Abstract
Corollary discharge (CD) is hypothesized to provide the movement information (direction and amplitude) required to compensate for the saccade-induced disruptions to visual input. Here, we investigated to what extent these conveyed metrics influence perceptual stability in human subjects with a target-displacement detection task. Subjects made saccades to targets located at different amplitudes (4°, 6°, or 8°) and directions (horizontal or vertical). During the saccade, the target disappeared and then reappeared at a shifted location either in the same direction or opposite to the movement vector. Subjects reported the target displacement direction, and from these reports we determined the perceptual threshold for shift detection and estimate of target location. Our results indicate that the thresholds for all amplitudes and directions generally scaled with saccade amplitude. Additionally, subjects on average produced hypometric saccades with an estimated CD gain <1. Finally, we examined the contribution of different error signals to perceptual performance, the saccade error (movement-to-movement variability in saccade amplitude) and visual error (distance between the fovea and the shifted target location). Perceptual judgment was not influenced by the fluctuations in movement amplitude, and performance was largely the same across movement directions for different magnitudes of visual error. Importantly, subjects reported the correct direction of target displacement above chance level for very small visual errors (<0.75°), even when these errors were opposite the target-shift direction. Collectively, these results suggest that the CD-based compensatory mechanisms for visual disruptions are highly accurate and comparable for saccades with different metrics.
Keywords: corollary discharge, saccade, visual error, visual perception, movement variability
the disruption to visual input that accompanies rapid eye movements (saccades) creates a complex problem for the visual system. Despite the shifts of the retinal image with each saccade, the visual scene is still perceived as stable and continuous, revealing that the brain actively and accurately integrates visual and motor information over multiple eye movements (for review, see Wurtz 2008). The neural mechanisms that underlie perceptual stability are assumed to require knowledge about the upcoming saccade movement vector and update of a map in retinotopic coordinates (Duhamel et al. 1992; Melcher and Colby 2008; Nakamura and Colby 2002; Sommer and Wurtz 2006; Wurtz 2008). Supported by neural and behavioral studies in nonhuman primates (Joiner et al. 2013; Sommer and Wurtz 2002, 2004a, 2004b) and humans (Bellebaum et al. 2005, 2006; Bridgeman et al. 1975; Duhamel et al. 1992; Joiner et al. 2010, 2013; Ostendorf et al. 2010), this remapping is hypothesized to be mediated by a corollary discharge (CD) signal. This internal signal does not generate the movement but is a copy of the efferent motor command sent to the muscles to produce movement (Sperry 1950; von Holst and Mittelstaedt 1950). The CD internally conveys the information of the impending saccade vector (movement amplitude and direction) to the required neural sensory areas to compensate for the impending changes in the retina position attributable to the upcoming eye movement (Duhamel et al. 1992; Gottlieb et al. 1998; Hall and Colby 2011; Nakamura and Colby 2002; Sommer and Wurtz 2006).
The CD of saccadic eye movements has been studied in the laboratory using behavioral tasks that examine motor behavior and perception across saccades. In a paradigm of the saccadic suppression of displacement developed by Bridgeman et al. (1975), subjects are required to make a saccade to a peripheral target following a central fixation period. During the movement, the target is extinguished and, in one variant of the task, immediately reappears at a displaced location. In a second task condition, the target reappears after a blank period following the saccade onset (e.g., 250 ms). After the target reappearance, subjects are required to report the direction of target displacement, and, on the basis of this two-alternative forced-choice decision, the resulting psychometric function provides an estimate of the perceptual threshold (the smallest displacement that is detected at greater than chance level) and bias (the displacement amount at which the probability of either directional report is at chance level, the perceived target location). It has been proposed that the accurate detection of target displacement in this task relies on the CD accompanying each saccade, allowing a comparison of the target location before and after the movement. This comparison has been shown to be affected by saccadic suppression; the threshold for detecting small target displacements is higher when the target immediately reappears at a shifted location than when a blank period precedes the target reappearance (Deubel et al. 1996, 2002). Additionally, it has been shown that the threshold for shift discrimination improves with exposure; there is a decrease in the perceptual threshold with an increase in the initial viewing period of the target (Zimmermann et al. 2013). As demonstrated by various studies, normal subjects report accurate estimates of target location and displacement (small bias and threshold measures) for a range of saccade amplitudes (Bridgeman et al. 1975; Collins et al. 2009; Deubel et al. 1996, 2002; Joiner et al. 2013; Ostendorf et al. 2010; Wexler and Collins 2014). Similar to end point variability, these perceptual thresholds have been shown to increase with movement amplitude for horizontal saccades (Bridgeman et al. 1975; Li and Matin 1990, 1997; Niemeier et al. 2003, 2007), suggesting that the brain optimally integrates premovement expectations of the visual scene with noisy sensory and motor information (Bays and Husain 2007; Niemeier et al. 2003, 2007; van Opstal and van Gisbergen 1989).
CD has also been studied with the “double-step task” developed by Hallett and Lightstone (1976). In this task, two targets are flashed successively and extinguished before the subject can perform the required eye movements. The task is conducted in the dark so that no visual feedback is possible, and the targets are arranged to ensure that correct performance requires internal information of the first saccade vector. If a subject were to make a saccade to the second target based solely on where that target fell on the retina instead of the CD-based updated eye location from the first movement, then the second saccade would not compensate for errors (overshoots and undershoots) in the first saccade. Several studies have demonstrated that both human subjects and monkeys are able to make trial-by-trial adjustments in the second saccade to adjust for errors on the first movement (Bellebaum et al. 2005; Joiner et al. 2010; Munuera et al. 2009; Quaia et al. 2010; Sommer and Wurtz 2002). It is unlikely that proprioception from the eye muscles plays a significant role in this process, as it has been shown that performance is not affected when these signals are eliminated and likely to be too slow to be effectively utilized (Guthrie et al. 1983; Lewis et al. 2001; Poletti et al. 2013; Wang et al. 2007). Thus the second saccade provides an assessment of the accuracy of the CD representation of the first eye movement. In our previous study that utilized this task, we showed that the CD of the first saccade conveyed accurate amplitude and direction movement information (Joiner et al. 2010). However, the trial-by-trial adjustment in the second saccade was significantly more accurate for primary horizontal saccades than for vertical, suggesting differences in the precision of the CD movement vector information.
As described above, the CD representation of movement amplitude and direction has been examined for perceptual behavior and motor performance. Specifically, the results of Li and Matin (1990, 1997) demonstrated that the accuracy of this representation for perception scales with horizontal movement amplitude. In our previous work, we showed that the accuracy of this representation for motor sequences is influenced by the direction of the initial eye movement (Joiner et al. 2010). This performance difference may be due to the neural origin of the respective motor commands; the motor signals and the associated CD for horizontal and vertical saccades are generated by distinct neural areas, which may result in divergent CD properties and behaviors when based on these respective signals (Leigh and Zee 2006).
On the basis of the collective examples above, it is not clear how the CD representation for both of these saccade metrics (amplitude and direction) influences visual perception and whether behavior remains consistent across these metrics. Probing how subjects account for saccade-induced disruptions to visual input for different movement metrics and relating this behavior to movement generation may provide a better understanding of how visual stability is accomplished. To determine the influence of saccade metrics, we designed a shift-detection experiment to examine the CD-based detection of transsaccadic target displacements for saccades to targets at different amplitudes (4°, 6°, and 8°) and directions (horizontal and vertical). We specifically investigated the systematic changes in perceptual threshold and bias for these different saccade vectors and the influence of movement-by-movement saccade variability and the resulting visual error on the detection of these transsaccadic target shifts.
MATERIALS AND METHODS
We recorded the eye movements of 10 healthy human subjects between the ages of 18 and 24 yr. Subjects had normal vision and were naive as to the aim of the study, which consisted of making perceptual judgments about the location of presented visual stimuli. Experimental protocols were approved by the Institutional Review Board of George Mason University, and informed consent was obtained from each participant. Subjects completed the experiments in multiple test sessions over 3 days and did not receive any training in the tasks before obtaining the experimental data.
Apparatus and measurement.
Eye movements were recorded using the Eyelink II eye tracker (head-mounted binocular eye tracker, 500-Hz temporal resolution, 0.2° spatial resolution; SR Research, Mississauga, Ontario, Canada). Stimuli were presented on a 19-in CRT-monitor (screen resolution 1,024 × 768 pixels; refresh rate 120 Hz) at a viewing distance of 62.5 cm. Subjects were seated in a dimly lit room in a stationary chair with their head stabilized by a chinrest. Stimulus presentation, eye movement, and manual keyboard response data acquisition were achieved using real-time experimental control software (Experiment Builder, SR Research). At the start of each experiment session, a nine-point gaze calibration was performed followed by a nine-point validation. Calibration and/or validation were repeated until the error was smaller than 1° on average, as previously done in studies conducted under these standard calibration settings (Peterson and Wong 2008; Wong and Peterson 2011).
Task and procedure.
In this experiment, each trial began with a central fixation cross (0.3° in extent). Subjects were required to maintain fixation on this cross for a variable period (random duration between 1,200 and 1,800 ms, Fig. 1A). After the fixation period, an initial target was presented at one of three amplitudes (4°, 6°, or 8°) and four directions (upward, downward, leftward, or rightward) from the fixation cross, 12 possible combinations (Fig. 1B). Subjects were required to make a saccade to the initial target. Once the eye position exceeded a virtual square window (3.2° in extent) around the fixation point, the target was extinguished and followed by a 250-ms blank period (Deubel et al. 1996). (We should note that previous studies, including our own, have compared perceptual performance with and without this gap period; Joiner et al. 2013; Ostendorf et al. 2010; Wexler and Collins 2014). We chose to concentrate on the condition with the blank because of our interest in the properties of the saccade CD rather than the effect of saccadic suppression on perception. In addition, previous results suggest that differences in perception attributable to CD are observable during this condition (Ostendorf et al. 2010). Therefore, all results presented here are from experiments conducted with a 250-ms blank period. The initial target then reappeared at a shifted randomized position in line with the movement (±0.5° collinear increments) between ±2.5°. The target shift was randomly drawn from a Gaussian distribution centered at 0°, with the smaller, less prominent shifts being sampled more than the larger, more detectable shifts (Fig. 1B). Following the reappearance of the target, the subject made a manual response using keyboard arrow keys (upward, rightward, downward, or leftward) to indicate the direction in which the target shifted. Subjects had to make this manual response within 3,000 ms. No instructions were given on reaction time, and all subjects were able to respond within the 3,000-ms time window. No feedback was given to subjects to indicate correct or incorrect responses. Each subject underwent three sessions of 312 trials each. The amplitude and direction of the initial target was randomly and equally distributed among all 12 possible combinations.
Fig. 1.
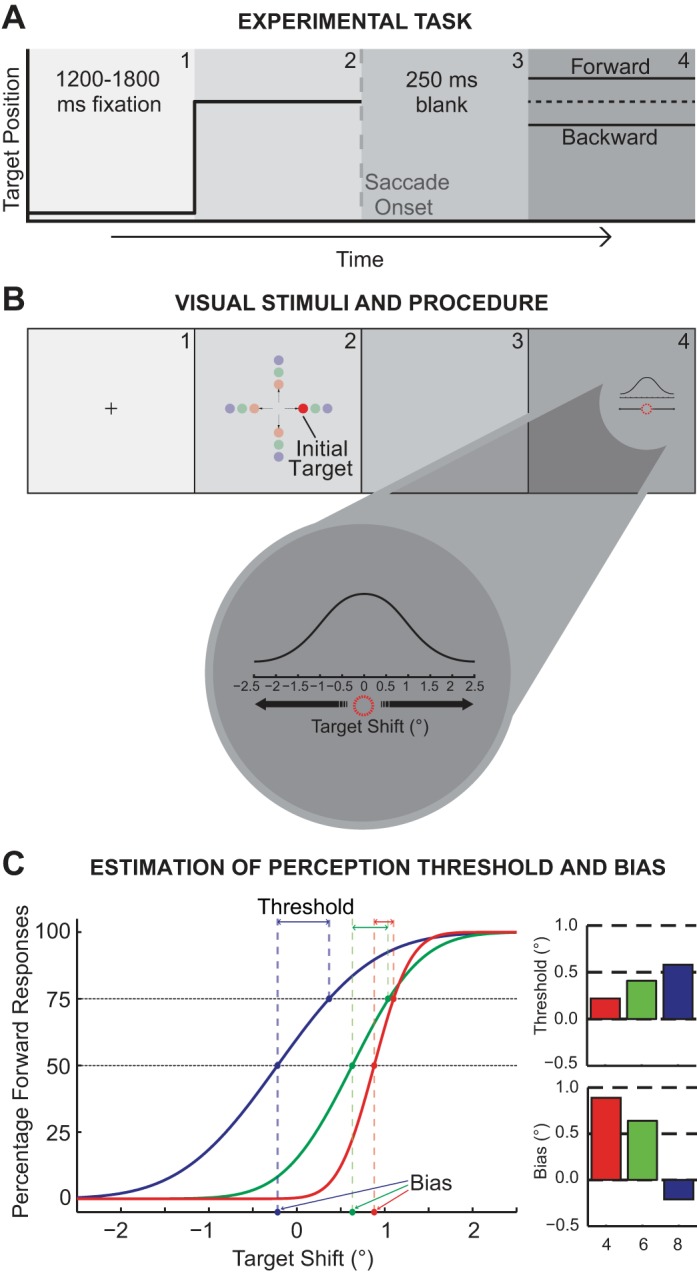
Transsaccadic shift-detection task. A: task procedure. Each trial began with a central fixation cross appearing for a variable period (1,200–1,800 ms), followed by appearance of an initial target. Subjects were required to make a saccadic eye movement toward this initial target, and the target was displaced during the saccade, reappearing at a new location after a 250-ms blank. B: task conditions. The initial target, T1, appeared randomly at 4°, 6°, or 8° horizontally or vertically from the central fixation point. The displaced target, T2, appeared randomly at shifted positions according the illustrated Gaussian distribution ranging from −2.5° to 2.5° shifts. After the appearance of the target at the shifted location, subjects were required to indicate the direction of the displacement (backward or forward) using arrow keys on the keyboard (upward, downward, leftward, and rightward). C: example psychometric curves. The frequency of forward responses on the y-axis is plotted as a function of target displacement on the x-axis. The manual response data were fitted to a cumulative Gaussian distribution to determine the psychometric function. 3 sample psychometric curves are shown with different slopes. 2 perceptual measures are derived, threshold and bias. The threshold, as shown by the bar chart, top, right, is computed as the difference in target displacement between the 50% and the 75% points of the sample psychometric curves. The perceptual bias was taken as the displacement from 0 at the point where the forward and backward responses were equal to 50%, as shown by the bar chart, bottom, right. In these example curves, the different conditions for each curve result in varying thresholds and biases.
Saccade measures.
Horizontal and vertical movements of at least one of the two eyes were recorded, and the resulting data were visualized, filtered, and analyzed offline using MATLAB v. 8.1.0 environment (Mathworks, Natick, MA). During the task, saccade initiation was detected when the saccade left the 3.2° square fixation window. For offline analyses, an eye movement was classified as a saccade if both eye velocity and acceleration exceeded 50°/s and 2,000°/s2 respectively. Saccade onset and offset were determined when the eye velocity and acceleration rose above and fell below these criteria, respectively. Saccade end points were examined for every amplitude and direction. Only trials where 1) the saccade was initiated within the fixation window and its distance exceeded 1/3 of the initial target amplitude and 2) the primary saccade end point was within the average eye position ± 2 SD were analyzed (on average >76% trials). These remaining trials were included in the analyses of both perceptual response and saccadic eye-movement data.
We determined the percent gain of the mean saccade amplitude for all target amplitudes and directions (Fig. 3D). This value was the ratio (scaled by 100) between the mean saccade amplitude and the initial target amplitude. A percentage >100% signified that the mean saccade amplitude exceeded the target amplitude (overshoot); a percentage <100% signified that the mean saccade amplitude fell short of the target amplitude (undershoot). The saccade error (Fig. 4) was the difference between the original target location and the primary saccade end point. Positive saccade errors represent hypometric movements (the target was in front of the saccade end point). The visual error was the difference between the eye position at the time of target reappearance and the shifted target location (Fig. 5).
Fig. 3.
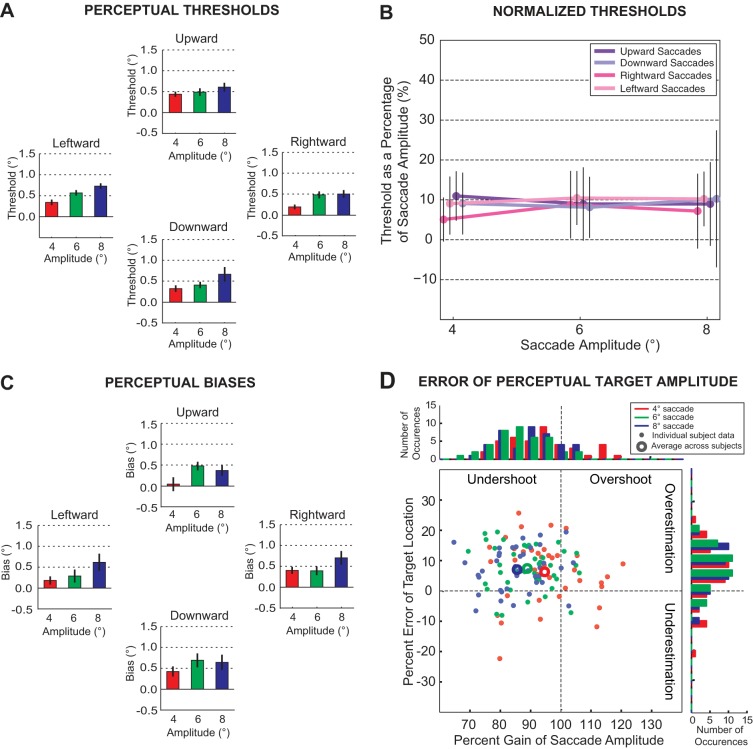
Perceptual performance for all subjects. A: perceptual threshold. Bar graphs represent the means and standard errors for perceptual threshold across subjects for all target amplitudes and directions (red: 4°, green: 6°, and blue: 8°). Saccade direction had no significant effect on threshold; however, perceptual threshold for all directions increased significantly with target amplitude. B: normalized threshold for each direction plotted against target amplitude. We derived normalized thresholds by scaling the perceptual threshold as a percentage of the mean primary saccade amplitude for each amplitude and direction. Mean normalized thresholds were ∼10% of movement amplitude. C: perceptual bias. Bar graphs represent the means and standard errors for perceptual bias (amount of displacement at chance level) across subjects for all target amplitudes and directions. D: percent gain of saccade amplitude and percent error estimation of target location. The percent error in the estimated target location is plotted as a function of percent gain in the saccade amplitude for all saccade amplitudes and directions. Horizontal and vertical movements are combined for each amplitude, and the larger symbols represent the respective mean percent gains in saccade amplitude and mean percent errors in target location estimation. The histograms to the right and above the plot are the respective distributions of the percent error and the percent gain.
Fig. 4.
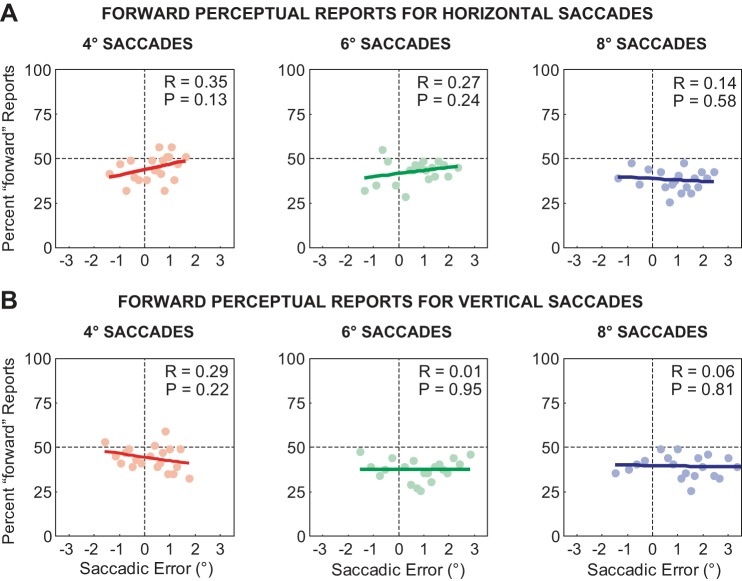
Relationship between saccade error and perceptual decision. The saccade error was the difference between the original target location and the eye position after the saccade. Positive saccade errors represent hypometric movements (the target was in front of the saccade end point). The percentage of forward perceptual reports is plotted as a function of saccade error for horizontal (A) and vertical (B) saccades. Each column displays the results for saccades to the 4°, 6°, and 8° targets. The regression line is represented by the thick colored trace and the respective correlation coefficient, and significance values are displayed in the top right of each plot.
Fig. 5.
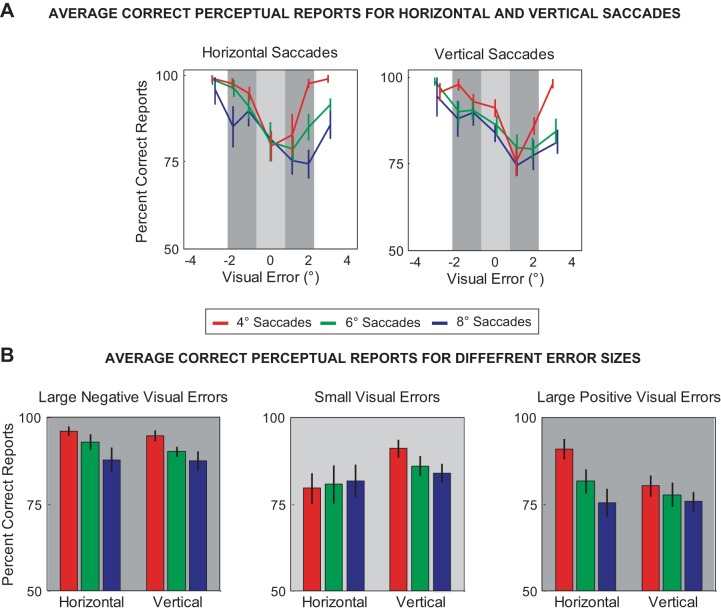
Visual error and perceptual judgment of shifted targets. The visual error with respect to the shifted target was quantified by taking the difference between the eye position at the time of target reappearance and the location of the shifted target. A: percentage of correct perceptual reports is plotted as a function of visual error for horizontal and vertical saccades for each target amplitude (red: 4°, green: 6°, and blue: 8°). B: bar graphs for the average percentage of correct reports for large negative (−2.25° to −0.75°), small (−0.75° to 0.75°), and large positive visual errors (0.75° to 2.25°). Error bars show SE.
Psychometric curves.
Keyboard responses indicating the subject's perception of the target shift were plotted as a psychometric function for each individual subject. As in our previous work (Joiner et al. 2013), the percentage of forward responses was plotted as a function of target shift. The data were then fitted with a cumulative Gaussian distribution as shown by the sample curves in Fig. 1C. From these curves, the perceptual bias and threshold were derived. The perceptual bias was taken as the displacement from 0 at the point where the percentage of forward responses was equal to 50%. The bias is the perceptual null location, the point where the forward and backward judgments occur with equal frequency. We take this point as the estimation of the postsaccadic judgment of the location of the presaccadic target. A positive bias indicated that the target location was perceived to be ahead of the actual target position; a negative bias indicated that the target location was perceived to be behind the actual position. We quantified the difference between the perceptual estimate (i.e., the bias) and the actual target location as a percent error of target location, the bias divided by the initial target amplitude scaled by 100 (Fig. 3D). For example, a perceptual bias of 0.5° for a 4° target location is a 12.5% error in target location (a 12.5% overestimation of the target location). Thus a percentage above 0 signified that the perceptual estimate exceeded the target amplitude (overestimation); a negative percentage signified that the perceptual estimate was lower than the target amplitude (underestimation).
The difference in shift size between the 50 and 75% points on the psychometric curve represented the perceptual threshold. This measure quantified the ability to perceive the target displacement; larger thresholds or smaller slopes of the psychometric function represented an increase in difficulty for accurately perceiving the target shift (Fig. 1C).
Statistical analysis.
The experimental data were not significantly different from a normal distribution (Lilliefors test, P > 0.06 in all cases). Statistical significance of multiple effects such as target eccentricity (4°, 6°, and 8°) and saccade direction (rightward, leftward, upward, and downward) on perceptual threshold and bias was determined by an ANOVA test. For significance, two-tailed t-tests and paired t-tests were used to compare results to set values or between conditions, respectively. All statistical analyses were performed using MATLAB and SPSS software (IBM SPSS Statistics, Version 20.0; IBM, Armonk, NY). For all tests, the significance level was 0.05.
RESULTS
Perceptual performance.
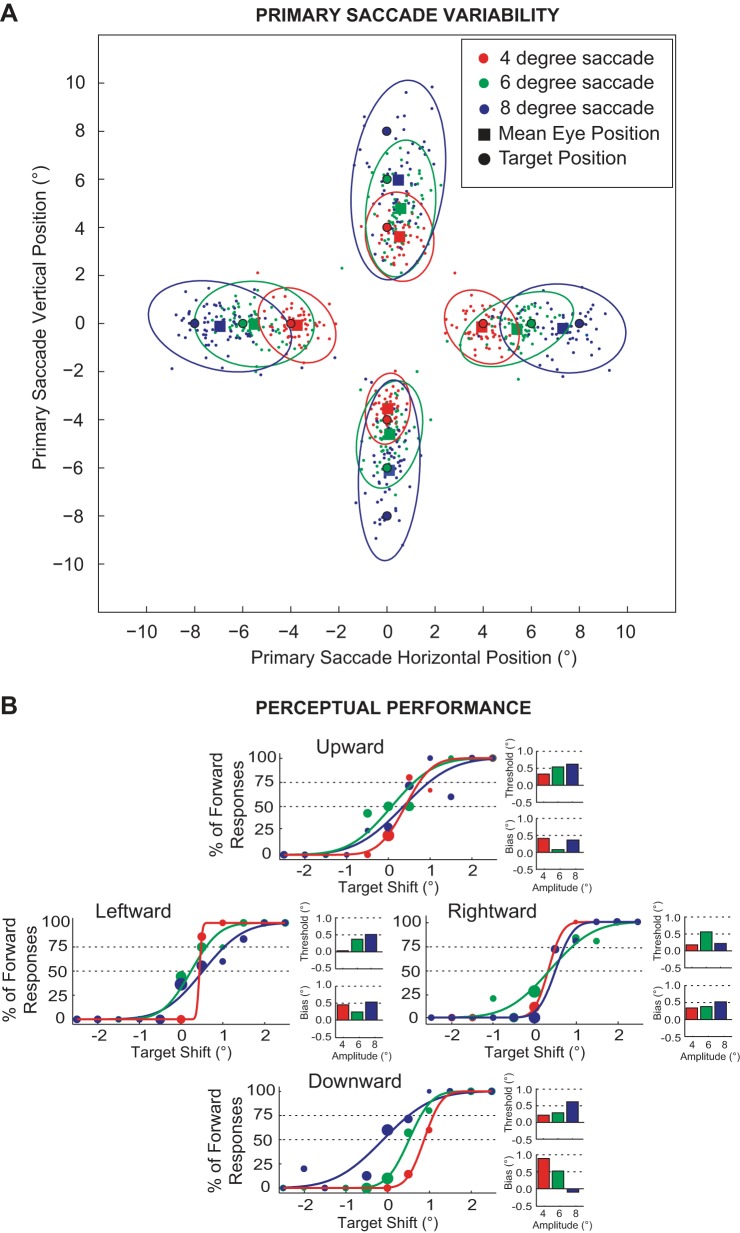
We examined the perceptual detection of transsaccadic changes in target location as subjects made eye movements to targets that required saccades of various amplitudes (4°, 6°, and 8°) and directions (horizontal and vertical). Unlike previous studies, we concentrated on this detection when the target shift followed a blank period, rather than a comparison of performance with and without the blank period, and the accompanying saccadic suppression of target displacement (see materials and methods). The primary saccade end points during this target-displacement detection task for a sample subject are presented in Fig. 2A. The colored small circles are the end points for individual saccades, and the ellipses represent 95% confidence intervals around these end points for each amplitude and direction. The filled circles and squares represent the target positions and mean saccade end points, respectively. Consistent with previous reports, saccade variability (Abrams et al. 1989; Leigh and Zee 2006; van Beers 2007) and undershoot (Collewijn et al. 1988; de Bie et al. 1987; Leigh and Zee 2006) scaled with saccade amplitude; both the size of the ellipses and the distance between the colored squares and respective circles scale with movement amplitude.
Fig. 2.
Perceptual performance and primary saccade eye positions for 1 subject. A: primary saccade variability. The primary saccade horizontal and vertical positions, along with the mean saccade amplitude (solid squares), are displayed. Colored filled circles represent the end points for individual saccades, and the ellipses represent 95% confidence intervals for each target amplitude (red: 4°, green: 6°, and blue: 8°) and direction (upward, downward, leftward, and rightward). B: perceptual performance measures. For each direction (upward, downward, leftward, and rightward), 3 psychometric functions (red: 4°, green: 6°, and blue: 8°) are shown with their respective perceptual threshold and bias measurements.
The psychometric curves and corresponding threshold and bias measurements for the same sample subject are shown in Fig. 2B. The functions are derived from the percentage of reported forward responses for the target-shift direction (see materials and methods). For all saccade directions, the slope of the psychometric functions largely decreased with the increase in movement amplitude (3 of the 4 movement directions), corresponding to an increase in the perceptual thresholds (Fig. 2B). Thus the subject had increasing difficulty distinguishing the direction of change in the target location, as the change occurred further from the initial fixation. Additionally, the bias (the postsaccadic target shift that resulted in 50% forward responses) was largely positive (11 out of the 12 cases, 91%) for all amplitudes and directions, indicating a mismatch between the estimated post- and presaccadic target location. Specifically, these positive bias estimates are compatible with a CD signal whose gain was <1 (subjects expected more undershoot than they observed postsaccadically).
The average perceptual threshold and bias measures across all subjects are summarized in Fig. 3, A and C, respectively. Note that all averages are above 0 and have low standard errors (≤0.22° in all cases), revealing that, despite the intersubject variability, behavior was sufficiently similar across subjects. Threshold measurements were significantly different between saccade amplitudes, but the thresholds were not significantly different between saccade directions (2-way ANOVA, P < 0.01 for the main effect of saccade amplitude and P = 0.73 for the main effect of saccade direction). Thus, similar to the sample subject, the difficulty in shift detection increased with saccade amplitude. To determine whether there was a systematic relationship between saccade amplitude and threshold, we compared the normalized threshold (Fig. 3B). For each subject, we scaled each threshold by that subject's mean saccade amplitude, in this case defining the threshold as a percentage of the movement length. We observed that these normalized thresholds were approximately constant across all movement directions and amplitudes. The normalized threshold was not significantly different across saccade amplitude or direction (2-way ANOVA, P = 0.947 for the main effect of saccade amplitude and P = 0.120 for saccade direction). Consistent with previous studies of horizontal movements, the threshold was ∼10% of the movement amplitude for all directions and amplitudes tested (Li and Matin 1990, 1997).
Similar to the threshold, the perceptual bias was significantly different between saccade amplitudes, but the bias was not significantly different between saccade directions (2-way ANOVA, P < 0.01 for the main effect of saccade amplitude and P = 0.88 for the main effect of saccade direction). This was true even though there were significant differences in the accuracy of the saccades; saccades to all targets generally fell short of the target with an increase in end point error with amplitude (1-way ANOVA, P = 0.03). The size of this error was significantly larger for saccade targets in the vertical plane than for the horizontal plane (paired t-test, P < 0.001). This is demonstrated by the data from the sample subject presented in Fig. 2. The mean saccade end points (colored squares) were closer to the saccade targets (colored circles) for the horizontal movements, especially at the larger amplitudes. However, the bias for this subject was generally positive for all movement directions and amplitudes. This was also true at the group level (Fig. 3C); when the saccade targets were not shifted (shift of 0°), subjects generally perceived the target as moving backward against the direction of the saccade (reporting a forward shift on <50% of the trials). Targets shifted a small distance in the direction of the saccade (generally <0.5°) were perceived as stationary (reporting a forward shift on ∼50% of the trials). This again indicates that overall the gain of the saccade CD was <1.
To examine the relationship between the perceptual bias and saccade metrics, we compared the percent error in the target location and the percent gain in the saccade amplitude. In Fig. 3D, we plot the percent error of the estimated target location (>0 overestimation, <0 underestimation) as a function of percent gain in the mean saccade amplitude (>100% overshoot, <100% undershoot) for all saccade amplitudes and directions. The former is the perceptual bias as a percentage of the target amplitude. The latter is the saccade amplitude as a percentage of the required movement amplitude. The histograms to the right and above are the respective distributions of the percent error and the percent gain, respectively. The mean percent error of the estimated target location was significantly <0 for all saccade directions and amplitudes (P < 0.001 for each amplitude and direction, 12 comparisons, 2-tailed t-test), but this percentage was not different between horizontal and vertical saccades for each amplitude (P > 0.09 for all 3 comparisons, paired t-test). Thus we combined the horizontal and vertical movements to specifically examine differences across movement amplitude. As shown in the figure, the majority of movements (100 out of 120 cases, 83%) undershot the target location (mean percent gain of 94.6 ± 3.8%, 88.7 ± 3.2%, and 85.4 ± 3.1% for 4 o, 6°, and 8°, respectively). However, despite regularly making movements less than the required target amplitude, subjects largely (99 out of 120 cases, 82%) overestimated the target location (mean percent errors of 6.5 ± 3.4%, 7.7 ± 2.4%, and 7.3 ± 2.3% for 4o, 6°, and 8°, respectively). Note that the mean percent error of the estimated target location is significantly >0 for all amplitudes (P < 0.01 for all three cases, 2-tailed t-test), but the percent gain in the mean saccade amplitude is significantly <100% (P < 0.01 for all 3 cases, 2-tailed t-test). As above, this again demonstrates that overall subjects undershot the target amplitude with a CD gain <1.
In summary, our results for all four cardinal directions were comparable to previous reports that examined horizontal saccades in human subjects (Collins et al. 2009; Deubel et al. 1996; Ostendorf et al. 2010) and monkeys (Joiner et al. 2013). The present results indicate that the ability to detect visual changes that occurred during the saccade proportionally increased in difficulty with movement amplitude. This difficulty appears to be a systematic function of the saccade amplitude, with the threshold of detection ∼10% of the movement amplitude for all directions. In addition, subjects made saccades that fell short of the initial target location but frequently overestimated this location, demonstrating a positive perceptual bias for targets in different directions and amplitudes.
Influence of saccade and visual errors on perceptual judgments.
As shown in Fig. 2A, there was movement-to-movement variability in the saccade end points that scaled with movement amplitude. We were interested in the influence, if any, of these movement amplitude fluctuations on perceptual performance. Previous work (Collins et al. 2009; Joiner et al. 2013; Ostendorf et al. 2010) has shown that the perception of transsaccadic target displacement is independent of variations in the saccade amplitude. This suggests that the CD signal of the saccade accounts for the inherent movement-to-movement variability. Here, we were interested in determining whether this independence was consistent across different movement directions, and more importantly, different saccade amplitudes attributable to the correlated changes in movement variability. Figure 4 shows the relationship between perceptual performance (the percentage of forward perceptual reports) and the saccade error (the difference between the original target location and the primary saccade end point). Positive saccade errors indicate that the target was in front of the saccade (hypometric saccade); negative values indicate that the target was behind the saccade (hypermetric saccade). As in our previous study (Joiner et al. 2013), we binned the saccade errors into 20 bins of equal size and then calculated the percentage of forward reports within each bin. In agreement with previous work (Collins et al. 2009; Joiner et al. 2013; Ostendorf et al. 2010), we found no significant relationship between perceptual judgments and saccade variability (P ≥ 0.13 in all cases). Importantly, this was true for saccades to targets located at different directions and amplitudes. Thus, although there was an increase in saccade variability with movement amplitude, perceptual performance remained independent of variations in the saccade amplitude.
The movement-to-movement fluctuations of the saccade amplitude and the random shift of the target (both in magnitude and direction) resulted in various visual errors at target reappearance that subjects could potentially use to guide the perceptual report. We therefore examined the influence of these visual errors on perceptual performance. The visual error was quantified by determining the vector between the eye position at the time of target reappearance and the shifted target location. Figure 5A plots the percentage of correct perceptual reports as a function of visual error for horizontal and vertical saccades. For each subject, we sorted the visual error within bins ∼1.25° wide and determined the average percentage of correct responses across subjects. In most cases, the percentage of correct responses for both horizontal and vertical saccades was greatest for the largest visual errors (≥±2.5°) but declined as the visual error decreased. Figure 5B displays the average percentage of correct perceptual reports across subjects for small (≤±0.75°) and large visual errors (negative: <−0.75°, positive: >0.75°) for the different saccade directions and amplitudes. As shown in the figure, the percentage of correct responses was significantly greater than chance level (50%, P < 0.01 in all cases), even for the small visual error range (≤±0.75°). For large errors (<−0.75° and >0.75°), we found a significant difference between movement amplitudes but not between movement directions (2-way ANOVA, P < 0.01 for the main effect of saccade amplitude and P ≥ 0.07 for the main effect of saccade direction, for both cases). That is, for the same error range, perceptual performance decreased with amplitude, but this decrease was similar for horizontal and vertical saccades. In contrast, for small visual errors between ±0.75°, we did not find a significant difference between movement amplitudes, but perceptual performance was significantly different between movement directions (2-way ANOVA, P = 0.79 for the main effect of saccade amplitude and P = 0.04 for the main effect of saccade direction). In other words, the perceptual response tended to be more accurate for vertical movements when subjects experienced these small visual errors, but there was not a consistent change in performance across movement amplitudes.
It should be noted that any significant difference in performance between movement directions and amplitudes for the different magnitudes of visual error reported above is nominal compared with the overall ability of subjects to report the correct shift direction (>75% in all cases). This overall similarity in performance makes it difficult to distinguish the contribution of the CD signal to the perceptual report, especially for large visual errors that likely provide a noticeable cue of the correct shift direction. A more revealing analysis of the CD accuracy is provided by examining the perceptual performance when small visual errors were in the direction opposite the shift direction. That is, we examined performance of subjects on trials when the correct perceptual response was not aligned with the limited visual information (visual errors ≤±0.75°). We found on this subset of trials that subjects were still able to report the correct shift direction significantly greater than chance level (>70% correct perceptual reports of for all amplitudes and directions, P < 0.01). Thus these results for visual errors suggest that subjects were not solely relying on retinal information to form the perceptual judgment and that the extraretinal information of the saccade provided by the CD is a highly accurate representation of the movement vector.
DISCUSSION
Understanding how humans perceive transsaccadic displacements of objects in the visual scene provides insights into the neural mechanisms that facilitate stable visual perception. In this study, we employed a forced choice task to quantify the effects of saccade amplitude and direction on the perception of transsaccadic changes in the saccade target location. We assessed two characteristics of perceptual performance, the perceptual bias (the postsaccadic estimate of the target location) and the perceptual threshold (the amount of target shift at which detection rose sufficiently above chance level). We found that the perceptual threshold 1) scaled with saccade amplitude with no significant main effect of direction and 2) was ∼10% of the saccade amplitude across the different saccade metrics studied. In addition, on the basis of the bias measures, we show that subjects mostly overestimated the target position despite the saccades generally falling short of the target amplitude. Finally, we examined the role of the saccade and visual errors on the ability to make a correct perceptual report of the transsaccadic target displacement. Our results show that 1) perception was independent of the saccade variability for all movement directions and amplitudes tested and 2) subjects were able to detect the target shift both when the visual error was in agreement and opposite to the target-shift direction at a level significantly higher than chance. Taken together, our experimental data suggest that the compensation for saccade-induced disruptions to visual input is a process that 1) has an uncertainty proportional to the magnitude of the motor signal and 2) is comparable for movements with different metrics.
Threshold linked to saccade generation.
The systematic increase in threshold with saccade amplitude is in line with several previous human and monkey visual perception studies. Li and Matin (1990, 1997) showed that the threshold for transsaccadic displacements in the direction of the saccade increased linearly with target amplitude for horizontal saccades ranging from 4° to 12°, Recently, Joiner et al. (2013) reported a similar increase in threshold over a comparable range of horizontal target amplitudes (8° to 16°) in monkeys. What does this systematic increase in perceptual threshold reveal about the underlying neural mechanisms? Recall that the threshold is the displacement amount required to perceive the target jump direction at a percentage greater than chance level. This amount should increase with any rise in noise associated with the decision process; i.e., a larger signal (target shift) is required to overcome any additional noise obscuring the detection of a target shift. A process with noise that increases in near constant proportion with the movement amplitude (signal-dependent noise, SDN) should demonstrate an approximately constant normalized relationship with the movement amplitude (a constant coefficient of variation). The finding that there is an approximate constant threshold percentage of 10% across the saccade amplitudes studied may reflect the SDN previously demonstrated for saccade generation (Goossens and van Opstal 2012). As shown in Fig. 2A, previous studies that examined the spatial variability of saccade end points demonstrated that, as the amplitude increased, there was an accompanying increase in motor command variance, reflected as an increase in saccade end point scatter (Abrams et al. 1989; Leigh and Zee 2006; van Beers 2007; van Opstal and van Gisbergen 1989). This reflects that the neural variability associated with the saccade motor command is linearly proportional to the mean command magnitude (SDN), as supported by several behavioral, physiological, and theoretical studies (Goossens and van Opstal 2012; Harris and Wolpert 1998, 2006; Hu et al. 2007; van Beers 2007, 2008). Thus the observed changes in perceptual threshold with amplitude may reflect the corresponding SDN associated with the respective motor commands; perceptual decisions based on the CD representation of increasingly noisy motor commands require larger external changes (target shifts) to form consistent judgments, but this increase is in near constant proportion to the original motor command.
Our finding that the thresholds for target-displacement detection were similar across movement directions is in agreement with our previous study that showed that subjects made accurate motor adjustments based on the CD of horizontal, vertical, and oblique saccades (Joiner et al. 2010). Although general performance in this paradigm was comparable between the two movement directions, the second saccade compensation in the double-step task following a vertical saccade was significantly less than that following a horizontal movement. This implied that the movement information conveyed by the CD of vertical movements was generally less accurate. On the basis of this finding and the different neural origins for the motor commands and presumably the CD for horizontal and vertical saccades (Leigh and Zee 2006), we initially hypothesized that transsaccadic perceptual performance would also be distinct when reliant on the CD of vertical saccades. Although we found no significant difference in the perceptual measures between the two movement directions, there are several differences between the two studies that may partially explain this inconsistency. First, although both tasks assess CD, there is a significant difference in the task requirements. In the double-step task, the subject must update the movement plan based on the CD information of the initial movement. In the perceptual task utilized here, the subject must make a perceptual judgment based on the CD of the initial movement. Thus, although the tasks may utilize the same internal CD signal, the task demands (motor planning vs. perceptual evaluation) require that this information be utilized differently. Thus the subsequent neural processes that use this CD information in each respective paradigm may negate any direct comparisons between tasks. Second, in the shift-detection task, the perceptual decision is made for foveal information, whereas, in the double-step task, the CD is used to make a movement to a peripheral goal. One possible method to reconcile this difference between tasks is to conduct a perceptual task during which the transsaccadic perceptual judgment is made for a peripheral target rather than at the fovea. This may reveal perceptual differences between the horizontal and vertical CD similar to that previously demonstrated for movement planning (Joiner et al. 2010).
Gain of the saccade CD and possible effects on the perception of visual space.
Our assessment of perceptual bias suggests that subjects misjudged the postsaccadic location of the target, often reported as a higher amplitude than the presaccadic distance. This overestimation was 1) present despite the saccades undershooting the saccade target and 2) similar across the three saccade amplitudes studied. Previous reports of target-shift detection with a postsaccade blank period have also shown positive bias measures from fitted psychometric curves (Collins et al. 2009; Ostendorf et al. 2010; Wexler and Collins 2014). A positive bias suggests that the gain of the saccade CD is <1. For example, when the targets were not shifted (shift of 0°), subjects generally perceived the target as moving opposite the movement direction. Thus, on the basis of the CD, subjects expected a greater undershoot than that experienced; a small forward shift is necessary for the subject to perceive that the target is stationary. Additionally, we found no significant relationship between the mean saccade gain and the perceptual bias (P > 0.06 in all cases), suggesting that the accuracy of the CD is not dependent on the accuracy of the saccade.
Although we did not assess the estimated target location during the initial fixation period, the positive perceptual bias exhibited by our subjects could also be interpreted as an expansion of visual space. That is, if there was an accurate initial estimation of the target location, the exhibited positive bias suggests that this estimated distance increased following the saccade. Such an expansion would be in disagreement with other transsaccadic perceptual studies that have shown compression of visual space at the saccade goal; flashed stimuli around the movement goal are perceived closer to the saccade end point than the true distance (Kaiser and Lappe 2004; Lappe et al. 2000; Morrone et al. 1997; Ross et al. 1997). This is true even for adapted saccades; perceptual localization of stimuli is influenced by the movement adaptation (Awater et al. 2005; Bruno and Morrone 2007; Collins et al. 2007; Zimmermann and Lappe 2010). Several differences in the experimental paradigms and how perception is assessed may account for the discrepancy with previous reports. First is the strong role saccadic suppression plays in the perception of visual space. The perceptual decision in the present study was made after the reappearance of the saccade target following a 250-ms blank period initiated at saccade onset, effectively reducing the saccadic suppression of displacement. Previous comparisons of target location with and without this blank period have shown differences in the postsaccadic estimate of the target location (Joiner et al. 2013; Wexler and Collins 2014). Therefore, the effect of saccadic suppression may prevent a direct comparison of perceptual reports between studies utilizing different types of postsaccadic presentations. Second, as described above, diverse visual stimuli have been used to probe perception during saccades. Unlike the present study during which the saccade goal is displaced during the movement, other studies examined the perceived location of flashed stimuli at different peripheral locations around the saccade goal (Awater et al. 2005; Bruno and Morrone 2007; Lappe et al. 2000; Ross et al. 1997). This difference, foveal vs. peripheral stimuli, likely contributes to the perceptual differences across studies. Finally, there are important variations in how the perceptual reports are given (Bruno and Morrone 2007). For example, in some studies, subjects reported the stimulus location following a saccade by placing a marker where they perceive the stimulus to be located, an unrestricted choice (Atsma et al. 2014; Honda 1989; Kaiser and Lappe 2004; Lappe et al. 2000). In contrast, other studies required a manual response or verbal report of the flashed location between restricted choices (Collins et al. 2009; Joiner et al. 2013; Ostendorf et al. 2010; Ross et al. 1997; Wexler and Collins 2014; Zimmermann et al. 2013). Despite the differences described above, most studies agree that saccades result in small but significant perceptual deviations of stimulus locations. However, it would be valuable to 1) obtain an initial estimation of the target location for the same experimental conditions of the present study to accurately quantify the effects on visual space perception and 2) quantify the extent that deviations in perceptual results are influenced by the experimental techniques used for assessment to directly compare behavior across studies.
Saccade and visual errors.
The saccades made by the subjects in our study were largely hypometric, often falling short (undershooting) of the desired target amplitude. As demonstrated previously, both the amount of movement undershoot and the saccadic end point variability scaled with movement amplitude (Abrams et al. 1989; Collewijn et al. 1988; de Bie et al. 1987; Leigh and Zee 2006; van Beers 2007; van Opstal and van Gisbergen 1989). Despite these mismatches between the saccade end point and target, the perceptual judgment of displaced target direction was largely accurate (thresholds typically <0.5°). In addition, we found that subjects demonstrated little relation between the error of the first saccade and the perception of the target location (Fig. 4), in agreement with previous studies (Collins et al. 2009; Joiner et al. 2013; Ostendorf et al. 2010). Importantly, this was true across saccade amplitudes, suggesting that the associated increase in the inherent movement variability was incorporated into the CD signal of the saccade on a movement-by-movement basis.
The results presented in Fig. 4 suggest that subjects did not use the movement end point to judge the target displacement direction. However, there was also a visual error between the saccadic end point and the reappearing shifted target that subjects could also utilize on which to base their decision. Thus the movement variability described above in combination with the unpredictability of the target shift allowed us to examine the influence of the postsaccadic visual error on perception. Although there were variations in performance for different directions and magnitudes of visual error, subjects reported the correct direction of the transsaccadic target shift significantly greater than chance level (>75% correct perceptual reports) for all saccade directions and amplitudes (Fig. 5). We found that this was true for small visual errors (≤±0.75°), for which the report would likely be a guess if totally reliant on such a limited amount of visual information. In addition, when these small errors were opposite the true shift direction (the shift direction and visual error were misaligned), performance was also significantly higher than the level expected when purely based on the visual error. This again suggests that subjects used the CD of the saccade to make the perceptual judgment rather than the visual displacement observed at target reappearance and that the CD provides a dependable and accurate representation of the saccade movement vector.
Conclusion.
Internal information on the metrics of impending saccades likely plays a critical role in the perception of a stable world. Transsaccadic perceptual tasks allow an assessment of the neural processes that compensate for the saccade-induced disruptions to visual input and the CD signals hypothesized to facilitate these mechanisms. In the present study, we report behavioral results that suggest that these processes are uniformly applied for different movement metrics (amplitudes and directions) and consistently independent of saccade variability. In addition, performance was significantly more accurate than that allowed by a simple guessing strategy and similar across different movement directions for the same magnitudes of visual error, even when the visual information was in conflict with the true environmental change. Future studies that systematically examine the combined effect of different saccade (horizontal, oblique, and vertical) and visual change directions (on and off axis) on perception may allow an estimation of the perceptual sensitivity field of these compensatory mechanisms and offer a more complete characterization of the underlying neural signals.
GRANTS
This work was supported by a grant from the National Eye Institute (R00 EY021252) to W. M. Joiner.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.B. performed experiments; S.B., L.C.J.B., and W.M.J. analyzed data; S.B., L.C.J.B., M.S.P., and W.M.J. interpreted results of experiments; S.B., L.C.J.B., and W.M.J. prepared figures; S.B., L.C.J.B., and W.M.J. drafted manuscript; S.B., L.C.J.B., M.S.P., and W.M.J. edited and revised manuscript; S.B., L.C.J.B., M.S.P., and W.M.J. approved final version of manuscript; W.M.J. conception and design of research.
ACKNOWLEDGMENTS
We are grateful for the advice and discussions with our colleagues Christian Quaia and Edmond Fitzgibbon.
REFERENCES
- Abrams RA, Meyer DE, Kornblum S. Speed and accuracy of saccadic eye movements: characteristics of impulse variability in the oculomotor system. J Exp Psychol Hum Percept Perform 15: 529–543, 1989. [DOI] [PubMed] [Google Scholar]
- Atsma J, Maij F, Corneil BD, Medendorp WP. No perisaccadic mislocalization with abruptly cancelled saccades. J Neurosci 34: 5497–504, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awater H, Burr D, Lappe M, Morrone MC, Goldberg ME. Effect of saccadic adaptation on localization of visual targets. J Neurophysiol 93: 3605–3614, 2005. [DOI] [PubMed] [Google Scholar]
- Bays PM, Husain M. Spatial remapping of the visual world across saccades. Neuroreport 18: 1207–1213, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellebaum C, Daum I, Koch B, Schwarz M, Hoffmann KP. The role of the human thalamus in processing corollary discharge. Brain 128: 1139–1154, 2005. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Hoffmann KP, Koch B, Schwarz M, Daum I. Altered processing of corollary discharge in thalamic lesion patients. Eur J Neurosci 24: 2375–2388, 2006. [DOI] [PubMed] [Google Scholar]
- Bridgeman B, Hendry D, Stark L. Failure to detect displacement of the visual world during saccadic eye movements. Vision Res 15: 719–722, 1975. [DOI] [PubMed] [Google Scholar]
- Bruno A, Morrone MC. Influence of saccadic adaptation on spatial localization: Comparison of verbal and pointing reports. J Vis 7: 1–13, 2007. [DOI] [PubMed] [Google Scholar]
- Collewijn H, Erkelens CJ, Steinman RM. Binocular co-ordination of human horizontal saccadic eye movements. J Physiol 404: 157–182, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Doré-Mazars K, Lappe M. Motor space structures perceptual space: Evidence from human saccadic adaptation. Brain Res 1172: 32–39, 2007. [DOI] [PubMed] [Google Scholar]
- Collins T, Rolfs M, Deubel H, Cavanagh P. Post-saccadic location judgments reveal remapping of saccade targets to non-foveal locations. J Vis 9: 29.1–29.9, 2009. [DOI] [PubMed] [Google Scholar]
- de Bie J, van den Brink G, van Sonderen JF. The systematic undershoot of saccades: A localization or oculomotor phenomenon? In: Eye Movements: From Physiology to Cognition, edited by O'Regan JK, Levy-Schoen A. New York, NY: Elsevier, 1987, pp. 85–94. [Google Scholar]
- Deubel H, Schneider WX, Bridgeman B. Post saccadic target blanking prevents saccadic suppression of image displacement. Vision Res 36: 985–996, 1996. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX, Bridgeman B. Trans-saccadic memory of position and form. Prog Brain Res 140: 165–180, 2002. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science 255: 90–92, 1992. [DOI] [PubMed] [Google Scholar]
- Goossens HH, van Opstal AJ. Optimal control of saccades by spatial-temporal activity patterns in the monkey superior colliculus. PLoS Comput Biol 8: 1–18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature 391: 481–484, 1998. [DOI] [PubMed] [Google Scholar]
- Guthrie BL, Porter JD, Sparks DL. Corollary discharge provides accurate eye position information to the oculomotor system. Science 221: 1193–1195, 1983. [DOI] [PubMed] [Google Scholar]
- Hall NJ, Colby CL. Remapping for visual stability. Philos Trans R Soc Lond B Biol Sci 366: 528–539, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PE, Lightstone AD. Saccadic eye movements to flashed targets. Vision Res 16: 107–114, 1976. [DOI] [PubMed] [Google Scholar]
- Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature 394: 780–784, 1998. [DOI] [PubMed] [Google Scholar]
- Harris CM, Wolpert DM. The main sequence of saccades optimizes speed-accuracy trade-off. Biol Cybern 95: 21–29, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H. Perceptual localization of visual stimuli flashed during saccades. Percept Psychophys 45: 162–174, 1989. [DOI] [PubMed] [Google Scholar]
- Hu X, Jiang H, Gu C, Li C, Sparks DL. Reliability of oculomotor command signals carried by individual neurons. Proc Natl Acad Sci USA 104: 8137–8142, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WM, FitzGibbon EJ, Wurtz RH. Amplitudes and directions of individual saccades can be adjusted by corollary discharge. J Vis 10: 1–12, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WM, Cavanaugh J, FitzGibbon EJ, Wurtz RH. Corollary discharge contributes to perceived eye location in monkeys. J Neurophysiol 110: 2402–2413, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M, Lappe M. Perisaccadic mislocalization orthogonal to saccade direction. Neuron 41: 293–300, 2004. [DOI] [PubMed] [Google Scholar]
- Lappe M, Awater H, Krekelberg B. Postsaccadic visual references generate presaccadic compression of space. Nature 403: 892–895, 2000. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The Neurology of Eye Movements, 4th ed. New York, NY: Oxford University, 2006. [Google Scholar]
- Lewis RF, Zee DS, Hayman MR, Tamargo RJ. Oculomotor function in the rhesus monkey after deafferentation of the extraocular muscles. Exp Brain Res 141: 349–358, 2001. [DOI] [PubMed] [Google Scholar]
- Li W, Matin L. The influence of saccade length on the saccadic suppression of displacement detection. Percept Psychophys 48: 453–458, 1990. [DOI] [PubMed] [Google Scholar]
- Li W, Matin L. Saccadic suppression of displacement: separate influences of saccade size and of target retinal eccentricity. Vision Res 37: 1779–1797, 1997. [DOI] [PubMed] [Google Scholar]
- Melcher D, Colby CL. Trans-saccadic perception. Trends Cogn Sci 12: 466–473, 2008. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Ross J, Burr DC. Apparent position of visual targets during real and simulated saccadic eye movements. J Neurosci 17: 7941–7953, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munuera J, Morel P, Duhamel JR, Deneve S. Optimal sensorimotor control in eye movement sequences. J Neurosci 29: 3026–3035, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Colby CL. Updating of the visual representation in monkey striate and extrastriate cortex during saccades. Proc Natl Acad Sci USA 99: 4026–4031, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeier M, Crawford JD, Tweed DB. Optimal transsaccadic integration explains distorted spatial perception. Nature 422: 76–80, 2003. [DOI] [PubMed] [Google Scholar]
- Niemeier M, Crawford JD, Tweed DB. Optimal inference explains dimension-specific contractions of spatial perception. Exp Brain Res 179: 313–323, 2007. [DOI] [PubMed] [Google Scholar]
- Ostendorf F, Liebermann D, Ploner CJ. Human thalamus contributes to perceptual stability across eye movements. Philos Trans R Soc Lond B Biol Sci 107: 1229–1234, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MS, Wong JH. Were you paying attention to where you looked? The role of executive working memory in visual search. Psychon Bull Rev 15: 372–377, 2008. [DOI] [PubMed] [Google Scholar]
- Poletti M, Burr DC, Rucci M. Optimal multimodal integration in spatial localization. J Neurosci 33: 14259–14268, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaia C, Joiner WM, FitzGibbon EJ, Optican LM, Smith MA. Eye movement sequence generation in humans: motor or goal updating? J Vis 10: 1–31, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Burr DC. Compression of visual space before saccades. Nature 386: 598–601, 1997. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science 296: 1480–1482, 2002. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol 91: 1381–1402, 2004a. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. II. Role of the SCMDFEF pathway in corollary discharge. J Neurophysiol 91: 1403–1423, 2004b. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature 444: 374–377, 2006. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol 43: 482–489,1950. [DOI] [PubMed] [Google Scholar]
- van Beers RJ. The sources of variability in saccadic eye movements. J Neurosci 27: 8757–8770, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers RJ. Saccadic eye movements minimize the consequences of motor noise. PLoS One 3: 1–8, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Opstal AJ, van Gisbergen JAM. Scatter in the metrics of saccades and properties of the collicular motor map. Vision Res 29: 1183–1196, 1989. [DOI] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt H. Das reafferenzprinzip. Wechselwirkungen zwischen zentralnervensystem und peripherie. Naturwissenschaften 37: 464–476, 1950. [Google Scholar]
- Wang X, Zhang M, Cohen IS, Goldberg ME. The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nat Neurosci 10: 640–646, 2007. [DOI] [PubMed] [Google Scholar]
- Wexler M, Collins T. Orthogonal steps relieve saccadic suppression. J Vis 14: 1–9, 2014. [DOI] [PubMed] [Google Scholar]
- Wong JH, Peterson MS. The interaction between memorized objects and abrupt onsets in oculomotor capture. Atten Percept Psychophys 73: 1768–1779, 2011. [DOI] [PubMed] [Google Scholar]
- Wurtz RH. Neuronal mechanisms of visual stability. Vision Res 48: 2070–2089, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann E, Lappe M. Motor signals in visual localization. J Vis 10: 1–11, 2010. [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Morrone MC, Burr DC. Spatial position information accumulates steadily over time. J Neurosci 33: 18396–18401, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]