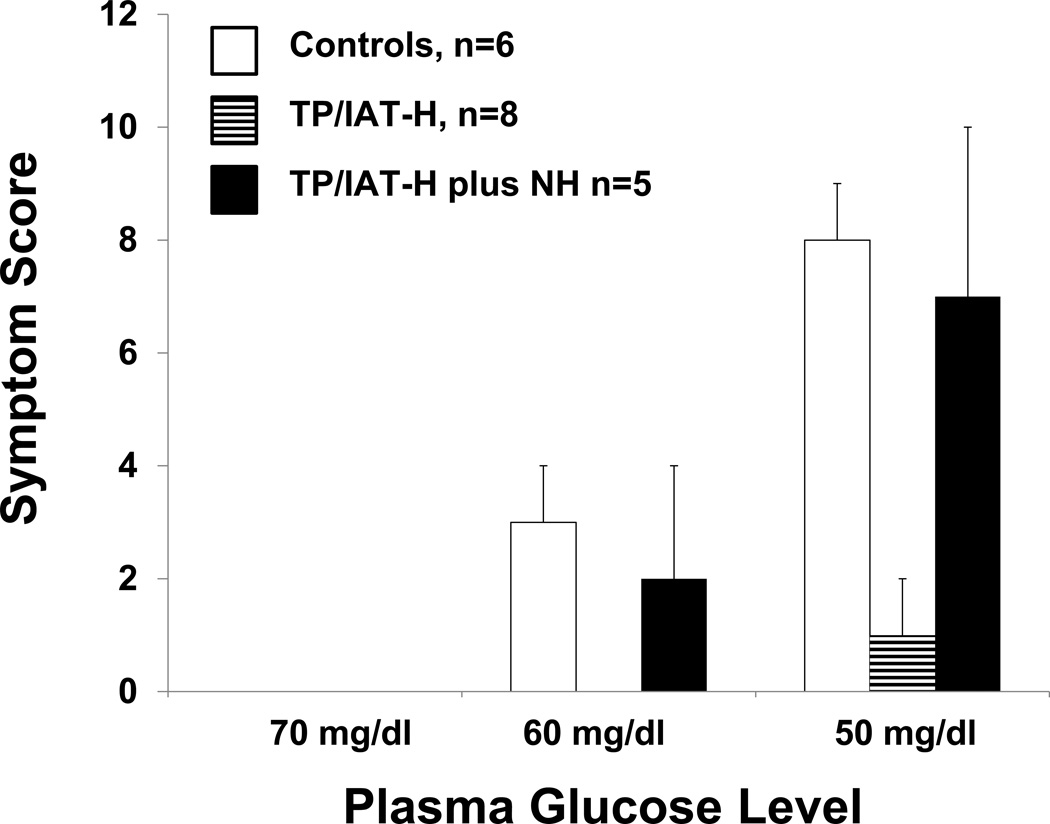
Symptom response scores to hypoglycemia in the TP/IAT-H group at the 50 mg/dl glucose nadir (1 +/−1; Fig 2.) were significantly less than both control group (8 +/− 1; p <0.01) and the TP/IAT-H+NH group (7 +/− 3; p<0.05). In the TP/IAT-H group, symptoms were both autonomic and neuroglycopenic in 2, autonomic only in 3, and absent in 4 subjects. In the TP/IAT-H + NH group, symptoms were both autonomic and neuroglycopenic in all 5 subjects.
Figure 2. Comparison of symptom response scores at plasma glucose levels of 70, 60, and 50 mg/dl during stepped hypoglycemic, hyperinsulinemic clamps.
Hypoglycemic symptoms occurred by the time control subjects reached glucose levels of 60 mg/dl and were more intense at the 50 mg/dl level. The TP/IAT-H group had minimal symptoms at the 50mg/dl glucose level and these scores were significantly less than found in the normal control group. The TP/IAT-H + NH group began having symptoms at plasma glucose levels of 60 that increased in intensity at the 50 mg/dl level where they exceeded the score for the TP/IAT group and were not significantly different than the normal control group. See Results for statistics.
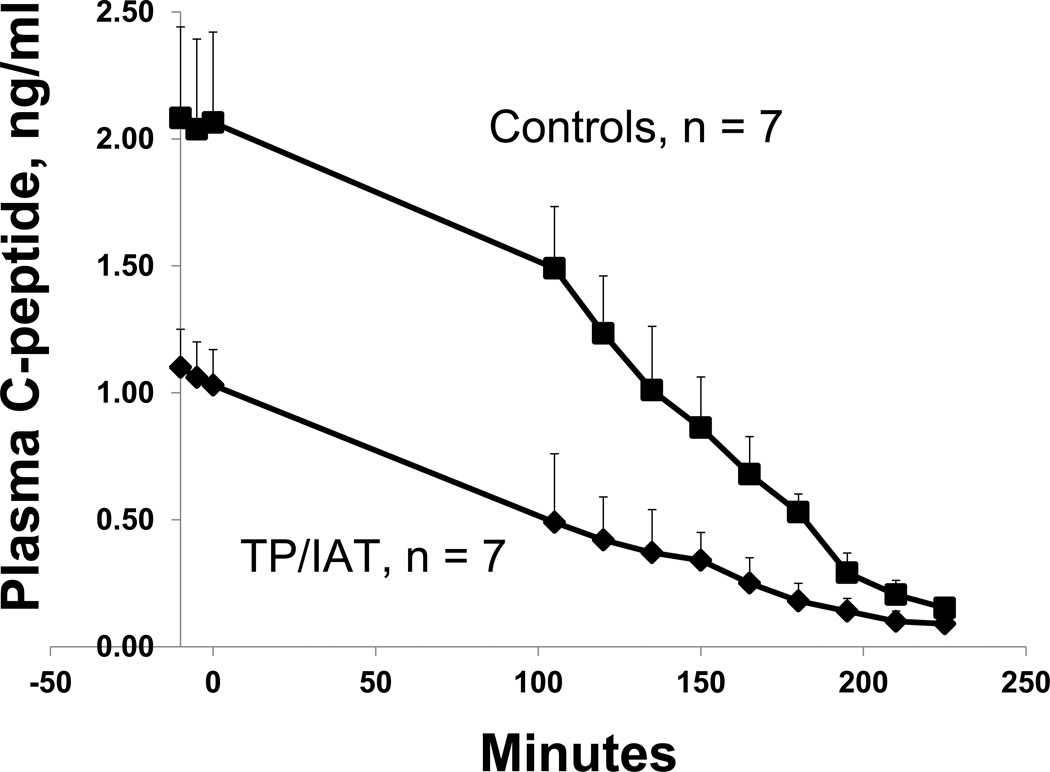
Baseline C-peptide levels (TP/IAT = 1.06 +/− 0.14; Control = 2.06 +/− 0.36; ng/ml; p<0.05) during the hypoglycemic clamp declined to nearly non-measurable levels in both the control and the TP/IAT groups at the 50 mg/dl glucose nadirs (0.09 +/− 0.04 and 0.15 +/− 0.06, respectively, p = ns; Fig. 3). This indicated that the transplanted islets appropriately ceased secreting insulin during hypoglycemia, thereby providing the insulin switch-off signal to the alpha cells required for release of glucagon during hypoglycemia (6, 7).
Figure 3. C-peptide suppression during hypoglycemic clamps.
Both the TP/IAT-H (n=5) and TP/IAT-H + NH (n=2) groups had significantly lower baseline C-peptide levels compared to the Control group (p<0.001). However, during the clamp both groups appropriately suppressed C-peptide secretion and by the end of the clamp both groups had levels that were almost completely suppressed and were not significantly different from one another.
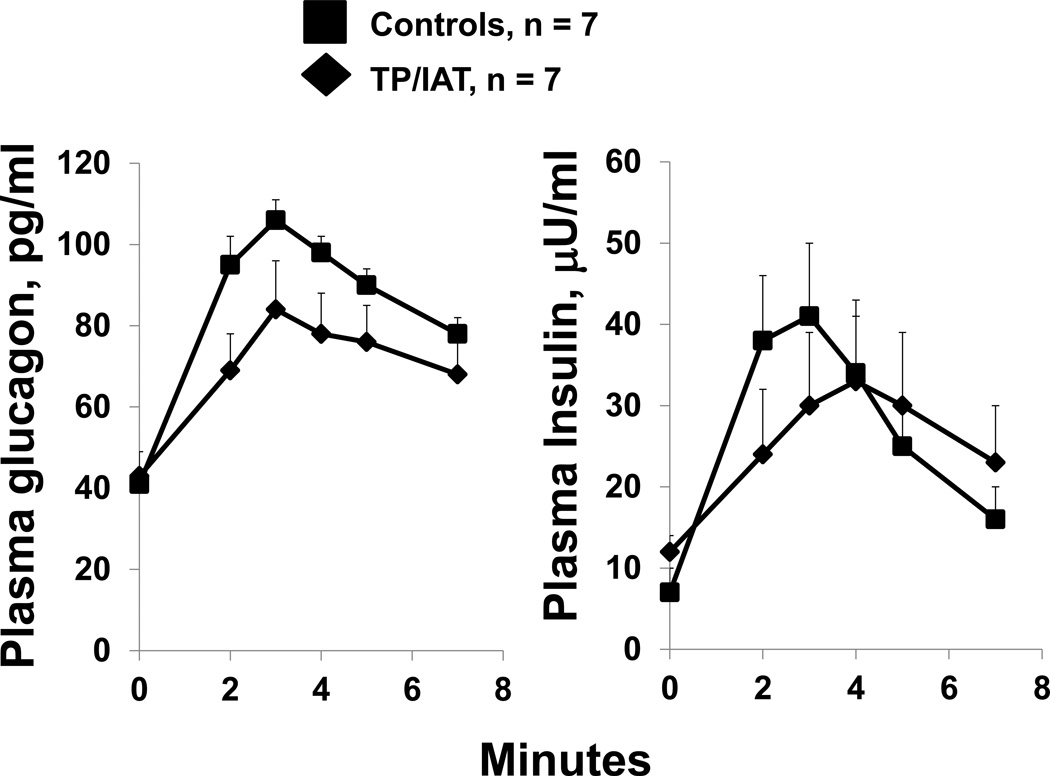
Baseline glucagon levels for the TP/IAT and Control groups were 43 +/− 6 and 41 +/−3 pg/ml, respectively, p=ns (Fig 4). All 7 TP/IAT recipients had acute glucagon responses to an intavenous arginine pulse. These values for the TP/IAT and Control groups were 37 +/− 7 and 63 +/− 6, respectively (p<0.05). Baseline insulin levels for the TP/IAT and Control groups were 12 +/− 1 and 8 +/− 1 µU/ml, respectively, p=ns (Fig 4). All 7 TP/IAT recipients had acute insulin responses to an arginine pulse. These values for the TP/IAT and Control groups were 21 +/− 8 and 30 +/− 5, respectively (p=ns).
Figure 4. Glucagon and insulin responses to intravenous arginine.
Acute glucagon and insulin responses to intravenous arginine were intact in the TP-IAT group. The glucagon response was significantly less than Control values (p<0.05) but the insulin response was not significantly different.
DISCUSSION
These results indicate that transplanted intrahepatic α-cells from non-diabetic humans did not provide the counter-regulatory glucagon responses to hypoglycemia normally provided by intra-pancreatic α-cells and that symptom awareness was virtually absent. The probability that this is a transplantation site-specific defect is strengthened by our finding that TP/IAT subjects who received not only intrahepatic, but also non-hepatic islets had virtually normal glucagon and symptom responses to hypoglycemia. These observations are consistent with previously reported animal studies that demonstrated intrahepatic islets failed to secrete glucagon normally during hypoglycemia, although they secreted glucagon in response to intravenous arginine (9, 10). As with the current work, islets transplanted into non-hepatic sites in animals had intact glucagon responses to hypoglycemia (9, 10). In the current studies all recipients had intact glucagon responses to intravenous arginine which could only have come from the intrahepatic alpha cells in the case of the recipients of intrahepatic islets only. These responses were normally timed, although tended to be lower than control responses, which is not surprising since the controls had more native islets, hence more alpha cells, than the recipients had autoislets.
Lack of glucagon secretion during hypoglycemia cannot by itself explain why islet autotransplant recipients initially become hypoglycemic post-transplant. This finding may relate in some cases to use by TP/IAT recipients of small amounts of long-acting insulin on a daily basis to diminish functional stress on their transplanted islets. However, only two of our TP/IAT recipients had been using small doses of exogenous insulin. Major clues may be that our recipients associate bouts of hypoglycemia with eating carbohydrate-rich meals and vigorous exercise. Ingestion of meals with robust insulin secretion is a possible cause when there is no glucagon secretion to offset declining glucose levels reaching the hypoglycemic range. Similarly, vigorous exercise with increased peripheral glucose uptake could result in hypoglycemia when glucagon release is absent, as glucagon is an important signal for increased hepatic glucose production, which prevents declining glucose levels from reaching the hypoglycemic range. Alternative explanations include the possibility that the procedure of islet isolation and/or placement of the isolated islets into the liver damages α- and/or β-cell function. The former does not seem likely because all TP/IAT recipients had glucagon responses to intravenous arginine and because it has been observed in rodents that absent glucagon responses of intrahepatic alpha cells can be recovered by prolonged fasting, and made to again disappear by re-feeding to increase liver glycogen stores(10). The latter does not seem likely because TP/IAT recipients had intact insulin responses to intravenous arginine and the expected decrement in C-peptide levels during hypoglycemic clamps. Those results suggest to us that when the normal glycogenolytic response by the liver during hypoglycemia floods the intrahepatic parenchyma, it also bathes the islets contained therein with glucose, which might negate hypoglycemic signals that intrahepatic α-cells receive from the systemic circulation. Another consideration is that intrahepatic islets are unlikely to establish meaningful central nervous system innervation, an important regulator of glucagon secretion from the native pancreas during hypoglycemia. However, this seems unlikely to be the explanation because we have previously shown that transplanted pancreases have normal glucagon responses to hypoglycemia despite the fact they are placed into the pelvic cavity where they are not likely to receive meaningful central nervous system innervation (11).). Finally, partial or complete duodenal resection and Roux-en-Y reconstruction, as performed during many bariatric procedures, are associated with risk of post-operative hypoglycemia, perhaps related to robust incretin responses to meals (12–14). Such increases in incretin hormone secretion could also contribute to reduced glucagon secretion by the intrahepatic transplanted ±-cells. Importantly, regardless of the initial cause of hypoglycemia in TP/IAT, lack of normal glucagon and symptom responses to hypoglycemia in recipients of intrahepatic islets only will exaggerate the degree and frequency of hypoglycemia. We also made the novel observation of diminution in the magnitude of symptom awareness of hypoglycemia during hypoglycemic clamps in TP/IAT recipients. The most common cause of decreased hypoglycemic awareness in humans is recurrent hypoglycemia (15–18), which supports our subjects’ reported histories of repeated bouts of hypoglycemia, confirmed in some by continuous glucose monitoring at home. It is notable that the degree of symptom awareness to hypoglycemia in TP/IAT recipients who received islets in non-hepatic sites as well as hepatic sites was comparable to that of normal control subjects.
The clinical importance of these observations pertains not only to intrahepatic islet autotransplantation after total pancreatectomy, but also to intrahepatic islet allotransplantation in patients with type 1 diabetes. We have previously reported defective glucagon secretion during hypoglycemia in type 1 diabetic recipients of allografted intrahepatic islets (5), and that they have diminished symptom awareness of hypoglycemia (5). Consequently, intrahepatic alloiset recipients, many of whom continue to use exogenous insulin on a daily basis (19), are at risk for not being able to counter-regulate hypoglycemia in a completely normal manner during daily living. There has been disagreement with our initial report of absent glucagon secretion during hypoglycemia in autoislet recipients (4) as well as with our published findings of absent glucagon secretion in recipients of intrahepatic alloislets (5). Rickels et al. studied alloislet recipients by performing hyperinsulinemic, hypoglycemic clamps and reported an initial suppression of glucagon followed by a modest glucagon rise towards but not reaching baseline by the end of the clamp (19). Those data replicated our own published earlier (5). However, since glucagon levels failed to rise above baseline values in both cases, no absolute rise in glucagon in response to hypoglycemia was demonstrated (Fig. 3A, Ref.5 and Fig. 2B, Ref.20). In terms of an explanation for the behavior of glucagon secretion during the clamps, since a rise in glucose levels are known to decrease glucagon secretion it seems likely that the suppression and then rise of glucagon levels towards baseline we both observed during clamps was due to the variable rate of intravenous glucose infusion – initially high and then lower - that is characteristic of the insulin clamp experiment. Ang et al. also reported a small (< 20 pmol/l) rise in glucagon levels during hyperinsulinemic, hypoglycemic clamps in type 1 diabetic recipients of alloislets (21). However, compared to their normal control subjects, this represented only an approximate 16% change in glucagon levels from baseline whereas our TP/IAT recipients who received islets in non-hepatic sites as well as hepatic sites had changes in glucagon levels from baseline that were equivalent to those of our control subjects.
We believe our data call for reconsideration of the exclusive use of hepatic sites for islet transplantation in humans. The hepatic site has been the preferred site because of historical precedent and attractive early experimental evidence of efficacy of this site in normalizing hyperglycemia (22). Understandably, concerns can be raised whether or not islets placed into the peritoneal cavity will be able to achieve vascularization and sufficient oxygenation to live and function. Nonetheless, non-hepatic sites, such as the peritoneal cavity, omental sacs, and serosal surfaces of gastro-intestinal organs have been shown to have hepatic portal venous drainage, as well as bone marrow and muscle, and are effective for islet transplantation (9, 22–27). More to the point, our current glucagon secretion data demonstrate that intraperitoneal islets can thrive and respond appropriately to hypoglycemia by releasing glucagon and suppressing C-peptide secretion for up to at least several years post-transplant. However, transplantation of a portion of the islets into an extra-hepatic site should be performed only with protocols careful to follow α-cell and β-cell function over time post-transplant to establish whether these sites allow islet function and longevity equivalent to the liver, and to determine the most promising non-hepatic sites.
In conclusion, these studies in human TP/IAT recipients demonstrate that they have absent glucagon responses and poor symptom recognition of hypoglycemia during hypoglycemic clamps and often experience frequent hypoglycemia post-transplant. This loss of symptom awareness is known to be very dangerous and increases the risk of hypoglycemic coma. Since transplanting a portion of the islets (approximately 100,000 – 150,000) in non-hepatic sites in combination with intrahepatic sites preserves the glucagon and symptom responses to hypoglycemia, this approach should be seriously considered when using the accepted procedure of intrahepatic islet transplantation.
Acknowledgements
We gratefully acknowledge support from NIH R01 39994 (RPR), American Diabetes Association Mentor-Based Grant (RPR), and NIH K23 DK084315 (MDB) and the University of Minnesota CTSA (8UL1TR000114). We appreciate the referral of 1 of the 14 recipients studied from James Markmann, MD, Ji Lei, MD, and Enrico Cagliero, MD at MGH.
Abbreviations
- TP/IAT
Total pancreatectomy/islet autotransplantation
- TP/IAT-H
TP/AIT-hepatic site
- TP/IAT-H + NH
TP/IAT-H plus non-hepatic site
- AGRarg
Acute glucagon response to arginine
- AIRarg
Acute insulin response to arginine
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Pezzilli R, Morselli Labate AM, Fantini L, Gullo L, Corinaldesi R. Quality of life and clinical indicators for chronic pancreatitis patients in a 2-year follow-up study. Pancreas. 2007;34(2):191–196. doi: 10.1097/mpa.0b013e31802e0301. [DOI] [PubMed] [Google Scholar]
- 2.Wehler M, Nichterlein R, Fischer B, Farnbacher M, Reulbach U, Hahn EG, et al. Factors associated with health-related quality of life in chronic pancreatitis. Am J Gastroenterol. 2004;99(1):138–146. doi: 10.1111/j.1572-0241.2004.04005.x. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland DE, Radosevich DM, Bellin MD, Hering BJ, Beilman GJ, Dunn TB, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214(4):409–424. doi: 10.1016/j.jamcollsurg.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pyzdrowski KL, Kendall DM, Halter JB, Nakhleh RE, Sutherland DE, Robertson RP. Preserved insulin secretion and insulin independence in recipients of islet autografts. N Engl J Med. 1992;327(4):220–226. doi: 10.1056/NEJM199207233270402. [see comments]. [DOI] [PubMed] [Google Scholar]
- 5.Paty BW, Ryan EA, Shapiro AM, Lakey JR, Robertson RP. Intrahepatic islet transplantation in type 1 diabetic patients does not restore hypoglycemic hormonal counterregulation or symptom recognition after insulin independence. Diabetes. 2002;51(12):3428–3434. doi: 10.2337/diabetes.51.12.3428. [DOI] [PubMed] [Google Scholar]
- 6.Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes/metabolism research and reviews. 1999;15(1):42–46. doi: 10.1002/(sici)1520-7560(199901/02)15:1<42::aid-dmrr1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Zhou H, Tran PO, Yang S, Zhang T, LeRoy E, Oseid E, et al. Regulation of alpha-cell function by the beta-cell during hypoglycemia in Wistar rats: the "switch-off" hypothesis. Diabetes. 2004;53(6):1482–1487. doi: 10.2337/diabetes.53.6.1482. [DOI] [PubMed] [Google Scholar]
- 6.Teuscher AU, Seaquist ER, Robertson RP. Diminished insulin secretory reserve in diabetic pancreas transplant and nondiabetic kidney transplant recipients. Diabetes. 1994;43(4):593–598. doi: 10.2337/diab.43.4.593. [DOI] [PubMed] [Google Scholar]
- 7.Robertson RP. Estimation of beta cell mass by metabolic tests: necessary, but how sufficient? Diabetes. 2007;56(1):2420–2424. doi: 10.2337/db07-0742. [DOI] [PubMed] [Google Scholar]
- 8.Hoeldtke RD, Boden G, Shuman CR, Owen OE. Reduced epinephrine secretion and hypoglycemia unawareness in diabetic autonomic neuropathy. Ann Intern Med. 1982;96(4):459–462. doi: 10.7326/0003-4819-96-4-459. [DOI] [PubMed] [Google Scholar]
- 9.Gupta V, Wahoff DC, Rooney DP, Poitout V, Sutherland DE, Kendall DM, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes. 1997;46(1):28–33. doi: 10.2337/diab.46.1.28. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, Zhang T, Bogdani M, Oseid E, Parazzoli S, Vantyghem MC, et al. Intrahepatic glucose flux as a mechanism for defective intrahepatic islet alpha-cell response to hypoglycemia. Diabetes. 2008;57(6):1567–1574. doi: 10.2337/db08-0137. [DOI] [PubMed] [Google Scholar]
- 11.Diem P, Redmon JB, Abid M, Moran A, Sutherland DE, Halter JB, et al. Glucagon, catecholamine and pancreatic polypeptide secretion in type I diabetic recipients of pancreas allografts. J Clin Invest. 1990;86(6):2008–2013. doi: 10.1172/JCI114936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patti ME, McMahon G, Mun EC, Bitton A, Holst JJ, Goldsmith J, Hanto DW, Callery M, Arky R, Nose V, Bonner-Weir S, Goldfine AB. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005 Nov;48(11):2236–2240. doi: 10.1007/s00125-005-1933-x. [DOI] [PubMed] [Google Scholar]
- 13.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia wih nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249–254. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 14.Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, Schneider BE, Holst JJ, Patti ME. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 15.Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest. 1993;91(3):819–828. doi: 10.1172/JCI116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitrakou A, Fanelli C, Veneman T, Perriello G, Calderone S, Platanisiotis D, et al. Reversibility of unawareness of hypoglycemia in patients with insulinomas. N Engl J Med. 1993;329(12):834–839. doi: 10.1056/NEJM199309163291203. [DOI] [PubMed] [Google Scholar]
- 17.Widom B, Simonson DC. Intermittent hypoglycemia impairs glucose counterregulation. Diabetes. 1992;41(12):1597–1602. doi: 10.2337/diab.41.12.1597. [DOI] [PubMed] [Google Scholar]
- 18.Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet. 1994;344(8918):283–287. doi: 10.1016/s0140-6736(94)91336-6. [DOI] [PubMed] [Google Scholar]
- 19.Ryan EA, Paty BW, Senior PA, Bigham D, Alfadhii E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 20.Rickels MR, Schutta MH, Mueller R, Markmann JF, Barker CF, Naji A, Teff KL. Islet cell hormonal responses to hypoglycemia after human islet transplantation for type 1 diabetes. 2005;54(11):3205–3211. doi: 10.2337/diabetes.54.11.3205. [DOI] [PubMed] [Google Scholar]
- 21.Ang M, Meyer C, Brendel MD, Bretzel RG, Linn T. Magnitude and mechanisms of glucose counterregulation following islet transplantation in patients with type 1 diabetes suffering from severe hypoglycemic ejpisodes. Diabetologia. 2014;57(3):623–632. doi: 10.1007/s00125-013-3120-9. [DOI] [PubMed] [Google Scholar]
- 22.Kemp CB, Knight MJ, Scharp DW, Ballinger WF, Lacy PE. Effect of transplantation site on the results of pancreatic islet isografts in diabetic rats. Diabetologia. 1973;9(6):486–491. doi: 10.1007/BF00461694. [DOI] [PubMed] [Google Scholar]
- 23.Kin T, Korbutt GS, Rajotte RV. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transplant. 2003;3(3):281–285. doi: 10.1034/j.1600-6143.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 24.Lau J, Mattsson G, Carlsson C, Nyqvist D, Kohler M, Berggren PO, et al. Implantation site-dependent dysfunction of transplanted pancreatic islets. Diabetes. 2007;56(6):1544–1550. doi: 10.2337/db06-1258. [DOI] [PubMed] [Google Scholar]
- 25.Merani S, Toso C, Emamaullee J, Shapiro AM. Optimal implantation site for pancreatic islet transplantation. Br J Surg. 2008;95(12):1449–1461. doi: 10.1002/bjs.6391. [DOI] [PubMed] [Google Scholar]
- 26.Rafael E, Tibell A, Rydenb M, Lundgrend T, Savendahle L, Borgstrom B, Arneloa B, Isaksson B, Nilsson B, Korsgren O, Permert J. Intramuscular Autotransplantation of Pancreatic Islets in a 7-Year-Old Child: A 2-Year Follow-Up. Am J Transplant. 2008;8:458–462. doi: 10.1111/j.1600-6143.2007.02060.x. [DOI] [PubMed] [Google Scholar]
- 27.Maffi P, Balzano G, Ponzoni M, Nano R, Sordi V, Melzi R, Mercalli A, Scavini M, Esposito A, Peccatori J, Cantarelli E, Messina C, Bernardi M, Del Maschio A, Standacher C, Doglioni C, Ciceri F, Secchi A, Piemonti L. Autologous pancreatic islet Transplantation in human bone marrow. Diabetes. 62:3523–3531. doi: 10.2337/db13-0465. 20113. [DOI] [PMC free article] [PubMed] [Google Scholar]