Abstract
Low frequencies of memory B cells in the peripheral blood make it challenging to measure the functional and phenotypic characteristics of this antigen experienced subset of B cells without in vitro culture. To date, reagents are lacking to measure ex vivo frequencies of dengue virus (DENV)-specific memory B cells. We wanted to explore the possibility of using fluorescently labeled DENV as probes to detect antigen-specific memory B cells in the peripheral blood of DENV immune individuals. Alexa Fluor dye-labeled DENV yielded viable virus that could be stored at −80°C for long periods of time. Using a careful gating strategy and methods to decrease non-specific binding, we were able to identify a small frequency of B cells from dengue immune individuals that bound labeled DENV. Sorted DENV+ B cells from immune, but not naïve donors secreted antibodies that bound intact virions after in vitro stimulation. Overall, Alexa Fluor dye labeled -DENV are useful reagents to enable the detection and characterization of memory B cells in DENV immune individuals.
Keywords: B cells, dengue, flow cytometry, labeled viruses
INTRODUCTION
Antigen-specific memory B cells circulate at very low frequencies in the peripheral blood. Memory B cells do not secrete antibodies, and to analyze the specificities of memory B cells, investigators have relied on the expansion and conversion of memory B cells into antibody-secreting cells by in vitro culture 1, 2. Several different stimuli, including Toll-like receptor (TLR) ligands, pokeweed mitogen, cytokine cocktails, CD40 ligation, and B cell receptor (BCR) crosslinking, have been used 3-6. Antibodies secreted by B cells can be measured in culture supernatants and the frequencies of antibody-secreting cells determined by the use of ELISPOT assays after in vitro stimulation. The stimulation condition used can impact the frequency of antibody-secreting cells as well as the functionality of distinct sub–populations, which makes comparisons across studies difficult 5. Recently, tetanus toxoid-specific antigen tetramers were generated and used to increase the avidity of BCR labeling and the brightness of staining of memory B cells 7. Staining procedures were optimized to minimize background, which enabled visualization and isolation of tetanus-specific memory B cells months to years after antigen had been cleared.
Dengue virus (DENV), a member of the flavivirus family, consists of four distinct serotypes, DENV-1-4. Most DENV infections are asymptomatic but in most symptomatic infections, cases present with acute febrile illness, dengue fever (DF). A small percentage of individuals develop dengue hemorrhagic fever (DHF), which is characterized by plasma leakage and bleeding tendency coincident with resolution of fever and clearance of viremia8, 9. Although age, nutrition status and viral factors have been implicated in DENV pathogenesis, prior T and B cell immunity are widely acknowledged as key determinants of susceptibility to DHF 10.
Significant effort has been spent recently to understand DENV-specific B cell responses in humans 11. There is massive expansion of plasmablasts during acute DENV infection, with frequencies reaching up to 50-80% of total B cell responses 12, 13. Several groups, including ours, have isolated and characterized human monoclonal antibodies (hMAbs) from memory B cells of DENV immune donors and vaccine recipients 14-21. Cross-reactive antibodies specific for the envelope (E), pre-membrane (prM) protein and non-structural protein 1 (NS1) with poor, moderate or potent neutralizing activity have been isolated. A number of hMAbs from DENV immune donors only bind epitopes detected on mature viruses and not on E produced as a soluble recombinant (rE) protein 22. All of the studies to date have used non-specific methods to activate antigen-specific memory B cells. Direct characterization of DENV-specific memory B cells employing antigen-specific reagents has not been performed to date.
Zhang et al. described a simple method to label DENV with Alexa Fluor succinimidyl ester dyes (AF-DENV) that yielded viable virus after labeling 23. We followed this procedure and speculated that AF-DENV would be a valuable tool to track DENV-specific memory B cells in immune individuals. We used multiparametric flow cytometry to identify DENV-specific memory B cells that bound intact viruses. We sorted DENV+ B cells and detected DENV-specific antibodies that bound intact viruses in supernatants from in vitro stimulated DENV+ B cells in immune, but not naïve, donors. Our data indicate that AF-DENV enable specific and sensitive ex vivo functional characterization of a subset of DENV-specific memory B cells that bind intact virions.
MATERIALS AND METHODS
Labeling of DENV preparations
Labeling of DENV with small Alexa Fluor (AF) dyes was performed according to the method of Zhang et al. 23. DENV was isolated from supernatants of Vero cells (multiplicity of infection [m.o.i.] = 0.1) grown in serum free medium. Supernatants were concentrated using Amicon filters (molecular weight [m.w.] cutoff, 100,000; (Millipore, Billerica, MA). Briefly, an aliquot of concentrated virus preparation was incubated with AF dye (Life Technologies, Carlsbad, CA, USA), which was reconstituted in freshly prepared 0.2 M sodium bicarbonate pH 8.3 to a final concentration of 100 μM dye. The reaction was stopped after 1 h at room temperature using 1.5 M hydroxylamine buffer for an additional hour at room temperature, and the labeled virus was separated from free dye by means of PD-10 columns (GE Health Care Bio-Sciences Corp, Piscataway, NJ) equilibrated in buffered aqueous solution. Supernatants from uninfected Vero cell cultures were concentrated on Amicon filters, supplemented with 2% fetal bovine serum (FBS) and labeled with AF dyes for use as a control in phenotyping experiments. Labeled virus (AF-DENV) and Vero supernatants (AF-VERO) were aliquoted and stored at −80°C. AF-DENV and AF-VERO stored in this manner were both viable and exhibited stable fluorescence for at least 6 months (data not shown).
Validation of reagents using antibody coated beads
Labeling efficiency as well as retention of binding capacity of AF virions was verified on all preparations. 100 ul aliquots of 3.0 micron beads bearing goat anti mouse IgG (Fc) (Spherotech, Inc MPFc-30-5) Lake Forest, Il) were incubated with the optimal concentration of mouse anti dengue 2H2 or 3H5 antibodies, washed and resuspended in 1 ml of PBS containing 10%FCS. Control beads were coated with mouse anti human CD8 antibody. Test samples of AF-labeled DENV-1-DENV-4 virions were incubated with 15 μl aliquots of functionalized beads for 30 minutes on a rotating platform at room temperature, washed two times and read on the flow cytometer. A pattern of reactivity was revealed to show both specificity of binding as well as relative fluorescence intensity of the labeled virion preparations.
Assessment of infectivity post labeling
Virion preparations were also assessed for infectivity following the labeling process. At multiple time points after infection, surface and intracellular AF signal was assessed. Aliquots of labeled preparations were UV inactivated on ice for 30 minutes using a model UVG-54 short wave UV lamp (Ultra Violet Products, San Gabriel, CA). Equal volumes of the untreated or UV inactivated virus (MOI 5-10) were used to infect U937 DCSIGN cells. Co-expression of the E protein with AF signal was evaluated to confirm entry and infection with AF-DENV preparations.
Staining of dengue naïve and immune PBMC with AF-DENV
PBMC from dengue immune and naïve donors were isolated from whole blood and cryopreserved. PBMC from naïve donors were selected from liquid nitrogen database entries or from volunteers questioned for recent febrile illness with travel to known DENV endemic areas. All research involving human participants was approved by the Institutional Review Boards at the University of Massachusetts Medical School. Written informed consent was obtained from each subject. Frozen PBMC were thawed quickly and resuspended in RPMI complete medium containing 20 μg/mL DNase (Life Technologies). The cells were pelleted and washed again in serum free RPMI. Cells were then labeled with LIVE/DEAD Near-IR Dead Cell stain (L10119; Life Technologies) per the manufacturer's recommendations, washed and returned to RPMI containing 10% FBS. All further incubations were performed at 4°C. PBMC were first incubated with an excess of AF-VERO supernatant for 10 min on ice, and then a titered amount of AF-DENV was added for an additional 30 min on ice. Following two washes in complete medium, cells were labeled for 30 min on ice with a cocktail of mouse anti-human MAbs: CD3-APC-H7, clone SK7; CD14-APC-H7, clone M0P9; CD19-PE-Cy7, clone SJ25C1 (BD Biosciences, San Jose, CA, USA); CD27-BV785, clone O323 (Biolegend, San Diego, CA, USA); and polyclonal rabbit anti-human IgM-PE (Dako, Carpinteria, CA, USA). Cells were washed twice, fixed in 1% Cytofix (BD Biosciences) and analyzed on a FACS Aria II μ equipped with Diva v7.0 and CS&T v2.0 software (BD Biosciences). A cytometer baseline for the instrument configuration consisting of four lasers (405, 488, 561, and 640 nm) with fixed octagonal/trigon optical filters using a 70-μm nozzle operating at 70 psi was generated with daily performance checks using CS&T beads (655050; BD Biosciences). Photomultiplier tube voltages and area scaling factors were optimized, and compensation spillover values were determined, using the applicable panel antibodies or reagents and mouse antibody capture beads (BD Biosciences). FCS 3.1 data files were collected to display and analyze rare B cell populations. Data analysis and transformation was performed using FlowJo v10 (Treestar, Ashland, OR, USA).
Magnetic cell sorting (MACS) isolation to enrich for antigen-specific B cells using AF-DENV
Total B cells were isolated from DENV immune and naïve donors using B cell isolation kits (Miltenyi Biotec, Auburn, CA USA). Cryopreserved PBMC (10-20 × 106) were thawed in RPMI containing 10% FBS and 20 μg/mL DNase and washed in complete medium. According to the kit instructions, cells were labeled with a cocktail of biotin-conjugated anti-CD2, -CD14, -CD16, -CD36, -CD43 and -CD235a antibodies for 10 min followed by addition of anti-biotin microbeads. Cells were washed and applied to LS columns (Miltenyi Biotec), saving the flow-through. Flow cytometry staining of the flow-through cells confirmed that 90% was B cells (data not shown). Total B cells were then labeled with a pre-titered amount of AF647-DENV on ice, and AF647+ and AF647– B cell fractions were isolated using anti-Cy5/anti-AF647 microbeads and MS columns (Miltenyi Biotec).
Enzyme-linked immunosorbent assay (ELISA) with supernatants from sorted memory B cells
Whole PBMC or isolated cell fractions (AF-DENV+ and total B) at approximately 500,000 cells/well were stimulated with 2.5 μg/mL R848 (Invivogen, San Diego, CA, USA) and 1000 U/mL recombinant human (rh) IL-2 (Peprotech, Rocky Hill, NJ, USA) in 96-well U-bottom microtiter plates. After five to seven days at 37°C and 5% CO2, cell culture supernatants were tested for total Ig production and DENV Ig by ELISA. Total IgG was measured by the Human IgG Quantitation Kit (E80-104; Bethyl Laboratories Inc., Montgomery, TX, USA). To measure DENV-specific IgG, ELISA plates were coated with 50 μL/well serum-free unlabeled DENV preparations and incubated for 18-24 h at 4°C. The plates were blocked with 1% bovine serum albumin (BSA) for 90 min, and supernatants were added to the wells for 1 h. Plates were washed with phosphate-buffered saline (PBS) containing 0.1% Tween-20. Goat anti-human IgG coupled to horseradish peroxidase (HRP; A80-104P; Bethyl Laboratories Inc., Montgomery, TX, USA) was added as the secondary antibody at a 1:20,000 dilution. Then, 100 μL TMB (3,3,5,5’-tetramethylbenzidine) Peroxidase Substrate (50-76-00; KPL, Gaithersburg, MD, USA) was added as the substrate. After approximately 20 min, the enzyme reaction was stopped by addition of 1 M hydrochloric acid, and the plates were read at 450 nm.
ELISpot Assay
Millipore ELISpot plates (cat. # MAIPSWU10) were pre-wetted with 70% ethanol and washed 5 times with distilled water. Wells were coated with 1×106 PFU of unlabeled DENV. To detect total IgG, wells were coated with 100μl of anti-human IgG (15μg/ml) (Mabtech, MT91/145). Plates were stored at 4°C overnight, washed with PBS (Gibco) and blocked with 100μl RPMI 1640/10% FBS for 30 minutes at room temperature (RT). The cells were washed to remove any bound IgG, counted (AF-DENV+ and total B cells) and 100 μl of cells were added to each well. Cell concentrations varied depending on the cell recovery. The plate was incubated at 37°C overnight. After washing, 100 μl of biotinylated monoclonal antibody directed against human IgG (1 μg/ml in PBS/0.5 % FCS; MT78/145, Mabtech) was added. Plates were incubated for 2 h at RT, washed and Streptavidin HRP (100μl of 1:1000 dilution in PBS/0.5% FBS) (BD Pharmingen) was added. Plates were incubated at RT for 1 h, developed with TMB substrate (100μl/well) (Mabtech) and analyzed using an ELISpot plate reader (CTL Immunospot reader).
RESULTS
Generation and binding of fluorescently labeled DENV to susceptible cell lines
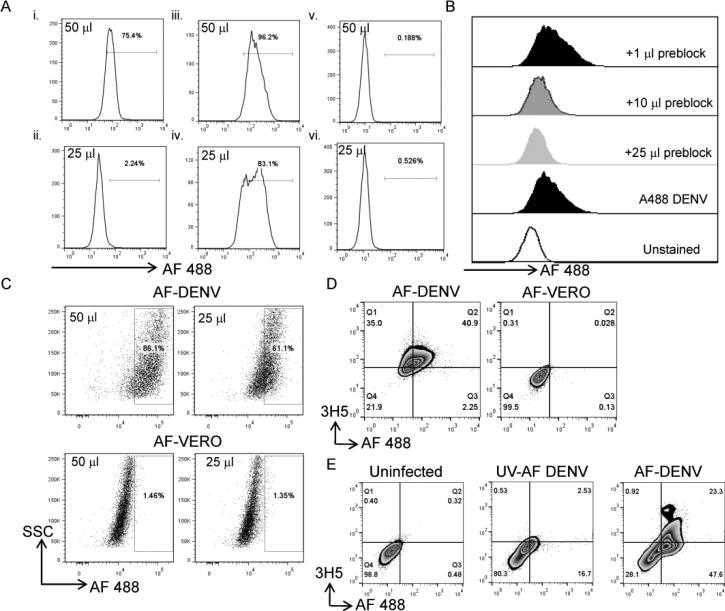
We conjugated Alexa Fluor dyes to DENV and control Vero cell supernatants (AF-DENV and AF-VERO, respectively) according to the method by Zhang et al. 23. We first evaluated binding of AF-DENV and AF-VERO to the DENV-susceptible cell lines U937-DCSIGN and U937. Different concentrations of AF-DENV and AF-VERO were added to U937-DCSIGN and U937 cells, and binding of the labeled viruses was assessed after incubation for 45 min at 4°C. We found moderate binding of AF-DENV to U937 cells (Figure 1Ai and ii). We found significant binding of AF-DENV (Figure 1Aiii and iv) and minimal binding of AF-VERO (Figure 1Av and vi) to U937-DCSIGN cells. To determine whether the fluorescence we detected on the surface of U937-DCSIGN cells was DENV-specific, we pretreated U937-DCSIGN cells with increasing concentrations of unlabeled DENV. Following the addition of AF-DENV, we found a decrease in fluorescence intensity in samples that were pretreated with excess unlabeled DENV (Figure 1B).
FIGURE 1. Binding characteristics of AF-DENV.
(A) Different concentrations of AF-DENV were added to the parental monocytic cell line U937 (i and ii) or to U937 transfected with DCSIGN (v and vi). AF-VERO binding was also assessed to U937-DC SIGN cells (iii and iv). Numbers adjacent to the gate in the histogram represent the percentage of AF-DENV+ or AF-VERO+ cells, respectively, in the corresponding cell lines. One of two independent experiments is shown. (B) Increasing concentrations of unlabeled DENV were added to U937-DCSIGN cells prior to the addition of AF-DENV. Histograms are shaded to emphasize the change in mean fluorescence intensity (MFI) with increasing concentration of unlabeled DENV. (C) U937-DCSIGN cells were infected with different concentrations of AF-DENV or AF-VERO, and intracellular fluorescence was assessed by flow staining 24 h later. Representative flow plots of one of three independent experiments are shown. (D) AF-DENV (left panel) and AF-VERO (right panel) -infected U937-DCSIGN cells were fixed and stained intracellularly for the dengue E protein using the 3H5 MAb 24 h post-infection. (E) Uninfected (left panel), UV AF-DENV (middle panel) and AF-DENV (right panel) -infected U937-DCSIGN cells were fixed and stained intracellularly for the dengue E protein using the 3H5 MAb 48 h post-infection.
We next infected U937-DCSIGN cells with AF-DENV (m.o.i. = 1) or AF-VERO and assessed intracellular fluorescence intensity 24 h later. We detected robust fluorescence in U937-DCSIGN cells infected with AF-DENV, but not AF-VERO (Figure 1C). We co-stained for the presence of DENV E protein using the 3H5 MAb and compared U937-DCSIGN cells infected with AF-DENV versus AF-VERO. Approximately 75% of the cells were positive for E protein in the AF-DENV-infected cultures. Of these cells, >50% were positive for both the E protein and AF dye (Figure 1D). To determine whether the AF-DENV preparations were infectious, we used UV inactivated AF-DENV and found minimal co-expression with the E protein (Figure 1E) in the UV AF-DENV preparations. Our data indicate that we were able to efficiently label DENV with AF dyes, and that the labeled virus could be recognized by receptors on susceptible cells or by virus-specific antibody.
Specific intracellular binding of AF-DENV to B cell lines that actively secrete antibodies
We previously established two Epstein-Barr virus (EBV)-transformed B cell lines that proliferated and maintained their secretion of DENV-specific antibodies 20. The B cell lines (#27 and #41) express CD19 and CD20 and secrete IgG antibodies that bind reduction-sensitive epitopes found on serotypes DENV-1 and -3, but not DENV-2 or -4. Since these B cell lines were actively secreting antibodies, we speculated that there would be minimal expression of the BCR on the surface of these cells.
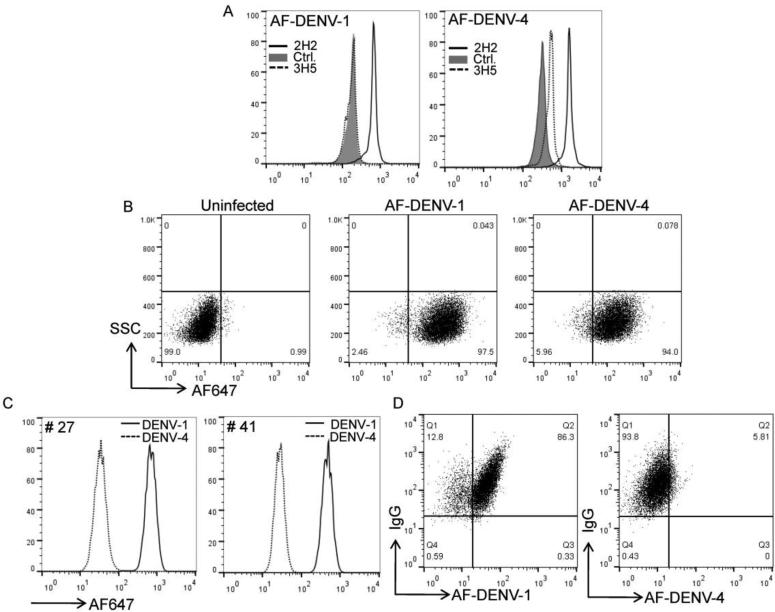
All AF-DENV preparations were initially tested for binding to control antibody or DENV-specific antibody coated beads. Both AF-DENV-1 and AF-DENV-4 preparations bound the 2H2 (flavivirus cross-reactive) antibody but not the control CD8 antibody bound to beads (Figure 2A). Minimal binding was detected when the AF-DENV-4 preparation was added to 3H5 (mostly DENV-2 specific) coated beads while no binding was detected with AF-DENV-1. We next assessed binding of AF-DENV-1 and AF-DENV-4 preparations to U937 DCSIGN cells. After incubation with labeled AF-DENV-1 or AF-DENV-4 for 3 hrs we found similar intensity of staining with both DENV-1 and -4 preparations when equivalent concentrations of virus were used (Figure 2B). The data indicate that we had efficiently labeled both DENV-1 and DENV-4 preparations which could be tested for binding to antigen-specific B cells.
FIGURE 2. Specificity of binding of AF-DENV to antibody-secreting cell lines.
(A) Binding of AF-DENV-1 and -DENV-4 to control, 2H2 (flavivirus cross-reactive) and 3H5 (DENV-2 specific) coated beads. (B) Binding of AF-DENV-1 and DENV-4 to U937-DCSIGN cells 3 h post incubation. (C) B cell lines #27 and #41 were permeabilized and stained with AF-DENV-1. Histograms represent overlays of AF-DENV-1+ stained cells compared to AF-DENV-4+ stained cells and demonstrate specific intracellular binding of cell lines #27 and #41 to AF-DENV-1. (D) Co-labeling of anti-IgG and AF-DENV in permeabilized B cell line #41. The concentrations of AF-DENV-1 and -4 were adjusted to be approximately equal numbers of virions.
We used AF-DENV-1 to assess surface and intracellular binding of DENV to the B cell lines #27 and #41. We found poor binding of AF-DENV-1 to the surface of B cell lines #27 and #41, suggesting that surface BCR levels were down-regulated on these antibody-secreting cells (data not shown). We then examined whether AF-DENV-1 could be used to detect specific intracellular IgG. We found significant staining of AF-DENV-1 in permeabilized B cell lines #27 and #41 (Figure 2A). When we compared the staining intensities of AF-DENV-1 and -4, we found significant binding of AF-DENV-1 to B cell line #27 and #41, but minimal binding to AF-DENV-4. Costaining with IgG resulted in the majority of cells positive for both AF-DENV-1 and IgG while no staining was detected for AF-DENV-4 (Figure 2B). Our data show intracellular binding of specific serotypes of AF-DENV to B cell lines #27 and #41 actively secreting antibodies.
Gating strategy to identify DENV-specific memory B cells
Memory B cells continue to circulate for many years after infection or vaccination, but their frequency is very low in the peripheral blood 1, 2. We asked whether AF-DENV could be used to identify these rare B cells without in vitro expansion. PBMC from dengue naïve and immune donors were thawed and stained with a panel of markers to identify memory B cells. The gating strategy we used initially selected cells within a generous lymphocyte gate, as defined by forward and side scatter profiles, and then selected singlet cells. Live cells were next selected by exclusion of the viability marker LIVE/DEAD Near-IR. B cells were enriched for by excluding CD3+ T cells and CD14+ monocytes. We next used antibodies to known B cell markers, CD19, CD27 and IgM, to discriminate class-switched memory B cells. The gating strategy allowed assessment of AF-DENV binding to memory B cells (CD19+CD27+IgM−) (Supplementary Figure 1).
Experiments to decrease non-specific binding of AF-DENV to memory B cells
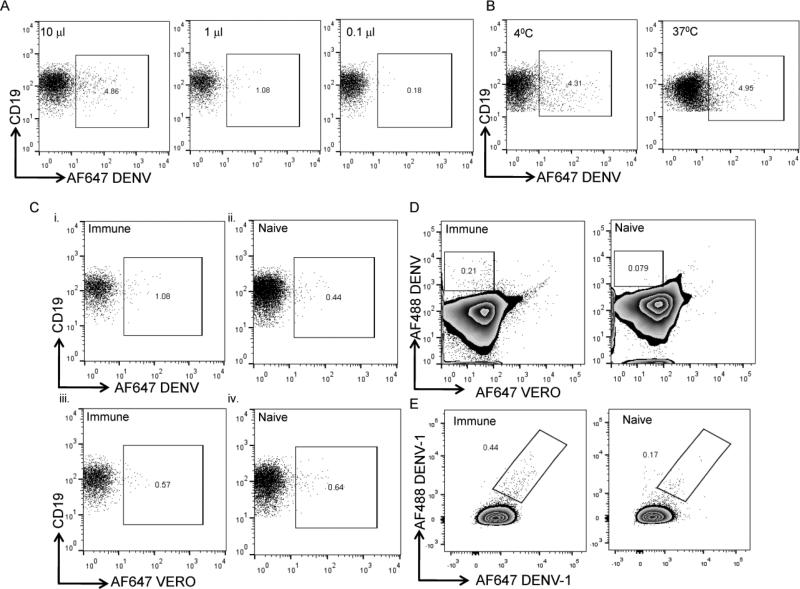
We first assessed binding of AF-DENV to memory B cells to ensure that binding activity could be titered out and to select a concentration that stained with reasonable frequency and signal intensity in order to test further binding conditions. Different concentrations of AF-DENV (between 0.1-10 μl) were initially tested for binding to memory B cells. We found that the staining intensity of AF-DENV varied according to the titration of labeled virus used (Figure 3A and data not shown). We next tested binding of AF-DENV to memory B cells at different temperatures (4°C and 37°C) and found an increased shift of the negative population at 37°C (Figure 3B). We also found significant binding of labeled DENV to monocytes at 37°C (data not shown). To prevent capping and shedding of BCR complexes on B cells and inhibit uptake by monocytes, all subsequent staining was performed at 4°C. For each serotype and preparation of AF-DENV we chose a concentration that had reasonable staining intensity and minimal background staining in naïve donors (typically between 1-5 μl of a 1-5×106 PFU/ml AF-DENV stock). Using carefully titrated AF-DENV, we assessed binding to memory B cells in PBMC from several naive and immune donors. When we first examined binding of AF-DENV to memory B cells, we found that a small frequency of CD27+IgM−CD19+ B cells bound AF-DENV in PBMC from both dengue immune and naïve donors (Figure 3Ci and ii); furthermore, we found that a small frequency of memory B cells bound AF-VERO (Figure 3Ciii and iv).
FIGURE 3. Optimization of staining conditions to identify memory B cells that bind AF-DENV.
Memory B cells were identified as live CD3−CD14−CD19+CD27+IgM– cells, as delineated in the gating strategy outlined in Supplementary Figure 1. (A) Different concentrations of AF-DENV were added to DENV immune PBMC at 4°C for 30 min. Frequencies represent AF-DENV+ cells within the CD19+CD27+IgM− B population. (B) Flow plots depict frequencies of AF-DENV+ memory B cells when binding was performed at 4°C or 37°C. (C) Representative flow plots of PBMC from immune and naïve donors showing frequencies of AF-DENV+ (i and ii) and AF-VERO+ (iii and iv) memory B cells. (D) Representative flow plots depicting the use of AF-VERO preparations to delineate non-specific staining of PBMC from immune and naïve donors. AF647-VERO+AF488-DENV+ memory B cells were excluded from the gating strategy to identify DENV+ B cells. Numbers within the gates in the dot plots represent the frequencies of AF488-DENV+ AF647-VERO−CD19+CD27+IgM− B cells in immune and naïve donors. (E) Numbers within the gates in the dot plots represent the frequencies of AF488-DENV-1+AF647-DENV1+ CD19+CD27+IgM− B cells in an immune and naïve donor.
We explored the potential of using unlabeled Vero cell supernatant as a pre-blocking step to reduce background staining in PBMC of naive donors and found a decrease in the frequency of B cells that bound labeled viruses (data not shown). However, this did not reduce background staining in memory B cells from naïve donors to acceptable levels. We speculated that the BCR of some memory B cells may recognize epitopes of the fluorochromes themselves in addition to Vero cell components. We included a pre-incubation step with a VERO preparation labeled to a different AF dye. We found that a small frequency of B cells in PBMC from both naïve and immune donors bound AF-VERO preparations. We therefore added a step to our gating strategy to exclude B cells that were VERO+ or VERO+DENV+ from our analysis in reporting the frequency of AF-DENV+ cells (Figure 3D) as virion-specific memory B cells. We sought to improve the specificity of memory B cell binding by using a single serotype of DENV that was labeled with two different fluorochromes. We found that memory B cells in an immune donor bound both forms of labeled DENV (Figure 3E). However, a lower frequency of memory B cells also bound both forms of DENV in a naïve donor. Together, our data indicate that we improved the specificity of binding of memory B cells to intact virions in dengue immune donors but we did not completely eliminate the binding to intact virions of a small frequency of memory B cells in dengue naive donors.
Binding of AF-DENV to a small subset of memory B cells
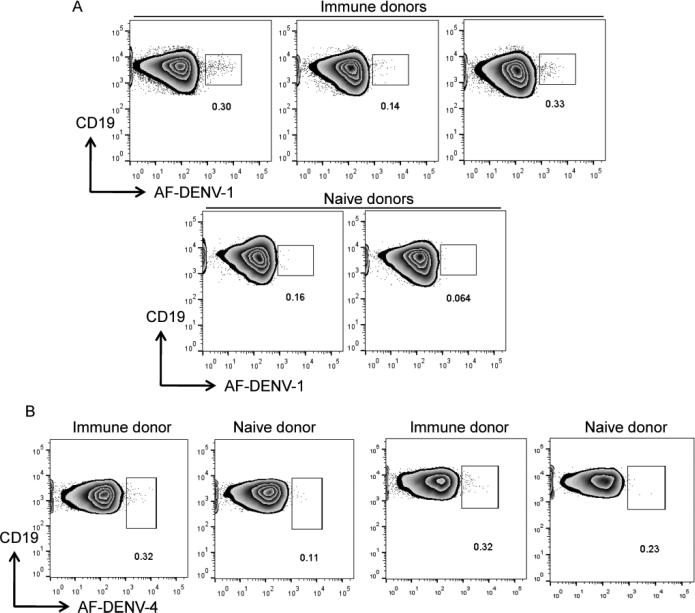
We thawed PBMC from dengue immune and naïve donors and used the gating strategy detailed in Supplementary Figure 1 to assess the frequency of memory B cells that bound labeled virions. Memory B cells that bound AF-DENV were brightly labeled, with an intensity of more than 1 log above background for the majority of cells. AF-DENV-1+ B cells represented 0.1-0.3% of CD19+CD27+IgM− B cells in immune donors, while frequencies of 0.005-0.1% were found in CD19+CD27+IgM− B cells from naive donors (Figure 4A). When we used AF-DENV-4 on PBMC from two donors who received a live attenuated DENV-4 vaccine several years prior 24, 25, we found detectible frequencies of AF-DENV-4+ memory B cells that were brightly labeled (Figure 4B). The staining intensity in PBMC from two naïve donors was lower; the frequencies of B cells that bound AF-DENV-4 were similar. We were thus able to identify a small, but detectible, population of memory B cells that bound to AF-DENV in PBMC from DENV immune donors.
FIGURE 4. Detection of AF-DENV+ memory B cells.
Using the gating strategy outlined in Supplementary Figure 1, frequencies of memory B cells that bound AF-DENV in immune and naïve donors are shown. Numbers within the gates in the zebra plots represent the frequencies of (A) AF-DENV-1+ and (B) AF-DENV-4+ B cells within the CD19+CD27+IgM− B cell population of immune and naïve donors.
AF-DENV+-sorted B cells secrete antibodies that bind intact virions
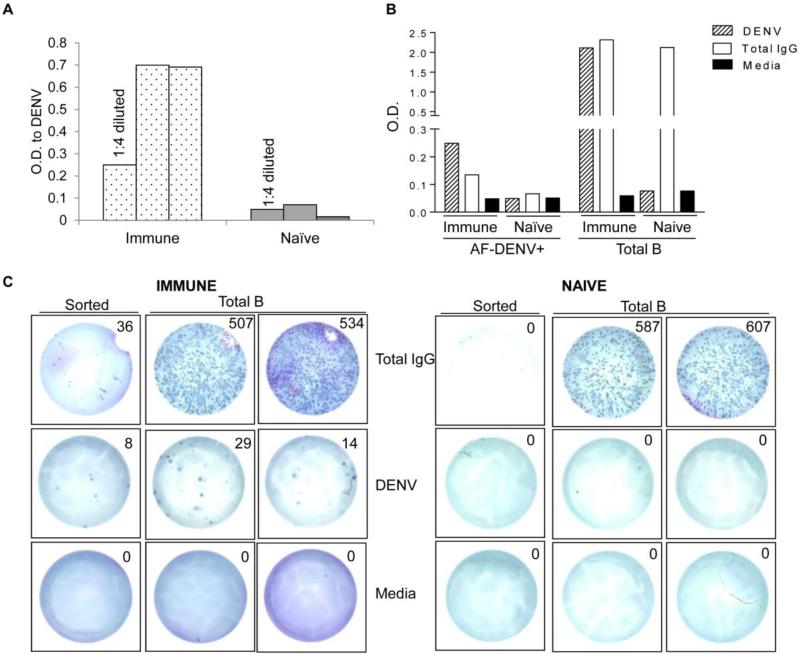
To determine whether B cells that bound AF-DENV were able to secrete DENV-specific antibodies, we sorted DENV+ B cells from PBMC of naïve and immune donors. Total B cells were first isolated by MACS and then labeled with AF-DENV. B cells that bound AF-DENV from naïve and immune donors were next isolated. Sorted AF-DENV+ B cells from 3 immune and 3 naïve donors were stimulated in vitro with the TLR ligand, R848, as memory B cells require in vitro stimulation to secrete antibodies. Supernatants collected five to seven days later were assessed for IgG antibodies that bound unlabeled DENV in an ELISA. Shown in Figure 5A are optical density (O.D.) values of DENV-specific antibodies from stimulated B cell supernatants from naïve and immune donors. We detected IgG antibodies that bound unlabeled intact DENV in supernatants from DENV+-sorted B cells in immune, but not in naïve donors. To determine whether the DENV-specific antibody concentration was similar to total IgG concentration in the sorted AF-DENV+ population we tested supernatants from stimulated, sorted AF DENV+ cells from a naïve and immune donor. We found O.D. values to DENV and total IgG were similar in the supernatant from AF DENV+ sorted cells from the immune donor with no DENV or total IgG responses in supernatants from AF DENV+ B cells from a naïve donor (Figure 5B). We found strong antibody responses to DENV in supernatants from total B cells in the immune donor but not in total B cells from the naïve donor. As expected, total IgG responses in supernatants from total B cells were high in both the immune and naive donor.
FIGURE 5. Sorted DENV+ B cells secrete Ig to DENV.
(A) AF-DENV+ B cells from immune and naïve donors (n=3) were isolated by MACS sorting. AF-DENV+ B cells were cultured with R848 for seven days, and supernatants were tested for the presence of DENV-specific antibody. Undiluted or the indicated dilution of supernatants from the stimulated cultures were tested by ELISA for recognition of DENV. (B) A 1:4 dilution of supernatant obtained from AF-DENV+ and total B cells from an immune and naïve donor were tested for binding to unlabeled DENV. Total IgG was assessed from the same supernatants in a separate ELISA. (C) Frequencies of antigen-specific B cells were measured by ELIspot assay in sorted AF-DENV+ and total B cells from PBMC of an immune and naïve donor. B cells were stimulated in vitro for 7 d with R848 and IL-2 as described in Materials and Methods. 2×104 total B cells from the immune and naïve donor were added to duplicate wells containing anti-human IgG, 1×106 PFU unlabeled DENV or media. For AF-DENV sorted B cell populations, cells were divided equally and added to ELISpot plates.
We next used ELISpot assays on sorted AF-DENV+ B cells and total B cells to more accurately enumerate the ratio of virus-specific to total IgG producing B cells in a naïve and immune donor. In the immune donor, we found a much higher percentage of sorted AF DENV+ B cells (approximately 25%) compared to total B cells (approximately 0.04%) were secreting DENV-specific Abs (Figure 5C). In the naïve donor, we found no DENV-specific B cells in either the total or sorted B cell populations. IgG secreting B cells in the total B cell population were similar in the immune and naïve donor.
DISCUSSION
The goal of this study was to determine if fluorescently labeled DENV could be used to identify antigen-specific human memory B cells in the peripheral blood. We found that a small frequency (<0.5%) of memory B cells bound AF-DENV in PBMC from dengue immune donors. The density of AF-DENV was high enough to allow isolation of memory B cells using MACS microbeads. Furthermore, sorted AF-DENV+-B cells produced specific immunoglobulin to DENV. Quantification and determination of total (non-antigen-specific) and antigen-specific memory B cells with surface immunoglobulin that can bind DENV can now be performed by including AF-DENV probes in multiparametric flow cytometry panels.
We were able to efficiently label DENV with AF dyes following the procedure of Zhang et al 23. We found that a low concentration of AF dye was sufficient to label virions effectively and maintain functional integrity of the virus preparation. High amounts of AF-DENV strongly bound a subset of B cells for analysis; however, we noticed binding to memory B cells in both naïve and immune donors. We also found that every labeled DENV preparation had to be carefully titrated for our B cell studies. We used relatively high starting numbers of donor PBMC (5-10 × 106) to identify rare virion-specific B cells in DENV immune versus naïve donors by flow cytometry in this study.
B cells are unique as they produce both secreted antibody and a membrane bound form as part of the BCR complex. Surface expression and secreted immunoglobulin from individual B cells has been studied by the use of antigen-specific labeling and flow cytometric analysis 7, 26-28. As described by several groups, there are a number of confounding variables when using detection reagents designed to track B cells 28-30. Non-relevant epitopes may be present, and B cells that bind whole virions may represent specificities for a range of epitopes, only some of which are of interest. We found a small frequency of B cells specific for epitopes against the AF dye itself, which contributed to the non-specific background staining seen in PBMC from naive donors. Furthermore, a small frequency of B cells bound some components in Vero cell preparations. Although we spent significant effort to reduce non-specific binding, we did not completely eliminate binding to memory B cells in PBMC of dengue naive donors. It is possible that BCRs from some B cells bound embedded host molecules and viral envelope lipids, thus contributing to the background staining.
We chose MACS sorting rather than fluorescence-activated cell sorting (FACS) to determine whether B cells that bound AF-DENV were able to secrete DENV-specific antibodies. While FACS can provide better purity and allow more possibilities for choice of markers, MACS is simpler to use. Furthermore, since the AF-DENV probes that we used contained live, infectious virions, sorting by FACS would have required BSL-3 conditions. Our data indicate that the MACS sorted AF-DENV+ cells contained a subset of antigen-specific B cells as these cells in immune but not naïve donors were able to secrete antibodies that bound unlabeled virions in an ELISA assay. However, the unsorted population also contained DENV-specific B cells that bound intact virions (data not shown). The labeling of intact virions with amine reactive dyes is likely to mask some B cell epitopes thus preventing all antigen-specific B cells from being picked up by our AF-DENV reagent. Furthermore, any antibody that recognizes the DENV envelope produced as a recombinant (rE) will also recognize intact virions in an ELISA.
The ELISpot assays that we performed on sorted B cells clearly indicate that the ratio of virus-specific to total IgG secreting cells on sorted AF-DENV+ B cells was much higher compared to total B cells in the same immune individual. We believe our findings that approximately 25% of sorted cells secreted DENV-specific antibodies in the immune donor are likely to be under-estimated. While we were easily able to count total B cell populations by trypan blue exclusion five to seven days later, few cells were counted in the AF-DENV+ culture for most experiments. We therefore divided the recovered B cells equally (DENV, media and total IgG) from stimulated AF-DENV+ cultures for the ELISpot assays. We cannot rule out the possibility that binding of AF-DENV to the BCR with subsequent MACS positive selection and culture altered the signaling cascade through the BCR and impacted the viability of sorted AF-DENV+ B cells. Future sorts and culture conditions will require further optimization. We also needed a relatively high number of PBMC from immune donors (40-50×106) to isolate about 2-3×106 total B cells and subsequently about 1×105 AF-DENV+ B cells to perform the ELISA and ELISpot assays. We were thus unable to use PBMC from our well established clinical studies in Thai children for these sorts where frequencies of AF-DENV+ B cells are higher (unpublished data). Together, our ELISA and ELISpot data indicate the utility and specificity of labeled intact DENV to enrich for antigen-specific B cells.
We envision use of AF-DENV for several applications. First, AF-DENV can help identify a subset of memory B cells available to respond to a second infection with DENV. Since prior B cell immunity is widely acknowledged as a key determinant of susceptibility to severe dengue disease (DHF), these reagents could be incorporated into ongoing clinical studies, where it would be informative to directly phenotype and track the kinetics of antigen-specific B cells prior to, during and after natural primary and secondary DENV infections. We are beginning to use these reagents on samples from our clinical studies in Thailand, where the number of PBMC available for research studies is limiting and do find detectible frequencies of AF-DENV-specific memory B cells. Second, a number of DENV vaccines are in development and require multiple doses to generate an effective antibody response to all four serotypes of DENV 31. AF-DENV can be used to track B cells prior to and following initial and booster doses of tetravalent DENV vaccination. Third, this approach allows for the production and detection of human antibodies secreted from memory B cells. Plasmablasts, on the other hand, only transiently circulate in the blood after infection or vaccination and are extremely fragile upon cryopreservation 12. Fourth, the reagents we have developed would be extremely useful for isolating and assessing the function of a key subset of antigen-specific B cells. A number of antibodies isolated from humans recognize complex quarternary epitopes present only on the intact virion 22. Fifth, these reagents can be used to track DENV-specific and cross-reactive B cells. In this study we used a single serotype of DENV to assess binding to memory B cells in immune donors. We have begun to assess binding of memory B cells to two different serotypes of DENV and find that some memory B cells bind only one serotype, while a smaller frequency of B cells binds both serotypes. Ultimately, the use of all four serotypes of DENV would be informative, but as with all multiparametric flow cytometry staining, compensation issues associated with using more than 12 colors in a single panel are not trivial.
Despite our efforts to reduce background staining, a small frequency of CD19+CD27+IgM− memory B cells in naïve donors bound these AF-DENV reagents. In every experiment performed, we used AF-DENV reagents on PBMC from donors who were in theory “naïve” to dengue infection as we believe they provide a stringent specificity control. However, it is challenging to truly identify dengue “naïve” individuals and some of our background staining may reflect cross-reactivity with other flaviviruses. When using AF-DENV reagents to phenotype B cells from patients with a history of prior dengue infection, care must be taken to include PBMC from appropriate naïve controls if available. For dengue vaccine studies, a pre-vaccine sample is important to include for accurate assessment of the increase in frequency of virion-specific B cells post vaccination.
The reagents we have developed will provide direct and detailed analysis of DENV-specific B cells in terms of their phenotype and kinetics of response during and after dengue infection or vaccination. Functional profiles of distinct subsets of B cells to shed light on neutralization/enhancing activity of secreted antibodies, intracellular protein or gene expression are needed to further our understanding of DENV-specific memory B cells. Identifying B cells that may be involved in protective (virion-specific reagents, domain I/II hinge or III E protein tetramers) or pathogenic (prM/NS1 tetramers) immune responses are key to understanding the immunological mechanisms that contribute to clinical DENV disease and will help guide vaccine development.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr. Alan L. Rothman for thoughtful discussions; Heather Friberg and Alejandro Ramirez for careful review of the manuscript.
This work was funded by the National Institutes of Health, U19 AI57319, and a discretionary fund grant U19 AI57234 from the NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: There are no potential conflicts of interest from any of the authors.
REFERENCES
- 1.Lanzavecchia A, Sallusto F. Human B cell memory. Curr Opin Immunol. 2009;21:298–304. doi: 10.1016/j.coi.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshida T, Mei H, Dorner T, Hiepe F, Radbruch A, Fillatreau S, et al. Memory B and memory plasma cells. Immunol Rev. 2010;237:117–39. doi: 10.1111/j.1600-065X.2010.00938.x. [DOI] [PubMed] [Google Scholar]
- 3.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–22. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Fecteau JF, Roy A, Neron S. Peripheral blood CD27+ IgG+ B cells rapidly proliferate and differentiate into immunoglobulin-secreting cells after exposure to low CD154 interaction. Immunology. 2009;128:e353–65. doi: 10.1111/j.1365-2567.2008.02976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henn AD, Laski M, Yang H, Welle S, Qiu X, Miao H, et al. Functionally Distinct Subpopulations of CpG-Activated Memory B Cells. Sci Rep. 2011;2:345. doi: 10.1038/srep00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henn AD, Rebhahn J, Brown MA, Murphy AJ, Coca MN, Hyrien O, et al. Modulation of single-cell IgG secretion frequency and rates in human memory B cells by CpG DNA, CD40L, IL-21, and cell division. J Immunol. 2009;183:3177–87. doi: 10.4049/jimmunol.0804233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franz B, May KF, Jr., Dranoff G, Wucherpfennig K. Ex vivo characterization and isolation of rare memory B cells with antigen tetramers. Blood. 2011;118:348–57. doi: 10.1182/blood-2011-03-341917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 9.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis. 1997;176:322–30. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 10.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nature Reviews Immunology. 2011;11:532–43. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 11.Silva WMPBWaAMd The Human Antibody Response to Dengue Virus Infection. Viruses. 2011;3:2374–95. doi: 10.3390/v3122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrammert J, Onlamoon N, Akondy RS, Perng GC, Polsrila K, Chandele A, et al. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J Virol. 2012;86:2911–8. doi: 10.1128/JVI.06075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Bates TM, Cordeiro MT, Nascimento EJ, Smith AP, Soares de Melo KM, McBurney SP, et al. Association between Magnitude of the Virus-Specific Plasmablast Response and Disease Severity in Dengue Patients. J Immunol. 2012;190:80–7. doi: 10.4049/jimmunol.1103350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, et al. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010;8:271–83. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–8. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schieffelin JS, Costin JM, Nicholson CO, Orgeron NM, Fontaine KA, Isern S, et al. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virol J. 2010;7:28. doi: 10.1186/1743-422X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, et al. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis. 2010;5:e1188. doi: 10.1371/journal.pntd.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SA, Zhou Y, Olivarez NP, Broadwater AH, de Silva AM, Crowe JE., Jr. Persistence of circulating B memory cell clones with potential for dengue virus disease enhancement for decades following infection. J Virol. 2011 doi: 10.1128/JVI.06335-11. doi:10.1128/JVI.06335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Setthapramote C, Sasaki T, Puiprom O, Limkittikul K, Pitaksajjakul P, Pipattanaboon C, et al. Human monoclonal antibodies to neutralize all dengue virus serotypes using lymphocytes from patients at acute phase of the secondary infection. Biochem Biophys Res Commun. 2012;423:867–72. doi: 10.1016/j.bbrc.2012.06.057. [DOI] [PubMed] [Google Scholar]
- 20.Friberg H, Jaiswal S, West K, O'Ketch M, Rothman AL, Mathew A. Analysis of human monoclonal antibodies generated by dengue virus-specific memory B cells. Viral Immunol;2012;25:348–59. doi: 10.1089/vim.2012.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costin JM, Zaitseva E, Kahle KM, Nicholson CO, Rowe DK, Graham AS, et al. Mechanistic study of broadly neutralizing human monoclonal antibodies against dengue virus that target the fusion loop. 2012 doi: 10.1128/JVI.02273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahala WM, Silva AM. The human antibody response to dengue virus infection. Viruses. 2011;3:2374–95. doi: 10.3390/v3122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang SL, Tan HC, Hanson BJ, Ooi EE. A simple method for Alexa Fluor dye labelling of dengue virus. J Virol Methods. 2010;167:172–7. doi: 10.1016/j.jviromet.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Gagnon SJ, Zeng W, Kurane I, Ennis FA. Identification of two epitopes on the dengue 4 virus capsid protein recognized by a serotype-specific and a panel of serotype-cross-reactive human CD4+ cytotoxic T-lymphocyte clones. Journal of Virology. 1996;70:141–7. doi: 10.1128/jvi.70.1.141-147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoke CH, Jr., Malinoski FJ, Eckels KH, Scott RM, Dubois DR, Summers PL, et al. Preparation of an attenuated dengue 4 (341750 Carib) virus vaccine. II. Safety and immunogenicity in humans. Am J Trop Med Hyg. 1990;43:219–26. doi: 10.4269/ajtmh.1990.43.219. [DOI] [PubMed] [Google Scholar]
- 26.Newman J, Rice JS, Wang C, Harris SL, Diamond B. Identification of an antigen- specific B cell population. J Immunol Methods. 2003;272:177–87. doi: 10.1016/s0022-1759(02)00499-4. [DOI] [PubMed] [Google Scholar]
- 27.Doucett VP, Gerhard W, Owler K, Curry D, Brown L, Baumgarth N. Enumeration and characterization of virus-specific B cells by multicolor flow cytometry. J Immunol Methods. 2005;303:40–52. doi: 10.1016/j.jim.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Kodituwakku AP, Jessup C, Zola H, Roberton DM. Isolation of antigen-specific B cells. Immunol Cell Biol. 2003;81:163–70. doi: 10.1046/j.1440-1711.2003.01152.x. [DOI] [PubMed] [Google Scholar]
- 29.Moody MA, Haynes BF. Antigen-specific B cell detection reagents: use and quality control. Cytometry A. 2008;73:1086–92. doi: 10.1002/cyto.a.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amanna IJ, Slifka MK. Quantitation of rare memory B cell populations by two independent and complementary approaches. J Immunol Methods. 2006;317:175–85. doi: 10.1016/j.jim.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol. 2011;29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.