Abstract
The polarization pattern of skylight provides a compass cue that various insect species use for allocentric orientation. In the desert locust, Schistocerca gregaria, a network of neurons tuned to the electric field vector (E-vector) angle of polarized light is present in the central complex of the brain. Preferred E-vector angles vary along slices of neuropils in a compasslike fashion (polarotopy). We studied how the activity in this polarotopic population is modulated in ways suited to control compass-guided locomotion. To this end, we analyzed tuning profiles using measures of correlation between spike rate and E-vector angle and, furthermore, tested for adaptation to stationary angles. The results suggest that the polarotopy is stabilized by antagonistic integration across neurons with opponent tuning. Downstream to the input stage of the network, responses to stationary E-vector angles adapted quickly, which may correlate with a tendency to steer a steady course previously observed in tethered flying locusts. By contrast, rotating E-vectors corresponding to changes in heading direction under a natural sky elicited nonadapting responses. However, response amplitudes were particularly variable at the output stage, covarying with the level of ongoing activity. Moreover, the responses to rotating E-vector angles depended on the direction of rotation in an anticipatory manner. Our observations support a view of the central complex as a substrate of higher-stage processing that could assign contextual meaning to sensory input for motor control in goal-driven behaviors. Parallels to higher-stage processing of sensory information in vertebrates are discussed.
Keywords: insect brain, central complex, E-vector signaling, context dependency
allocentric orientation in space is believed to guide adaptive locomotor behavior in both vertebrates and invertebrates (Frost and Mouritsen 2006; Mouritsen 2001). In particular, a variety of insect central-place foragers and migrants rely on sky-compass signals for spatial learning in local settings or when bridging long distances (Merlin et al. 2011; Mouritsen 2001). A subject's bearing relative to the sun provides a straight compass cue if corrected for time of day, but direct view of the sun is often obscured by clouds or objects in the nearby environment. Hence, the benefit of orientation via sun compass information strongly depends on the capability to conclude the position of the sun from indirect cues such as the sky polarization pattern. The scattering of direct, unpolarized sunlight in the atmosphere (Rayleigh scattering; Strutt 1871a,b) results in linear polarization of light from the blue sky with a pattern of electric field vector angles (E-vector angles) suited to indicate the course of the solar meridian relative to the subject's longitudinal body axis (Fig. 1A). Thus if seen as a pattern of E-vectors across the sky (Bech et al. 2014) or integrated with the chromatic or intensity gradient to distinguish between the solar and antisolar sky hemispheres (Heinze and Reppert 2011; Pfeiffer and Homberg 2007), the celestial E-vector pattern can signal one's bearing relative to the sun. If referred to directly (i.e., neither time-compensated nor integrated with additional cues), it can still serve to keep a steady course over moderate time scales.
Fig. 1.
Celestial compass cues and neural substrates of polarization vision in the locust brain. A: when the sun (yellow) is not visible, its position may still be inferred from the intensity gradient of skylight in conjunction with the pattern of polarization. The latter is related to solar position in that the course of the solar meridian represents a line of symmetry for the pattern of electrical field vectors (black bars), which are arranged in concentric circles around the sun. The degree of polarization (bar thickness) is highest along a circle at 90° angular distance from the sun. B: frontal diagram of the brain of the desert locust. Bilateral pathways of polarization-sensitive neurons from the optic lobes converge onto a polarization-vision network in the central complex. An anterior pathway (red neuropils) connects the dorsal rim area of the lamina and medulla (DRLA and DRME) via the anterior lobe of the lobula (ALO), the anterior optic tubercle (AOTU), and the medial (MBU) and lateral (LBU) bulb to the lower division of the central body (CBL) of the central complex. A parallel pathway (yellow neuropils) originating in the ALO is connected via the AOTU and lateral accessory lobe (LAL) to the upper division of the central body (CBU). The superior medial protocerebrum (SMP) is connected to the CBU via the anterior bundles (AB). Finally, projections from the accessory medulla (AME) extend to the posterior optic tubercle (POTU) and likely target tangential neurons entering the protocerebral bridge (PB; green). CA, calyx of mushroom body; LA, lamina; ME, medulla. Together with the LAL, the MBU and LBU make up the lateral complex (LX). C: basic types of polarization-sensitive neuron of the central complex. Columnar neurons connect distinct slices of the PB to the CBU (CPU neurons) or CBL (CL neurons) of the central body and have additional arborizations in the lateral complexes. Tangential neurons invade many or all slices within the CBL (TL) or the PB (TB). A courtesy of Dr. Keram Pfeiffer, B modified from Pfeiffer and Homberg (2014), and C modified from Müller et al. (1997) and Heinze and Homberg (2007, 2008) with permission.
Evidence from behavioral, anatomic, and physiological approaches suggest that the desert locust, Schistocerca gregaria, relies on the polarization pattern of the blue sky during orientation tasks (Homberg 2004; Homberg et al. 2011). When presented with linearly polarized light from a zenith-centered source, tethered flying locusts strive for polarotactic orientation to the E-vector angle that would correspond to steering a steady bearing to the sun (Mappes and Homberg 2004). In line with this, neural substrates for the E-vector-dependent reception of polarized skylight and a polarotopic representation of its E-vector pattern have been identified in the locust brain (Heinze and Homberg 2007, 2009; Heinze et al. 2009; Vitzthum et al. 2002). Polarization-sensitive dorsal rim areas in both compound eyes connect to pathways of polarization-sensitive interneurons. Some of these neurons show additional tuning to wavelength and azimuthal direction of unpolarized light suited to integrate information from the chromatic gradient to provide an unambiguous compass signal (el Jundi et al. 2014; Pfeiffer and Homberg 2007). These bilateral pathways converge onto a polarization-vision network in the central complex, a set of midline-spanning neuropils in the insect brain known to play a role in motor control and visual spatial orientation (Neuser et al. 2008; Ofstad et al. 2011; Pfeiffer and Homberg 2014; Ritzmann et al. 2012; Triphan et al. 2010; Fig. 1B). Neuropils of the central complex include the lower and upper division of the central body (CBL and CBU, respectively) as well as the protocerebral bridge (PB); polarization-sensitive neurons in the central complex have been categorized according to their branching patterns. Columnar neurons connect distinct slices of the PB to the CBU (CPU neurons) or CBL (CL neurons) of the central body and have additional arborizations in the lateral accessory lobes, the presumed main input and output relays of the network (Heinze and Homberg 2008). Tangential neurons invade many or all slices within the CBL (TL) or the PB (TB). The putative processing hierarchy is TL-CL-TB-CPU (Heinze et al. 2009). Figure 1C illustrates the subtypes TL2, CL1, TB1, CPU1, and CPU2 relevant here. Investigations into the physiology and anatomy of the polarization-vision network in the central complex revealed polarotopic representations of mean preferred E-vector angles that span the width of central-complex neuropils and cover 180° in E-vector angle within each hemisphere of the PB (Heinze and Homberg 2007).
A standard signal for measurement of E-vector tuning is polarized light directed onto the dorsal rim areas, generated by light passing through a steadily rotating polarizer (e.g., Heinze and Reppert 2011; Vitzthum et al. 2002). Under a natural sky, this signal bears an exteroceptive indication of ego-motion: a locust walking or flying under a natural sky will encounter such rapid changes in angle between its longitudinal body axis and the E-vector orientation in a patch of the sky only during rotation about its vertical axis. Such yaw movement may serve an initial orientation that could precede ongoing motion on a steady course or reorientation when drifted off course. In contrast, while on course (i.e., aligned to the polarization pattern as striven for by tethered flying locusts), the angle between E-vector orientation in a patch of the sky and the animal's longitudinal body axis should not vary substantially on moderate time scales.
In the present approach, we measured the responses of polarization-sensitive neurons of the locust central complex to sustained presentation of polarized light with stationary E-vector, a condition representing persistent alignment with the celestial E-vector pattern. Moreover, we complemented earlier insights into characteristics of E-vector tuning by comparing response amplitudes at preferred and antipreferred E-vector angles to levels of ongoing activity (OA) and by analysis of the range of polarization sensitivity across cells of a respective type and of the specificity of response profiles for direction of E-vector rotation. Cell types studied here cover the putative input, intrinsic, and output neurons of the central-complex polarization-vision network. The response characteristics we analyzed in conjunction with the span of processing levels sampled here open a novel perspective on E-vector signaling in the polarization-vision network of the central complex in the locust brain.
MATERIALS AND METHODS
Experimental animals and preparation.
Locusts were reared in crowded indoor cultures at 28°C under a 11:13-h light-dark regime. Data included in the final analysis covered 45 neurons from 45 adult gregarious animals. Before preparation, animals were immobilized by cooling at 4°C for 15 min. To ease subsequent handling, legs and wings were cut off, and the animals were mounted to a metal holder using dental wax, which along with instant glue (cyanoacrylate) was also used for wound closure. The frontal brain surface was accessed by excision of the frons integument (including antennae and ocelli) and partial removal of the subcuticular fat body and tracheal air sacs. To reduce movements of the brain, muscles connected to the antennae and mouthparts as well as the anteriormost esophagus were transected, the gut was removed through an abdominal incision, and a wire loop waxed to the ventral head capsule was positioned to support the brain from posterior. Finally, the neural sheath was incised and partly removed to ease brain tissue penetration by the recording electrode. During preparation, locust saline (Clements and May 1974) was used to replace fatty hemolymph and keep the brain immersed. All animal procedures were in compliance with the guidelines of the European Union (Directive 86/609/EEC) and the German Animal Welfare Act.
Stimulation.
Neural activity was measured in a Faraday cage open to one side. All light sources outside of the cage were covered with red filters to prevent interference with controlled stimulation. In addition to polarized light, we presented objectlike patterns of unpolarized light on a cathode-ray-tube (CRT) screen (DP2070SB 22-in. CRT; Mitsubishi, Tokyo, Japan) as well as combinations of both (work in progress). As a result, most responses to polarized light included here (173 out of 214 responses to rotating polarizer, 109 out of 113 responses to stationary polarizer) were recorded under a dim, unilateral widefield illumination by the CRT (covering −45 to 60° in azimuth and −32 to 28° in elevation within the left laterofrontal visual field), but none of the periods evaluated here coincided with the presentation of visual objects against this uniform gray background.
The widefield CRT screen emitted 4.3 × 1013 photons·cm−2·s−1, which is within the intensity range of small-field chromatic stimuli considered as unpolarized compass signals in several studies (e.g., el Jundi et al. 2011; Kinoshita et al. 2007) but had no measurable effect on photon flux within the spectral range of the blue polarized-light signal. All light measurements were performed with a digital spectrometer (USB2000; Ocean Optics, Dunedin, FL) with its detector head at the position of the compound eyes, directed toward the CRT display and source of polarized light, respectively.
The polarized-light stimulus was generated from a blue light-emitting diode [11-mm inside diameter (ID), measured spectral range 421.6–524.3 nm, peak 461.11 nm, 1015 photons·cm−2·s−1; ELJ-465-617; EPIGAP Optoelektronik, Berlin, Germany]. It passed a remote-controlled rotatable linear polarizer (20-mm ID; HN38S; Polaroid, Cambridge, MA) positioned in the zenith of a locust's head at 60-mm distance (visual angle 19°, common velocity of filter rotation 30°/s).
Intracellular recording and tracer injection.
Membrane potentials were recorded with two Ag-AgCl wire interfaces, one being immersed in the saline solution to act as a reference electrode, the other inserted into a sharp micropipette for intracellular measurement. The latter were drawn from borosilicate capillaries [0.75-mm ID, 1.5-mm outside diameter (OD); Hilgenberg, Malsfeld, Germany] with a Flaming/Brown filament puller (P-97; Sutter Instrument, Novato, CA), their tips loaded with a solution of Neurobiotin tracer (4% in 1 M KCl; Vector Laboratories, Burlingame, CA) and shanks filled with 1 M KCl connected to the Ag-AgCl wire interface. Impedances in tissue ranged from 50 to 200 MΩ. Raw signals were amplified, band-passed (×10, 20 Hz to 20 kHz; SEC 1L/H amplifier; npi electronic, Tamm, Germany), digitized (16 bit/11.1 kHz; Power1401 mkII converter run with Spike2 software; both Cambridge Electronic Devices, Cambridge, United Kingdom), and stored for offline analysis in custom-written MATLAB software (MathWorks, Natick, MA). For live monitoring, the amplified signal was fed into an audio monitor and an oscilloscope (Hameg HM 205-2).
Histology: visualization of cell morphologies.
At the end of a respective recording session, Neurobiotin was injected through the recording pipette via application of 0.5- to 2-nA depolarizing currents for 1–15 min. Brains were dissected out, fixed overnight at 4°C in a solution of 4% paraformaldehyde, 0.25% glutaraldehyde, and 0.25% picric acid in 0.1 M phosphate-buffered saline (PBS), rinsed in PBS (4 × 15 min), and incubated with Cy3-conjugated streptavidin (1:1,000; Dianova, Hamburg, Germany) in 0.1 M PBS with 0.3% Triton X-100 detergent (PBT) for 3 days at 4°C in the dark. After rinsing in PBT (2 × 30 min) and PBS (3 × 30 min), preparations were dehydrated in an ascending ethanol series (H2O; 30, 50, 70, 90, 95, and 100% ethanol, 15 min each), cleared in a solution of methyl salicylate in ethanol (1:1, 30–45 min) followed by pure methyl salicylate (45–60 min), and finally mounted in Permount (Fisher Scientific, Pittsburgh, PA).
For identification of cell morphologies, the whole mount brain preparations were scanned confocally [Leica TCS SP5 confocal laser scanning microscope (CLSM); Leica Microsystems, Wetzlar, Germany] at 1,024- × 1,024-pixel resolution and either ×10 or ×20 magnification (Leica oil-immersion objectives HC PL APO ×10/0.40 and HCX PL APO ×20/0.70, respectively) with Cy3 fluorescence being excited by a diode-pumped solid state (DPSS) laser at 561 nm. In many cases, autofluorescence of the tissue allowed us to contour relevant neuropils as well. Confocal image stacks were then processed in Amira 5.3.3 (FEI Visualization Sciences Group, Merignac, France) and Corel Photo-Paint (X3 V 13.0.0576; Corel, Ottawa, Ontario, Canada) to generate and edit two-dimensional projection views. For detailed analysis of hitherto undescribed morphologies, the respective preparations were rehydrated and sectioned as described by Heinze and Homberg (2008). Briefly, brains were incubated in xylenes (2–4 h) to remove the embedding medium and were rehydrated in a decreasing ethanol series (100, 95, 90, 70, 50, and 30%, 15 min each). After rinsing with 0.1 M PBS (4 × 20 min), they were embedded in albumin-gelatin. The preparations were fixed overnight at 4°C in 8% buffered formaldehyde. On the following day, the embedded brains were cut into 130-μm-thick sections with a vibrating-blade microtome (Leica 1200S) and rinsed with 0.1 M PBS (4 × 15 min) before they were preincubated overnight in normal goat serum (NGS; 1:20) and 0.1 M PBT. Afterward, the sections were incubated for 5 days with anti-synapsin (1:50, monoclonal, provided by E. Buchner, University of Würzburg, Germany), streptavidin (1:1,000), and NGS (1:100) in 0.1 M PBT at 4°C. The sections were then washed in 0.1 M PBT (2 × 20 min) and 0.1 M PBS (3 × 20 min) before they were incubated in goat-anti-mouse-Cy5 (1:300) and streptavidin-Cy3 (1:1,000) in 1% NGS in 0.1 M PBT over 3 days at 4°C in darkness. The sections were rinsed again in 0.1 M PBT (2 × 20 min) and 0.1 M PBS (3 × 20 min), dehydrated in an increasing ethanol series, transferred to a mixture of ethanol (100%) and methyl salicylate (1:1), and finally cleared in pure methyl salicylate and embedded with Permount between two glass coverslips (24 × 60 mm).
The relevant brain sections were scanned with a CLSM (Leica TCS SP5) with a ×20 objective (HCX PL APO lambda blue ×20/0.70 IMM UV, working distance 260 mm; Leica). The Cy3 signal was detected with a DPSS (561 nm) laser, whereas the Cy5 fluorescence was detected with a HeNe (633 nm) laser.
Criteria for inclusion in final data analysis.
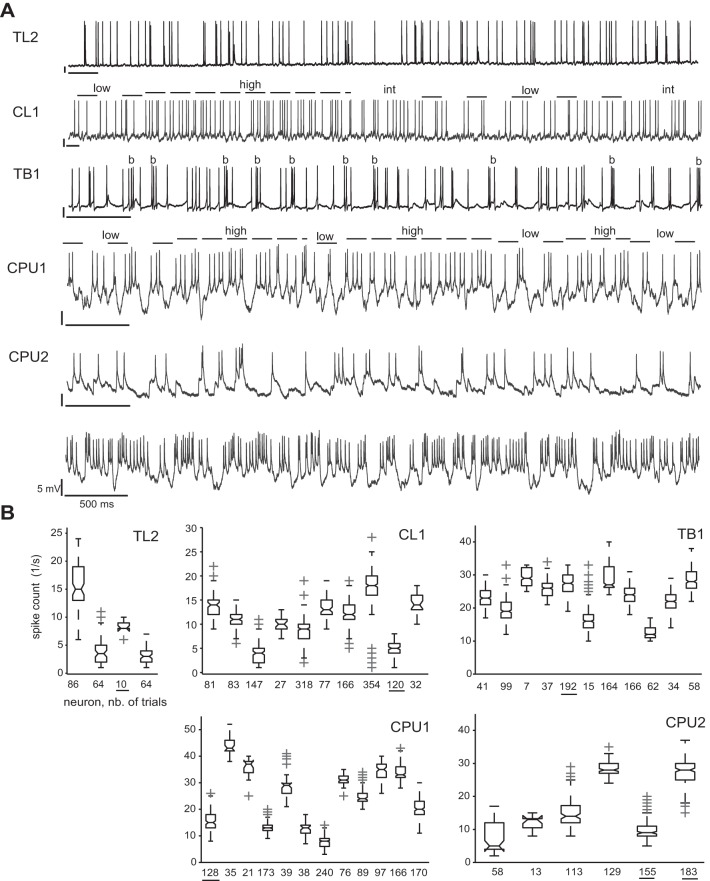
Physiological data were included in the final analysis only if the recorded neuron was successfully labeled. In some cases, more than one neuronal cell was stained, probably owing to leakage of Neurobiotin into neighboring neurons. In these cases, data were included in the analysis if characteristic patterns of OA that had previously been determined for distinct cell types could be clearly assigned to one of the labeled morphologies (see Fig. 2 for example traces). To define the spiking patterns of OA typical for a respective cell type, we evaluated experiments that provided both distinct single-cell labeling and several minutes of OA (data not shown). Basic statistics covered the distributions of spike counts (1,000-ms bin width; Fig. 2B) and interspike intervals. Along with these numerical measures, action potential wave forms (spike amplitudes and widths, double spikes) and dynamics of subthreshold activity added to the cell-type-specific physiological profiles. For instance, the OA of CPU neurons is marked by alternation between brief states (500–700 ms) of low, intermediate, and high spike rates, whereas action potentials are relatively low in amplitude, occasionally occur as doublets, and ride on pronounced dynamics of subthreshold activity marked by fast hyperpolarizations.
Fig. 2.
Complexity and variability of ongoing activity (OA). A: intracellular recordings revealed cell-type-specific levels and dynamics of OA. OA was comparatively low and rather uniform in TL2 neurons. Statelike dynamics were observed in CL1 neurons with rapid or gradual transitions between states of high and low firing rate (dashed lines) that lasted up to several seconds. Gradual transitions include intermediate (int) states in overall rate. TB1 neurons showed moderate average levels of OA marked by bursts (b) of 50- to 100-ms duration. Overall levels of OA were particularly dependent on the individual recording in CPU1 and CPU2 neurons, as illustrated by the 2 traces recorded from different CPU2 neurons and evident from ranges of box plots in B. In both subtypes, statelike variability of local firing rates (shown for CPU1; same indexing as for CL1) were observed, whereas state durations were substantially lower than in CL1 neurons, commonly between 500 and 1,000 ms. Bars = 5 mV, 500 ms. B: box plots showing the distribution of spike counts in OA for different cells of a respective type. Each box depicts data from an individual neuron. Numbers below the x-axis specify (in seconds) the total duration of OA evaluated. Thus ranges of box plots reflect the within-cell dynamics of local spike rate at 1,000-ms resolution as observed over the period of recording sessions. Because the latter varied between 10 and 45 min, so does the total duration of OA recorded. Underlined numbers mark data from neurons shown in A.
Data preprocessing for final analysis.
Instantaneous firing rates were estimated by means of Gaussian-smoothed peristimulus time histograms [PSTHs; MATLAB implementation as adopted from Jude Mitchell's code (MATLAB function compute_gauss_smooth, accessible at http://www.snl.salk.edu/∼jude/sfn2008/index.html)] by Kreuz et al. (2011) under avoidance of biasing at the edges of the peristimulus time window. Circular statistics (MATLAB toolbox by Berens; see Berens 2009) were computed to quantify relationships between firing rates and E-vector angle. For this purpose, corresponding E-vector angles as inferred from orientations of the polarizer were assigned to individual spikes that occurred during rotation of the polarizer, thus obtaining distributions of spike angles. Where necessary for a respective test, transformations from axial to circular scale were performed by doubling spike angles.
Response measures: polarization responsiveness.
The present study covers a variety of response features, including the principle responsiveness to E-vector angle (polarization responsiveness) and the amplitude of the E-vector response in terms of the amplitude of spike-rate modulation. We rated polarization responsiveness by correlation strength [CS; i.e., the strength of the association between the E-vector angle and spike rate during full (360°) rotations of the polarizer]. This association was quantified by means of a circular-linear correlation analysis (Berens 2009), which, analogous to the linear-linear case, provides a circular-linear correlation coefficient and its P value. The correlation coefficient can be squared to obtain the coefficient of determination (r2) that ranges from 0 to unity and quantifies the proportion of variance shared by the two variables. The size of r2 can be rated in an objective manner by means of the conventional scale for effect sizes. For instance, an r2 exceeding 0.25 indicates that >25% of the observed variability in spike rate during polarizer rotation can be explained in terms of a dependency on E-vector angle, which, by convention, corresponds to a strong effect. In other words, r2 is positively related to the portion of the entire range of E-vector angles (i.e., 0–179°) across which spiking covaried with E-vector angle: it reflects the overall degree to which spiking changed in correlation with the change in the E-vector angle. The criterion for principle responsiveness to E-vectors was the statistical significance of r2 (at α = 0.05 and β = 0.2). As a measure of polarization responsiveness, CS as quantified by r2 is strict but independent from the amplitude of the response. The latter is a desirable feature that is met neither by the commonly applied Rayleigh test for general deviation from circular uniformity nor by tests for significance of the median angle (see Fisher 1995; Zar 1999).
Response measures: preferred E-vector angle and vector strength.
Whereas CS can indicate polarization responsiveness, it does not reflect more specific response features. In particular, r2 1) neither indicates the preferred E-vector angle 2) nor distinguishes between actual periods of 180 and 360° and 3) is insensitive to the amplitude of the spike rate modulation that constitutes the response to a rotating polarizer; a perfect correlation can be observed under minimal or, likewise, under most pronounced deviations from the ongoing activity of the neuron. To address the first and second points, we plotted PSTHs for visual inspection of the E-vector tuning and estimated the preferred E-vector angle (Φmax; scaled 0° to 179°) as the angle of the mean (not to be confused with peak spiking) vector of a spike angle distribution pooled across clockwise and counterclockwise rotations of the polarizer (30°/s). The antipreferred angle, Φmin, was defined as the angle perpendicular to Φmax. The length of the mean vector, |r|, quantifies vector strength (VS) and ranges from 0 to unity (Ashida et al. 2010). It becomes 1 if and only if all of the vectors are of the same direction and is closely related to circular variance (S) by S = 1 − |r|. Here, we refer to it as a measure of response amplitude. It reflects the degree to which spiking was concentrated around Φmax. Importantly, the same VS may be observed for very different response profiles. For instance, a particular value of |r| could result from a rather silent neuron increasing its spike rate considerably at Φmax or by a neuron with higher OA that responds with a moderate decrease in spike rate at Φmin and a moderate increase at Φmax. If the same modulation amplitude, say half the difference in spike rate between Φmax and Φmin, is superimposed on different levels of ongoing spiking activity, |r| is positively related to the resulting amplitude of spike-rate modulation.
Response measures: information per spike.
VS is related to the peakedness of a response to a periodically modulated stimulus. It reflects the degree to which spiking is locked to a certain phase of the stimulus or here to the preferred E-vector angle. Thus high values of |r| indicate a high level of probability that a single spike observed under rotation of the polarizer coincided with the presentation of Φmax. Skaggs and colleagues (1993, 1996) have proposed a more straight measure of the information a spike that was observed during periodic stimulation holds over the phase of the stimulus. This measure of information per spike (IpS) was applied in an analogous manner to quantify the information (in bits per spike) an individual spike observed during the rotation of the polarizer provides over the acute E-vector angle. As is the case for VS, IpS may be particularly high for responses of neurons that are effectively blind to virtually each but their preferred E-vector angle (i.e., in cases with relatively low CS).
Comparison with OA: effective response amplitudes.
Each response measure described above ignores the degree to which a putative response actually differs from the level and pattern of ongoing activity of the respective cell. OA was defined as the activity recorded under the absence of any controlled stimulation except for widefield illumination by the CRT display if this was also given during presentation of polarized light. Sections regarded as OA were selected carefully to exclude off-responses to preceding presentation of polarized light as well as effects of other visual stimuli tested. To assess effective response amplitudes (i.e., the degree to which spike rates at Φmax and Φmin effectively differ from OA), we normalized response spike rates to different levels of OA that were observed in the respective neuron. Here, the underlying rationale is to regard a response as robust to the degree to which it stands out against extreme states of the OA of the cell, importantly, against states that deviate from the median OA in the same direction as the (putative) response does. Thus a high effective response amplitude at a respective E-vector angle implies that spiking at that angle substantially differs from the particular level of OA that is closest to the putative response. More general analyses were performed on responses normalized to the median OA of the respective neuron.
Types of data plot.
Box plots were laid out as follows: boxes range from the first to the third quartile (Q1 and Q3, respectively); maximum whisker ranges are from Q1 − 1.5 * (Q3 − Q1) to Q1 and from Q3 to Q3 + 1.5 * (Q3 − Q1), respectively. Plotted whiskers extend to the adjacent value (i.e., the most extreme data value that is not an outlier). Outliers are plotted using cross-shaped markers. Notches indicate the 95% confidence interval of the median [i.e., 2 medians are significantly different at the 5% level if their intervals do not overlap (note that in results, statements on statistical significance of differences between medians rely on this indication)]. Bubble plots are 1-dimensional scatter plots that use marker size to indicate frequency of observations: the diameters of the circular markers are linearly scaled to the absolute frequency at which a value defined by the center of the marker was observed. Bubble plots were occasionally drawn in addition to box plots to visualize the sampling size and symmetry of individual distributions.
RESULTS
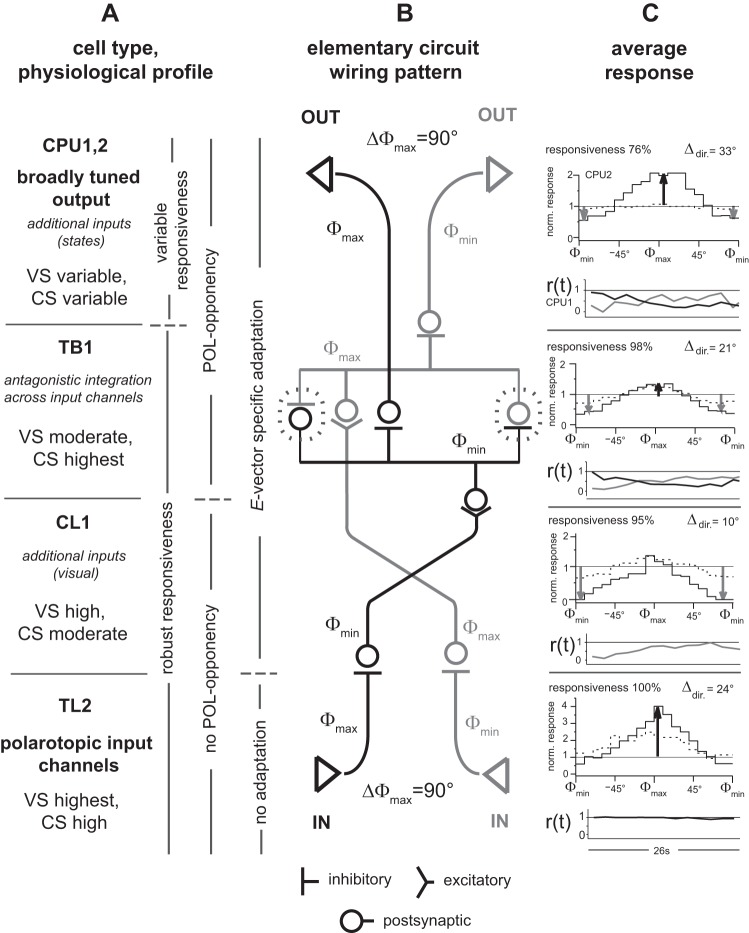
The results are organized according to response features. Within each section, the cell types (see Fig. 1) are addressed with respect to the putative processing hierarchy, TL-CL-TB-CPU. Data from 45 out of 100 recordings from central-complex neurons met the criteria for inclusion in the final analysis. Unless stated otherwise, analyses of responses to the rotating polarizer were confined to cases with significant correlations between firing and E-vector angle. Table 1 provides a brief summary of features of OA and the most basic response characteristics of central-complex neurons. Figure 13 contains a schematic of the main findings of responses to polarized light and depicts strongly related aspects of the discussion.
Table 1.
Ongoing activity and response characteristics of central-complex neurons
| Cell Type | No. | Ongoing Activity | Response, Φmin | Response, Φmax | Polarization Opponency | Adaptation |
|---|---|---|---|---|---|---|
| TL2 | 4 | Low, uniform | Not robust | Strong, robust | No | No |
| TLU1 | 1 | Low, uniform | Not robust | Strong, robust | No | No |
| CL1 | 9 | Prolonged states | Strong, robust | Not robust | No | Yes |
| TB1 | 9 | Bursts | Moderate, robust | Moderate, robust | Yes | Yes |
| CPU1 | 12 | Brief states | Variable | Variable | Yes | Yes |
| CPU2 | 6 | Brief states | Variable | Variable | Yes | Yes |
| TL6 | 1 | High, uniform | None | Strong | No? | Yes |
Φmax, preferred E-vector angle; Φmin, anti-preferred E-vector angle.
Fig. 13.
Summary of main findings. In A–C, upward reading corresponds to going downstream along the presumed hierarchy of processing, TL-CL-TB-CPU. A: cell types at consecutive stages of processing, their putative functional relevance, and related response features. Statements in italics are hypothetical (see discussion). Statements on CPU neurons give a summary across CPU1 and CPU2 if not indicated otherwise as in C. B: simplified scheme of the elementary circuit for the generation of conditional, broadly tuned signaling of the polarization plane (CPU neurons) via antagonistic integration (TB1; dashed contours) across opponent, narrowly tuned input channels (TL2 and CL1), as proposed in Fig. 12. C: tuning to E-vector angle and time course of responses to preferred and/or antipreferred angles. Top subplots: responses to a rotating polarizer. These tuning plots show medians of binned (10°) spike counts normalized to median spike count in OA and plotted against the angular distance to the mean spike angle (Φmax). Data summarize responses obtained from clockwise and counterclockwise rotations but differentiate between stronger (solid line) and weaker (dashed line) responses. At this point, responses, including those that lacked significant correlation, were ranked according to VS; stronger (weaker) responses correspond to the upper (lower) half of the amplitude-ranked data set. Black (gray) vertical arrows indicate responses near Φmax (Φmin) that proved robust compared with very high (very low) levels of OA (these comparisons not shown here); responsiveness indicates proportion of responses with significant correlation between spiking and E-vector angle; Δdir. indicates angular difference between direction-specific Φmax values. For details on data sets and measures, see Figs. 4, 5, 8 (for robustness of responses near Φmax and Φmin, weaker vs. stronger responses, and rotation-of-direction specificity, respectively), and 9 (for response time courses). Note that the anticipatory effect in direction-of-rotation-specific responses is even higher for the E-vector angles at peak spike rates, which are not shown here (Fig. 9D). Bottom subplots: r(t) depicts the average (median) time course of excitatory (black lines) or inhibitory (gray lines) responses to stationary E-vector angles over 26 s. To capture the mere time course of the responses, binned (2-s) spike rates were normalized to the within-response median rate for TL2 neurons and to the within-response maximum for all other types of neuron. As expected from their E-vector tuning, responses to stationary angles were exclusively excitatory in TL2 neurons, exclusively inhibitory in CL1 neurons, and E-vector opponent in TB1 and CPU neurons. Responses of TL2 neurons were tonic, whereas responses to the specific E-vector angle in all other types of neurons quickly adapted.
Ongoing activities.
The OA of polarization-sensitive neurons showed several cell-type-specific characteristics. These comprised bursting in TB1 neurons (ca. 50- to 100-ms burst duration) and alternation between firing-rate states of higher duration (ca. 500-1,000 ms) and very high duration (up to tens of seconds) in CPU and CL1 neurons, respectively (Fig. 2A). Within the scope of this study, the consideration of OA will be largely restricted to distributions of spike counts at 1,000-ms bin width (Fig. 2B). These were relatively low and least variable in TL2 neurons at the input stage of the network except for one in a total of four cells that showed a moderate level of OA and higher variability (Fig. 2B). All cell types downstream from TL2 neurons showed substantial variability in spike count both within a cell (over time) and across cells of the same type, including many cases statistically significant at α = 0.05 with minimal overlap of interquartile ranges in box plots. Thus in addition to the cell-type-specific complexities in spike pattern, cells of the same type had substantially different median activity levels, which persisted throughout the time range of a recording. Along the putative hierarchy of processing (TL-CL-TB-CPU), the lower bounds and ranges of OA spike-count distributions tended to increase. All within-cell spike count histograms differed from normal distribution with statistical significance at P ≪ 0.01 as verified by two-tailed Kolmogorov-Smirnov tests (Massey 1951). To take this into account, we preferred rank statistics of spike counts (median and 2.5th and 97.5th percentiles) for normalization of E-vector responses.
Profiles of E-vector tuning.
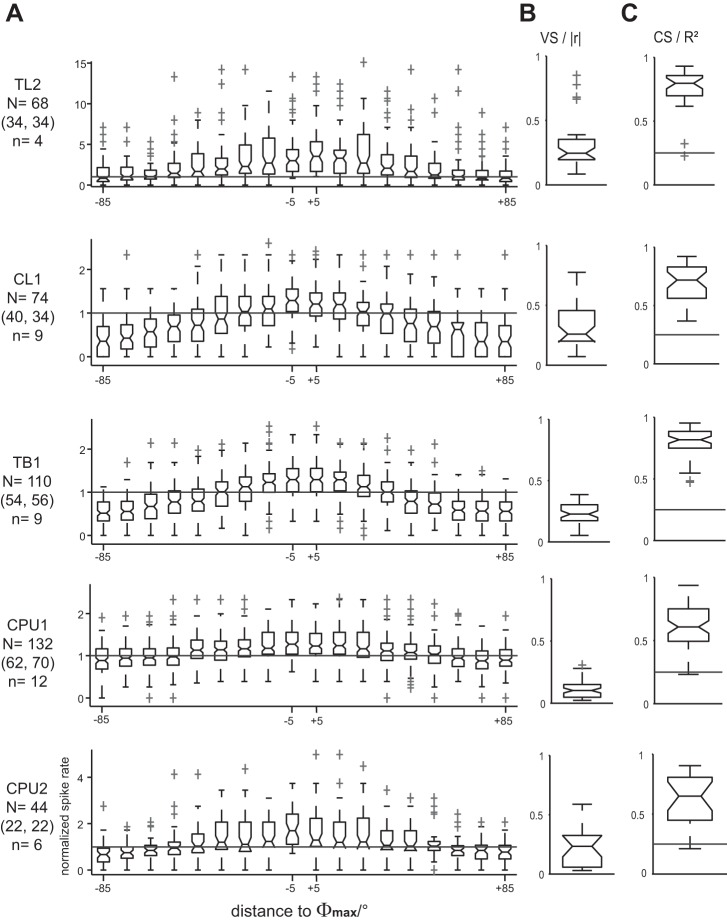
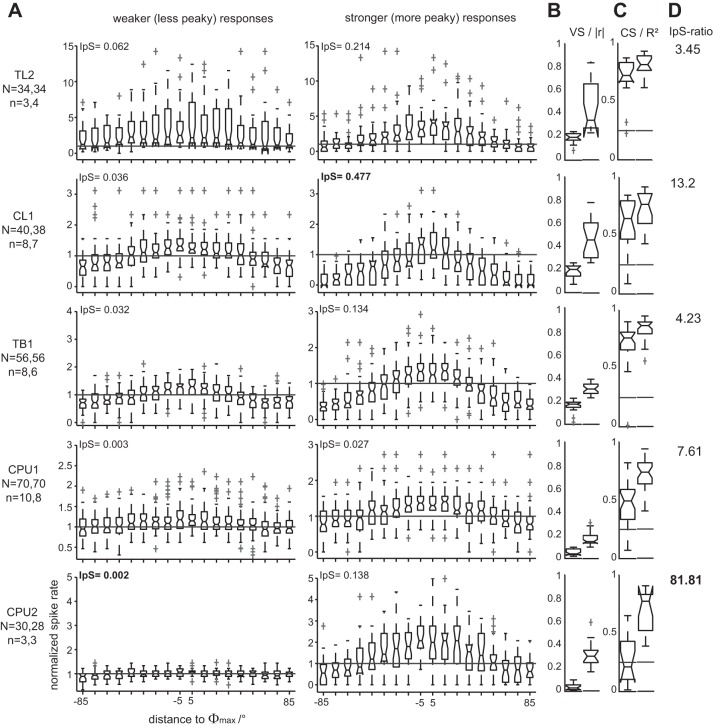
To capture the characteristic E-vector tuning of each cell type, we evaluated responses to full rotations of the polarizer. Binned (10°) spike angles were pooled across clockwise and counterclockwise rotations. Corresponding spike rates were normalized to the median of the OA of each respective cell, and their binned distributions were box-plotted against the angular distance to the Φmax value (Fig. 3A), which was individually calculated for each response. Concurrent with the increase in OA along the processing hierarchy, average response amplitudes in terms of VS decreased from the input stage (TL2 and CL1 neurons) over intermediate-stage TB1 neurons to neurons at the output stage (CPU1 and CPU2). This is illustrated by the box plots in Fig. 3B and by the vertical distances of response rates to the median level of OA (horizontal line) shown in Fig. 3A. Notably, a prominent change occurred from CL1 to TB1 neurons: the distribution of |r| values from TB1 cells was less dispersed, more symmetrical, and shifted toward lower values compared with those of CL1 neurons (Fig. 3B). Although the overall spread of |r| is comparable for TB1 and CPU1 neurons, its median is significantly lower in the latter than in all other types of polarization-sensitive neurons considered here. By contrast, |r| was substantially more dispersed again in CPU2 neurons. Still, values of individual responses obtained from CPU1 and CPU2 neurons at the output stage matched the enhanced levels observed in TL2 and CL1 neurons near the input stage, as reflected by the overlapping ranges of the box plots in Fig. 3B.
Fig. 3.
Electric field vector (E-vector) tuning of central-complex neurons. A: tuning profiles were obtained from N responses of n cells to a polarizer rotated at 30°/s. Spike rates in 36 nonoverlapping bins spanning the entire period of polarizer rotation were normalized to the median spike rate of OA and box-plotted against the corresponding distance of the respective bin to the preferred E-vector angle (Φmax). To capture the profile of each cell type, data were pooled across responses to clockwise and counterclockwise polarizer rotation (N numbers in parentheses) that showed significant correlation between spiking and E-vector angle. The cell-type-specific modulation depth of E-vector tuning is reflected by the angle-specific distance of response rates to the horizontal reference line that indicates the median level of OA. Some outliers that were consistent with the respective tuning profiles are omitted for the sake of appropriate axis scaling. B and C: the peakedness (vector strength, VS) and overall E-vector dependency (correlation strength, CS) of tuning profiles were quantified by calculating the length of the mean vector (|r|) and coefficient of determination (r2) for each response, respectively. Note that VS decreases from CL1 to TB1 neurons, whereas CS increases. Horizontal lines in C indicate the 0.25 threshold level for statistically strong effects.
In addition to VS, the CS was calculated for each individual response to quantify the extent to which the firing rate could reflect the acute E-vector angle on average (i.e., as measured across the response to an entire rotation of the polarizer). Figure 3C shows the respective box plots of r2 values. Throughout cell types, minimum values of r2 were well above (in TL2, CL1, and TB1 neurons) or still very close (in CPU1 and CPU2 neurons) to the conventional lower threshold of 0.25 for large effect size, indicating strong correlations between firing and E-vector angle. Highest scores and least interquartile dispersion were observed in TL2 and TB1 neurons. For these, median values of r2 were similar and significantly higher than in all other types of neurons considered here, amounting to 79 and 82% of explained variability, respectively. Thus the substantial reduction in VS from CL1 neurons to TB1 neurons coincided with a strong and statistically significant increase in CS: tuning profiles became flatter, but spiking became correlated with E-vector angle over a wider range of angles. At the output stage, variability of CS was increased, whereas the ranges of r2 were virtually congruent in CPU1 and CPU2 neurons and medians of the data pooled across cells were lower than in upstream cell types.
Amplitudes of E-vector tuning.
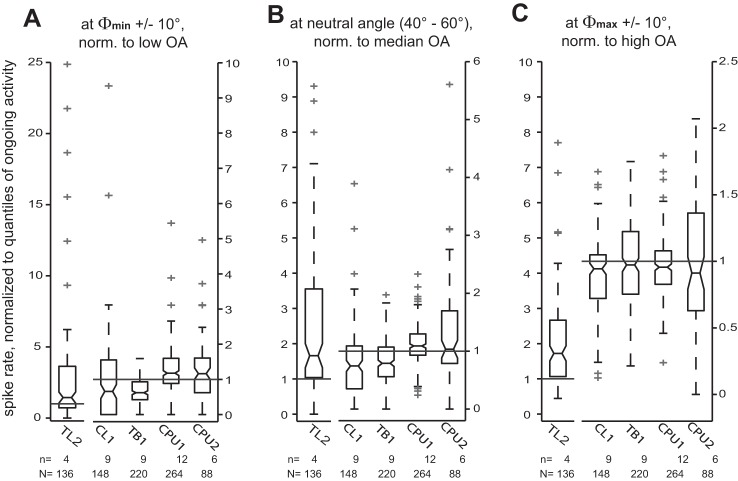
In the previous subsection, we described the profiles of E-vector tuning for different cell types on the basis of responses to a rotating polarizer that were normalized to the median spike count of the OA of each respective cell. It is important to note that this approach does not suffice to estimate the effective amplitude of the rate-coded responses to E-vector angles because the OA of the neurons exhibits substantial dynamics at cell-type-specific time scales (Fig. 2). To address this, we have normalized the response spike rates at Φmin ±10°, at intermediate angles (40–60° distance to both Φmax and Φmin), and at Φmax ±10° to very low levels of OA (2.5th percentile of the spike count distributions shown in Fig. 2), median, and very high levels of OA (97.5th percentiles), respectively (Fig. 4). At the input stage, E-vector tuning was effectively unidirectional [i.e., responses to the rotating polarizer were robust only at Φmax (in TL2 neurons) or Φmin (in CL1 neurons)]. The robust responses of TL2 neurons to their preferred E-vector angle and of TB1 neurons to their antipreferred angle constituted the most consistent robust deviations from OA. Thus the response of an individual TL2 neuron to its preferred E-vector angle provides a robust and strong input to the polarization-sensitive network of the central complex, whereas the TL2 neurons encountered here were effectively unresponsive to antipreferred angles. Their median spike rate near Φmin even exceeded the low states of OA. By contrast, in TB1 neurons, the unidirectional tuning of upstream neurons had apparently been transformed into an E-vector opponent response, which is less strong but shows higher overall robustness, although being more robust at Φmin compared with Φmax, where half of the responses fell below the high state of OA. Again, CPU2 neurons showed increased variability in response pattern but were still capable of robust opponent responses as reflected by overlapping ranges of box plots in Fig. 4. CPU1 neurons showed less robust response opponency than CPU2 cells: the proportion of responses that withstood comparison with extreme states of OA was lower at both Φmin and Φmax.
Fig. 4.
Effective amplitudes of E-vector tuning. To explore how putative E-vector responses compare with OA, we normalized spike rates near the antipreferred E-vector angle (Φmin; A), intermediate angles (B), and preferred E-vector angle (Φmax; C) to very low (2.5th percentile), median, and very high (97.5th percentile) levels of OA (horizontal lines), respectively. The underlying spike count samples are the same as for the tuning profiles in Fig. 3, but N numbers of the box plots shown here are doubled as a result of rebinning of the spike counts from 10° resolution to a bin size of 20°. Reference levels of ongoing activity were measured for each individual cell considered here. Some outliers are omitted for the sake of appropriate axis scaling. In all subfigures, the scaling of the y-axes for TL2 cells (left y-axes) differs from that of the other cell types (right y-axes). Responses of TL2 and CL1 neurons are unidirectional: TL2 neurons show robust excitation at Φmax but effectively lack an inhibition at Φmin; in contrast, CL1 neurons show robust inhibition at Φmin but no robust excitation at Φmax. All types of neurons downstream to CL1 appear capable of more robust response opponency, whereas scatter is highest in CPU2 neurons.
In TL2 neurons, median spike rates at neutral E-vector angles were comparable with high-level OA. In all types of neurons downstream from TL2, spike rates at these angles were substantially closer to median than to low- or high-level OA, as expected for stimulus-unrelated spiking. In particular, the average distance to median OA decreases along the putative hierarchy of processing. It shall be noted that the levels of OA to which we normalized the responses were measured in the absence of the additional illumination by polarized light and thus at a lower overall ambient light level than the E-vector responses.
Cell-type-specific spans of polarization sensitivity.
As outlined above, the CS of responses to the rotating polarizer varied to different degrees in the types of neurons encountered here with a tendency toward increased spans of r2 at the output stage of the network (Fig. 3C). Spans of VS of these correlation-significant responses appeared less related to processing stage, being comparable between CL1 and CPU2 neurons and among TL2, TB1, and CPU1 neurons, respectively. To assess the full cell-type-specific spans of polarization sensitivity, we reanalyzed the data under inclusion of cases that lacked significant correlation between spiking and E-vector angle. All putative responses from a respective cell type were amplitude-ranked according to their VS. The sets of ranked data were then split along their medians into their lower and upper halves, corresponding to weaker and stronger responses. Figure 5 illustrates how tuning profiles (normalized to median OA), VS, and CS of both subsets compare with each other within each cell type.
Fig. 5.
Cell-type-specific spans of sensitivity to E-vector angle. To assess spans of sensitivity to E-vector angle, we amplitude-ranked all responses from a respective cell type according to their VS. The sets of ranked data were then split into their lower and upper halves, corresponding to weaker responses (A, left) and stronger responses (A, right), respectively. For compact plotting, rates were box-plotted against the corresponding distance of the respective bin to Φmax. Some outliers that were consistent with the respective response profiles are omitted for the sake of appropriate axis scaling. For each subset, information per spike (IpS; bits per spike; see insets in A) and distributions of |r| (B) and r2 (C) were calculated. Left and right box plots in B and C correspond to the data from weaker and stronger responses, respectively. Horizontal lines in box plots of r2 indicate the 0.25 threshold level for statistically strong effects. Note that both the difference in steepness of tuning (A and B) and in CS (C) between weaker and stronger responses increases steadily along the putative hierarchy of processing. In CPU2 neurons, nearly half of the weaker responses lack significant correlation between spiking and E-vector angle (P values of r2 not shown). Concurrently, the increase in IpS (IpS-ratio; D) from weaker to stronger responses is lowest for TL2 neurons at the input stage and still relatively low in TB1 neurons while being 20-fold higher in CPU2 neurons.
As previously observed for the correlation-significant responses alone, the overall spans of r2 across weaker and stronger responses tended to increase along the processing hierarchy (TL-CL-TB-CPU), although the span was lower in TB1 than in CL1. The tuning profile of stronger responses of TB1 neurons shows the strongest association between spike rate and E-vector, resulting in an opponency of responses to Φmax and Φmin unparalleled in consistency by all other cell types (Fig. 5A). This is accompanied by high values of r2 with narrow spans that show small overlap between weaker and stronger responses in TB1 neurons (Fig. 5C). Cases that lack significant correlation were not encountered in TB1 neurons except for a single outlier out of 56 cases. Concurrently, response amplitude in terms of VS was more stable than in upstream neurons and still relatively high for the subset of weaker responses (Fig. 5B). Taken together, the differentiated analysis confirmed that TB1 neurons provide a very reliable polarization signaling over the entire range of E-vector angles. Moreover, 5 out of a total of 9 TB1 neurons contributed both stronger and weaker responses, indicating a substantial within-cell component of the low overall variability.
The spans of CS as well as its separation between weaker and stronger responses were highest in CPU neurons. Most strikingly, weaker responses of CPU2 neurons had the flattest response profile (Fig. 5A) with a median VS close to 0 and lacked significant correlation in 8 out of 15 cases (P values not shown). Here, cells that provided stronger and weaker responses, respectively, were not identical (14 stronger and 15 weaker responses, both groups covering 3 cells). Thus a major component of the large overall response variability in CPU2 neurons arises from differences between cells or from states of responsiveness as prolonged as the duration of a typical recording. Notably, median spike rates of OA were up to 5-fold higher (5 vs. 28 spikes per second) in the less responsive neurons (Fig. 6). For the subsets of stronger responses, median VS and CS were equal for TB1 and CPU2 neurons.
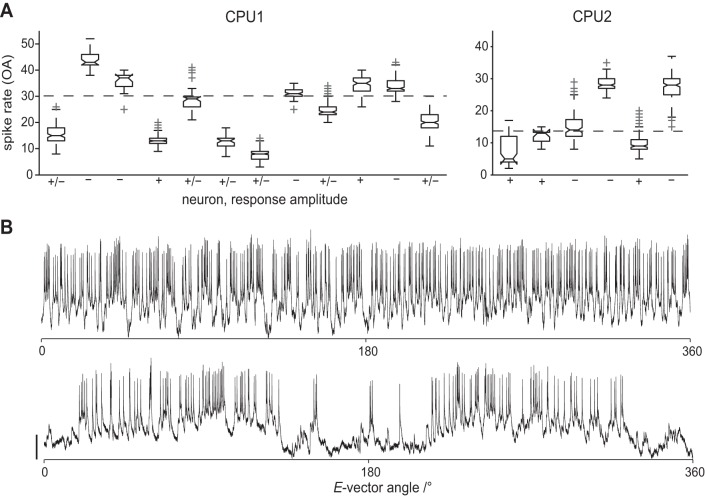
Fig. 6.
Sensitivity to E-vector angle is related to OA in CPU neurons. In CPU neurons, the average sensitivity to E-vector angle covaried systematically with the overall level of OA of the individual cell. Within-cell distributions of spike rates in OA shown in Fig. 2 are plotted again in A. Here, +, −, and +/− mark data from neurons that contributed exclusively stronger (+), exclusively weaker (−), or both stronger and weaker (+/−) responses as rated by VS. Horizontal dashed lines indicate approximate threshold levels of ongoing activity that separate the subpopulations of exclusively weakly and exclusively strongly responding cells, set by visual inspection. B: traces recorded from a less (top) and a more (bottom) sensitive CPU2 neuron (corresponding to the 2 rightmost box plots in A) during polarizer rotation at 30°/s. Bar = 5 mV.
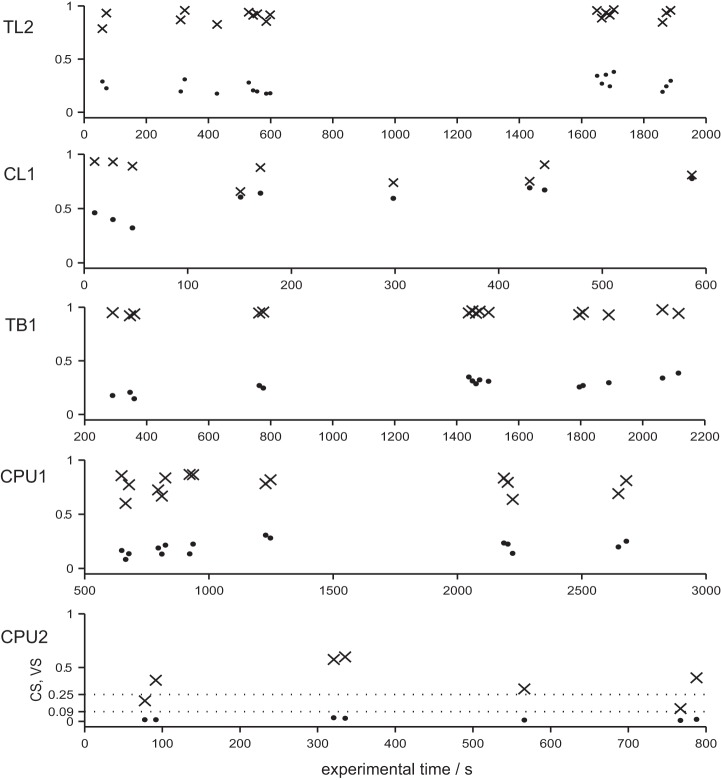
To illustrate the range of within-cell (co-)variability of VS and CS, we plotted the individual values of |r| and r2 from several rotations of the polarizer against the time course of recordings lasting at least 600 s (Fig. 7). These plots show again that variability of CS (i.e., of responsiveness to E-vector angles) is small in TB1 and relatively high in CPU2 neurons. As expected, the richness in IpS was positively related to the peakedness of the tuning curves and thus to VS, but it was rather independent from CS (Fig. 5, A–C). The lowest values were computed for weak responses of CPU neurons accompanied by the lowest median vector strengths. A maximum value of 0.48 bits per spike was obtained for stronger responses of CL1 neurons, which were also highest in median VS. The increase in IpS-ratio (Fig. 5D) from weaker to stronger responses is lowest for TL2 neurons at the input stage (×3.45) and still relatively low in TB1 neurons (×4.23) but 20-fold higher in CPU2 neurons (×81.81). Table 2 provides an overview of responsiveness to polarization plane in terms of significant correlation between firing and E-vector angle during presentations of a rotating polarizer (note that the strength of the effect as measured by r2 is omitted).
Fig. 7.
Within-cell variability of polarization sensitivity. To illustrate within-cell variability of polarization-sensitivity, VS (values of |r|; dots) and CS (values of r2; × marks) for full rotations of the polarizer (30°/s) were plotted against the time course of exemplary recordings. As a conventional scale for r2, dotted lines mark the threshold levels for medium- and large-sized effects, corresponding to at least 9 and 25% explained variability, respectively. Note that variability of CS (i.e., of principle responsiveness to E-vector angles) over time is small in the TL2 and TB1 neurons, whereas it is relatively high in the 2 CPU neurons.
Table 2.
Polarization-plane responsiveness of central-complex neurons
| Cell Type | ntotal | ncorr. sig. | ncorr. n.s. | %n corr. sig. | Ntotal | Ncorr. sig. | %Ncorr. sig |
|---|---|---|---|---|---|---|---|
| TL2 | 4 | 4 | 0 | 100 | 34 | 34 | 100 |
| CL1 | 9 | 8 | 0 | 88.9 | 39 | 37 | 94.9 |
| TB1 | 9 | 8 | 0 | 88.9 | 56 | 55 | 98.2 |
| CPU1 | 12 | 10 | 0 | 83.3 | 70 | 66 | 94.2 |
| CPU2 | 6 | 4 | 0 | 66.7 | 29 | 22 | 75.9 |
ntotal, Total number of cells; ncorr. sig.(ncorr. n.s), number of cells that contributed exclusively responses with (non-) significant correlation between E-vector and spiking; Ntotal, total number of responses, including those that lacked significant correlation; Ncorr. sig., number of responses with significant correlation. N values refer to full (360°) rotations of the polarizer. Note that the mere significance of the correlation does not indicate its strength.
Rotation-direction specificity and anticipatory features of responses to rotating polarizer.
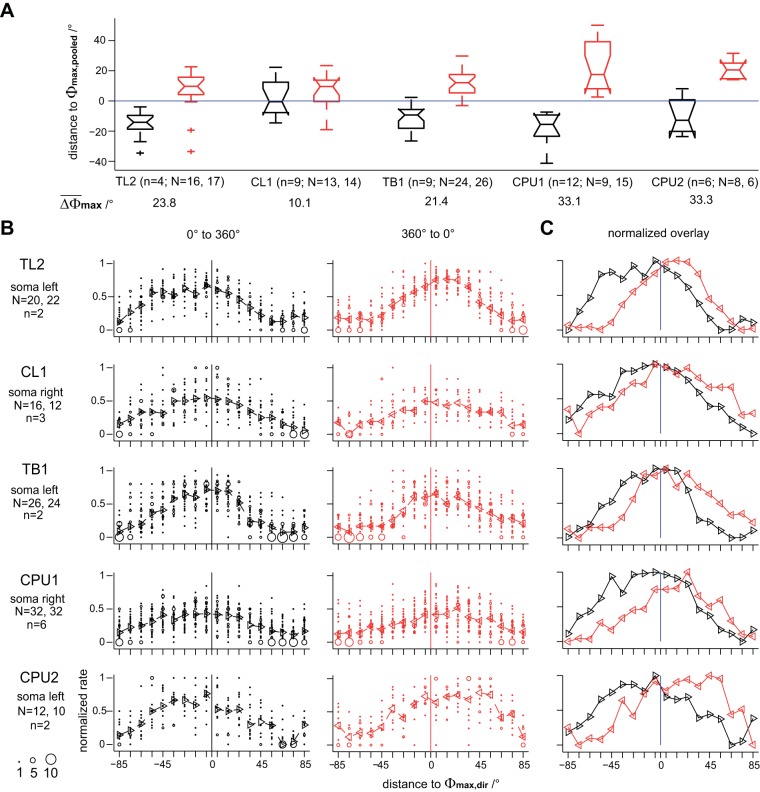
All above-mentioned analyses (except for plots in Fig. 7) were performed on response data pooled across clockwise rotations (0 to 360°) and counterclockwise rotations (360 to 0°) of the polarizer. Next, we inspected the responses to the rotating polarizer for rotation-direction specificity and, within each direction, for symmetry of spiking around Φmax. Here, we consider the overall Φmax value for data pooled across directions of rotation (Φmax,pooled) to be an estimator of the actual preferred E-vector angle of a neuron.
In all types of neurons, the Φmax values of individual responses differed from Φmax,pooled in a consistent, rotation-direction-specific manner (Fig. 8A): in the vast majority of cases, individual Φmax values preceded the passage of Φmax,pooled. Thus their deviance from Φmax,pooled is not a mere result of latency and is in fact suited to anticipate future E-vector angles. Median deviations from Φmax,pooled amounted to about 10–20° in absolute value at 30°/s rotation velocity except for a far lower median value for clockwise rotations in CL1 neurons. The difference between median Φmax values of both directions of rotation was highest in CPU1 and CPU2 neurons (∼33° both), pointing to a particularly pronounced anticipation of future E-vector angles near the output stage of the network. For the analysis of symmetry of spiking around Φmax, we first grouped responses within each cell type according to direction of rotation and, to avoid bias by brain-side effects, according to soma position in terms of brain hemisphere. Figure 8, B and C, shows bubble plots and medians of normalized response rates for a selected soma position for each cell type. To capture the time course of responses, the spike rates at binned distances to Φmax were additionally normalized to the maximum across cells and trials. In general, spiking in the individual responses was not concentrated symmetrically around Φmax but skewed to peak in advance of its passage, thus further promoting a possible anticipation of future E-vector angles. Here, it is important to bear in mind that Φmax values denote the mean of a respective distribution of spike angles, which is not necessarily equivalent to the E-vector angle of peak spiking. In the most prominent cases, again obtained from CPU neurons, the E-vector angle of peak spiking preceded Φmax,pooled by ∼45°, equivalent to 1.5 s at 30°/s rotation velocity.
Fig. 8.
Responses to rotating polarizer depend on direction of rotation in an anticipatory fashion. In all types of neuron, the preferred E-vector angles of individual responses (Φmax values) differed from the overall mean across both directions (Φmax,pooled) in a consistent, rotation-direction-specific manner. A: distributions of angular distances between Φmax,pooled and Φmax obtained under clockwise (black box plots) and counterclockwise (red box plots) rotations of a polarizer, respectively. Difference angles (ΔΦmax) were obtained from N responses of n cells of a given cell type and rescaled to a range of [−90°; 90°] before plotting. To include strong responses only, cases with nonsignificant median spike angle were excluded (Rayleigh test, α = 0.05). In the vast majority of cases, individual Φmax values preceded the passage of Φmax,pooled. B: bubble plots of spike rates at binned (10°) distances to the Φmax values of the individual responses (Φmax,dir). Data were obtained from N responses (including weaker responses; significant correlation only) of n cells to clockwise (black; left) and counterclockwise (red; middle) rotation of the polarizer, rescaled to an angular distance range of [−90°; 90°] and normalized to peak rate. Triangles and dashed lines indicate median values. C: to ease comparison, median values for both directions were normalized to their maxima and replotted together.
Responses to linearly polarized light with stationary E-vector angle.
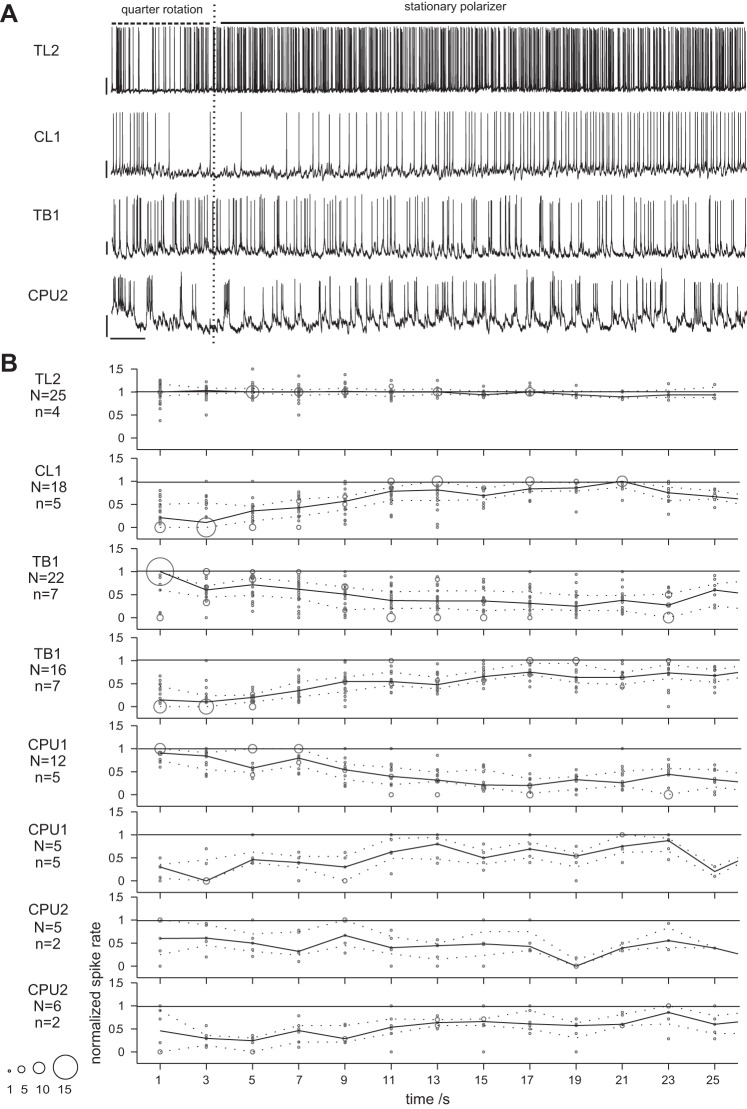
Above, we provided various characterizations of how spiking in different types of polarization-sensitive neurons is tuned to E-vector angle, as rated from responses to continuous rotations of the polarizer. To explore how E-vector angles are represented over time, we analyzed spiking activity recorded during presentation of polarized light with stationary orientation of the polarizer, lasting for about 20–30 s (Fig. 9A). In the majority of cases, we kept presenting the polarized light after tuning measurement and stopped subsequent rotations of the polarizer at positions that evoked a pronounced response. This approach had two advantages over a simple lights-on situation. First, continuous presentation of polarized light prevented interference by polarization-unrelated lights-on/lights-off responses. Second, a directly preceding response to the rotating polarizer indicates that the respective cell was actually in a polarization-sensitive state when the E-vector rotation stopped (i.e., when the stationary E-vector stimulus began). Because of the anticipatory character of responses to the rotating polarizer, the tested E-vector angles that evoked strong responses often differed substantially from the Φmin,pooled or Φmax,pooled values (see Fig. 8). As a consequence, it would have been misleading to group response data according to the distances of the stationary angles to these. Instead, we grouped recorded activity into putative cases of excitatory response, inhibitory response, or no response in a data-driven manner independent from the E-vector angle tested. For the tonic responses of TL2 neurons, classification relied on how the median spike rates compared with OA. If the median spike count of a putative response binned at 1-s resolution was equal to or higher than the third quartile of the spike count distribution obtained at the same resolution for OA, it was considered an excitatory response (25 out of 28 cases). Median spike rates equal to or lower than the 1st quartile of the OA data would have resulted in classification as an inhibitory response but have not been observed. For all other types of neurons, classification was based on the mere time course of spiking. Here, a simple criterion that was obtained from visual inspection of raw traces sufficed to allocate 89 out of 93 cases to either of 2 types of response. If the 1st occurrence of maximum (minimum) spike count in a 5-s bin PSTH fell within the 1st 10 s after stimulus onset, a sample was rated an excitatory (inhibitory) response. Otherwise, the spiking pattern was classified as neutral. Finally, binned spike rates were normalized to their overall median in case of tonic responses from TL2 neurons or, as for all other types of neuron, rescaled to a common interval of [0, 1].
Fig. 9.
Responses to presentation of linearly polarized light with stationary E-vector angle. A: exemplary responses to a stationary polarizer (15-s stimulus duration) preceded by a quarter rotation (90°, 3 s) of the polarizer. Dashed (solid) horizontal lines indicate periods with rotating (stationary) polarizer; vertical dashed lines indicate end of polarizer rotation. No response of a CPU1 neuron is shown because spike rates of pronounced responses were too high for resolved plotting. Bars = 10 mV, 1 s. B: time course of responses to a stationary polarizer. Within each cell type, data were pooled across N responses from n neurons and bubble-plotted for better visualization of scatter. To capture the mere time course of the responses, spike rates were normalized to the within-response median rate for TL2 neurons and to the within-response maximum for all other types of neuron. Solid line plots show median values; dashed line plots show lower and upper quartiles. Note that sampling size generally decreases toward later time bins. As expected from their E-vector tuning, responses to stationary angles were exclusively excitatory in TL2 neurons, exclusively inhibitory in CL1 neurons, and E-vector opponent in TB1 and CPU neurons. Responses of TL2 neurons were tonic, whereas responses to the specific E-vector angle in all other types of neuron quickly adapted.
In CL1 neurons, 5 out of 23 cases were actually rated excitatory according to the above-mentioned criterion (data not shown). We consider these to be false positive classifications, which might have arisen from the inherent dynamics of OA typical for CL1 neurons (cf. Fig. 2) and could have been promoted by low sample sizes in the later phase of the response time windows (down to 1 or 2 values for 15 s after stimulus onset and later).
Figure 9B illustrates the time courses of responses as captured by normalized spike rates binned at 2-s resolution. With regard to their direction (excitatory vs. inhibitory), responses to stationary E-vector angles matched the observations on the effective amplitudes of responses to polarizer rotation. TL2 neurons showed robust excitatory responses but no pronounced inhibitory modulation of spiking was observed, whereas the opposite was the case in CL1 neurons. By contrast, TB1 and CPU neurons proved capable of response opponency again with response courses being more coherent within a respective type of response in TB1 neurons, reflecting the differences in response variability between TB1 and CPU. In terms of time course, the most striking change again occurred at the early stage transition from TL2 to CL1 neurons: responses to stationary E-vector angles were tonic in TL2 neurons, whereas stimulus-specific adaptation (SSA) was encountered at all downstream stages of E-vector signaling. On average, the adapting responses faded to 50% in normalized amplitude within 6–10 s in CL1 and 8–12 s in TB1 and CPU neurons. Transitions to OA-like spiking occurred about 16–20 s after stimulus onset.
Novel types of polarization-sensitive neurons of the central complex.
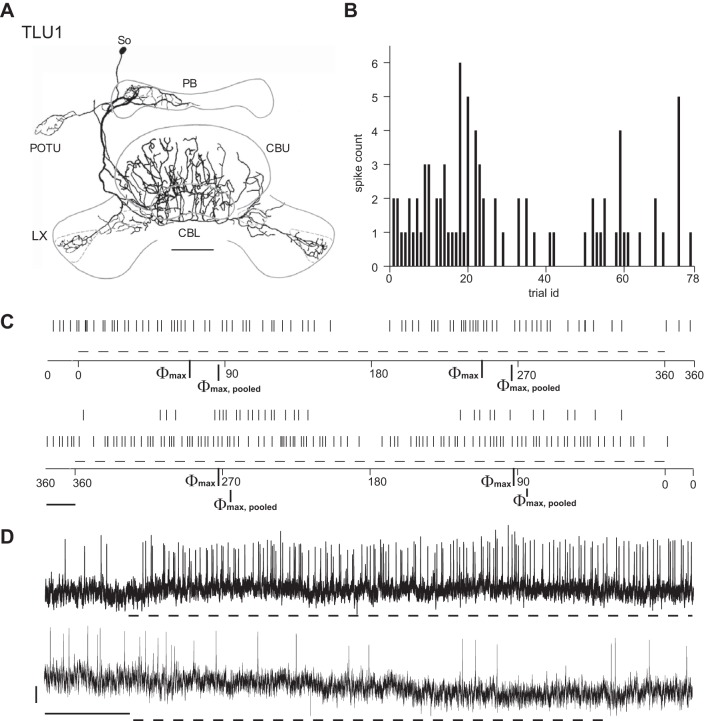
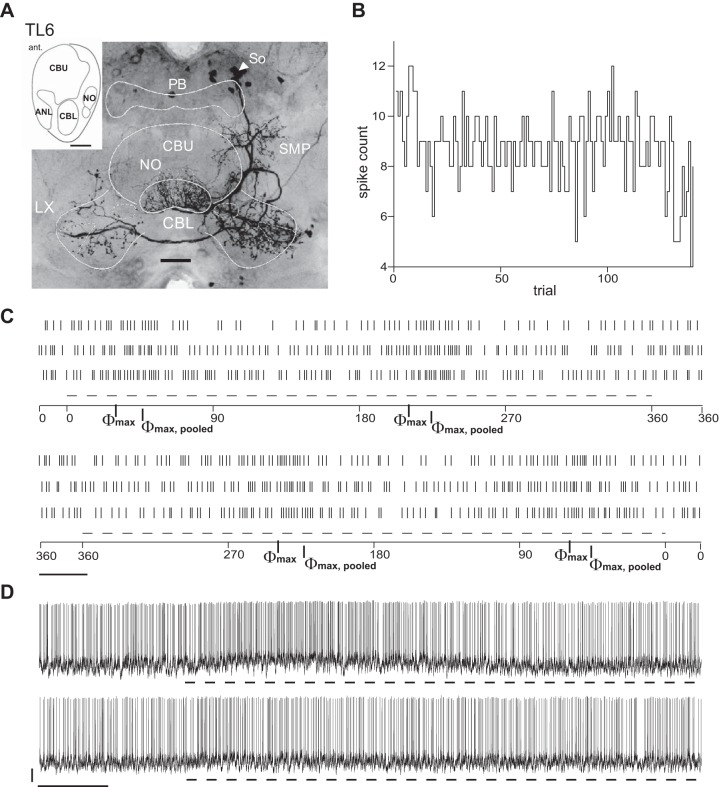
Two hitherto undescribed polarization-sensitive neurons of the central complex were termed TLU1 (Fig. 10) and TL6 (Fig. 11). Each of these neurons was recorded and stained only once. Both neurons have widefield ramifications in several substructures of the central complex and the lateral complexes. TLU1, a tangential neuron of the CBL and CBU, is the first polarization-sensitive neuron with tangential arborizations in the CBU reported so far and might serve an internal feedback role. Putative input regions with fine and smooth neurite endings include a hemisphere of the PB and one of the posterior optic tubercles, both ipsilateral to the cell body in the pars intercerebralis (Fig. 10A). Beaded and thus likely presynaptic endings invade the CBL, layers 1 and 2 of the CBU, and both lateral complexes. TL6 is a novel subtype of tangential neuron of the CBL. It has smooth ramifications in the superior medial protocerebrum, the noduli, and the CBL and wide beaded and thus presumably presynaptic endings in both lateral complexes (Fig. 11A).
Fig. 10.
Morphology, OA, and E-vector responses of the TLU1 neuron. A: reconstruction of the morphology of the cell. Putative input regions with fine and smooth neurite endings include a hemisphere of the PB and 1 of the POTU, both ipsilateral to the soma (So) in the pars intercerebralis. Beaded and thus likely presynaptic endings invade the CBL, layers 1 and 2 of the CBU, and both LX. B: spike counts in 1,000-ms trials of OA plotted against trial number and hence the time course of the experiment, although trials were not evenly distributed over time. The OA of TLU1 is low, including several trials with no spiking at all. C: raster plots of spiking responses to clockwise (top) and counterclockwise (bottom) rotations of the polarizer at 18°/s. Dashed lines beneath indicate periods of steady polarizer rotation. Between rotations, presentation of polarized light was preserved. Bar = 1 s. Φmax values for clockwise/counterclockwise rotations were 68/93°; Φmax,pooled was 86°. D: responses to polarized light with stationary E-vector angle. Dashed lines beneath the traces mark stimulus time windows. Traces show response at 35° (top) and 80° (bottom) distance to Φmax,pooled. Bars = 2 mV/5 s. The response is tonic and more pronounced near Φmax,pooled as expected from the data in C.
Fig. 11.
Morphology, OA, and E-vector responses of the TL6 neuron. A: projection view of the fluorescent labeling of the cell. TL6 has its soma located in the pars intercerebralis and arborizes in the ipsilateral SMP, throughout the CBL, the anterior lip (ANL; see schematic showing sagittal view of the central complex, inset) anterior to the CBL, and the paired noduli (NO) posterior to the central body. Endings in the SMP, CBL, and noduli appear smooth and relatively fine and thus presumably constitute input sites. Ramifications with beaded and thus presumably presynaptic endings span both LX, being more dense in the LX ipsilateral to the soma. B: spike counts in 1,000-ms trials of OA plotted against trial number and hence the time course of the experiment, although trials were not evenly distributed over time. C: raster plots of spiking responses to clockwise (top) and counterclockwise (bottom) rotations of the polarizer at 30°/s. Dashed lines indicate periods of steady polarizer rotation. Between rotations, presentation of polarized light was preserved. Bar = 1 s. Φmax values for clockwise/counterclockwise rotations were 31/59°; Φmax,pooled was 46°. TL6 appeared capable of a polarization-opponent response. D: responses to polarized light with stationary E-vector angle. Dashed lines mark stimulus time windows. Traces show responses at Φmax,pooled (top) and Φmin,pooled (bottom), respectively. Bars = 5 mV/5 s. The response at Φmax,pooled is transient. Although the TL6 neuron appeared capable of polarization-opponent responses as rated from the responses shown in C, no reduction in spike rate was observed under the presentation of the E-vector angle corresponding to Φmin,pooled.
Physiologically, TLU1 resembled neurons at the input stage, whereas TL6 shared more properties with intermediate and output stage neurons of the central complex. Similar to TL2 neurons at the input stage, TLU1 showed sparse background spiking and a strong, tonic response to its preferred E-vector angle (Φmax,pooled), whereas the response amplitude around Φmin,pooled appeared to be substantially lower, pointing to a lack of true polarization opponency (Fig. 10, B–D). In both neurons, responses to a rotating polarizer strongly depended on direction of polarizer rotation. TL6 spiked more frequently and showed rather moderate yet polarization-opponent responses to a rotating polarizer and a phasic response at its Φmax,pooled, reminiscent of polarization-sensitive cells at the intermediate (TB1) and output (CPU1 and CPU2) stages of the central-complex network. In contrast to TB1 and CPU neurons, however, TL6 showed no response to stationary presentation of its Φmin,pooled angle (Fig. 11, B–D). The processes of TLU1 and TL6 invade neuropils of the central complex and neighboring regions in highly unique patterns. Their complex, wide-spanning arborizations might serve to integrate various inputs or to impose operational states on the network.
DISCUSSION
From sensory input to premotor output in the locust central-complex polarization-sensitive network: the gross picture.
Our study provides novel insights into the dynamics of E-vector signaling at different stages of the polarization-vision network in the central complex of the locust. In TL2 neurons of the CBL, robust responses to E-vector angles are confined to a narrow range around Φmax. Responses are tonic as suggested by earlier recordings using shorter stimuli (Vitzthum et al. 2002), distinct from the rather low and regular OA, and relatively constant in informational content of the individual spike. This should establish a reliable representation of E-vector angles across the population of TL2 neurons at the input stage of the network.
The excitatory responses of TL2 neurons are fed onto ascending subtypes of CL1 neurons in an inverting manner, probably via GABAergic output of TL2 neurons, as suggested by Homberg et al. (1999). The average level of OA was higher in CL1 neurons than in TL2 neurons, and their inhibitory responses adapted rapidly. As a consequence, it appears to be the absence of spiking that holds more reliable E-vector information in the CL1 neurons encountered here. In line with this notion, their responses to the rotating polarizer were marked by increased variability (and thus by lower reliability) at Φmax compared with Φmin, particularly within the group of strongly responding neurons. A general increase in response variability from TL2 to CL1 neurons may trace back to superimposition by the more variable, statelike OA typical for CL1 neurons.
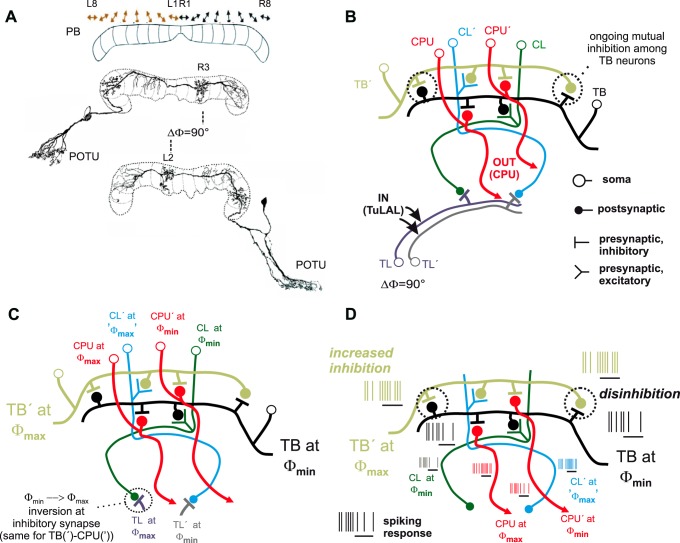
At the intermediate stage of the network, tangential neurons of the PB (TB1) responded more robustly to both Φmin and Φmax, which is referred to as polarization opponency. Here, the association between the spiking of the individual neuron and the acute E-vector angle was strong and relatively stable for responses to the rotating polarizer. Both phenomena stabilize the compasslike polarotopic mapping of E-vector angles across the PB reported by Heinze and Homberg (2007). They may arise from antagonistic integration across CL1 neurons with opponent tuning (Fig. 12). In individual CL1 neurons, the difference between extreme states of OA readily matches the difference in spike rate between E-vector responses at Φmax and Φmin. Thus the mere observation of the spiking of an individual CL1 neuron cannot suffice for unambiguous signaling of E-vector angles. In theory, this ambiguity could be resolved by inhibitory coupling within pairs of TB1 neurons, whereat each of two paired TB1 neurons would receive input from a CL1 neuron via noninverting synapses. If the two CL1 neurons are tuned to Φmin angles 90° apart, their antagonistic integration should result in the polarization opponency found in TB1 neurons (Fig. 12D). This antagonistic integration might also smooth out the pronounced statelike variability of OA that interferes with polarization signaling in CL1 neurons. In addition to explaining how polarization opponency in TB neurons arises, the model unravels the redundancy of the polarotopic representation across the width of the PB as a mere byproduct of the wiring that establishes mutual inhibition among TB neurons. In other words, the second, “redundant” representation of another full 180° consists of those arborizations making up the inhibitory TB-TB connections.
Fig. 12.
A mutual inhibition model of polarization-opponent E-vector responses in TB1 neurons. Robust polarization opponency of E-vector responses might arise from mutual inhibition among TB1 neurons that receive input from opponently tuned CL1 neurons. A: polarotopy in the PB (top) and relevant morphological features of TB1 neurons. The PB holds a redundant polarotopic representation of E-vector angles, covering 2 × 180° across the 16 vertical slices of the neuropil (corresponding to 180° across 8 slices per hemisphere). Double arrows symbolize the Φmax values of TB neurons that have varicose and hence putatively presynaptic terminals in the respective slices of the PB sketched beneath. Each TB1 neuron has 2 distinct columns of presynaptic arborizations lying 8 slices apart from one another and hence in different hemispheres of the PB. Smooth and thus presumably dendritic endings span 3 neighboring slices in each hemisphere with the proximalmost (relative to soma position) of the 3 lying 1 slice distal to the respective varicose column. The particular TB1 neurons shown here are tuned to Φmax values 90° apart. According to the general morphology described above, their presynaptic columns lie 4 slices apart, being congruent with slices that hold dendritic columns of the putative partner TB neuron. B: presumed synaptic wiring among basic types of central-complex neurons involved in the model. Input to the network is provided onto TL neurons by TuLAL neurons connecting the anterior optic tubercles to the lateral accessory lobes (see Fig. 1). The model posits inhibitory synapses between TL and CL neurons as well as within pairs of TB neurons and between TB and CPU neurons with synaptic partners being tuned to Φmax values 90° apart as sketched in C. D: hypothetical network response, as expected from the wiring pattern and resultant tuning relationships depicted in B and C, to an E-vector that matches Φmax for the input TL neuron to the left in the diagram and Φmin for the second one, labeled TL′. Black horizontal lines beneath the stylized spike trains mark stimulus time windows (adaptation to further ongoing stimulation not shown). In particular, the TB neuron for which the stimulus E-vector angle matches Φmin receives reduced excitatory input from its partner CL neuron and increased inhibitory input from its partner TB neuron (TB′) for which, in turn, the same stimulus E-vector angle corresponds to Φmax. The reduced activity of the TB neuron at its Φmin releases its partner TB′ from inhibition via the TB-TB′ synapse, thus adding enhancement to the excitatory input TB′ receives from its partner CL′. Note that the activity of CL′ at its Φmax is comparatively high but not distinct from higher levels of its ongoing activity, whereas the mechanism of mutual inhibition/disinhibition among TB neurons provides a basis for truly polarization-opponent responses downstream to CL neurons.
The enhancement in CS at the transition from CL1 to TB1 neurons is accompanied by both a stabilization of informational content and a reduction in response amplitude in terms of overall VS (Fig. 13A). This is suggestive of a CS-VS tradeoff to be a crucial early step to bundle a distributed sensory representation into pooled premotor output (of CPU neurons), which is meaningful over the entire range of E-vectors, even if its overall VS is lower compared with the input stage. Furthermore, this observation speaks against alternative models that could assume a process of reading out the compass by thresholding the responses of TB1 neurons and comparing the result across slices of the PB because the thresholding of response rates would be hampered by the reduction in response amplitude.
A second inversion of responses presumably occurs at the transition from TB1 to CPU neurons near the output stage of the network, as indicated by the near 90° phase shift in the polarotopy between TB1 and CPU neurons that arborize in the same slice of the PB (Heinze and Homberg 2007; Fig. 13B). In CPU neurons, the variability of responses was particularly high with respect to general responsiveness (i.e., CS), response amplitude, and IpS. At this stage, average response amplitudes are negatively related to the average levels of OA of the cells. This is suggestive of a masking of polarization responses by high-level OA that lasts throughout the 15- to 45-min period of a recording session. In CPU2 neurons, the resultant span of response strength ranges from effective unresponsiveness to a pronounced polarization opponency. This resembles tuning profiles of polarization-sensitive descending neurons (Träger and Homberg 2011) and is in concert with the variability of polarotactic responses to modulations of zenithal E-vector angle observed in tethered flying locusts (Mappes and Homberg 2004) and in crickets walking on an air-suspended ball (Brunner and Labhart 1987).
A working hypothesis on central-complex function.
E-vector signaling at the output stage of the central-complex network appears to be governed by variations in responsiveness in terms of lasting responsiveness states that are related to the level of OA. Such modulation of higher-stage responsiveness to exteroceptive cues could assign behavioral meaning to these cues. Future studies should strive to identify the modulators. These might depend on experience and lifestyle and be related to circadian rhythms, feeding states, and locomotor states such as resting, flying, or walking. The indicators of these states could comprise representations of exteroceptive cues such as airflow or visual flow as well as idiothetic information from proprioceptive feedback or motor-efference copies. First insights into this subject were provided by recordings from tethered flying locusts that revealed flight-correlated activity changes in neurons of the lateral accessory lobes, the main input-output relays of the central-complex network (Homberg 1994). Notably, the neurons that changed activity in a flight-correlated manner included CPU2 neurons. CPU2 neurons arborize in the CBU (see Fig. 1) where they might receive sensory information that signals locomotor state and thus, presumably, the acute behavioral relevance of compass information (el Jundi et al. 2010). The morphological complexities of the newly discovered TLU1 and TL6 neurons are also in line with the notion of the central-complex network as a site for complex integration and modulation (Figs. 10 and 11).
In addition to lasting responsiveness states, we have observed response features suited to link compass signaling to the behavioral goal to stay oriented. Responses to constant stimuli in terms of polarized light with stationary E-vector angle were marked by SSA in all cell types downstream of TL2 neurons (Fig. 13C). This may correlate with the tendency to steer a steady course relative to a polarization pattern that was observed in tethered flying locusts (Mappes and Homberg 2004): as long as the locust stays on course, the neurons no longer modulate their firing as a function of E-vector angle. Responsiveness to varying E-vectors that, under a natural sky, indicate changes in heading direction was generally preserved but more variable at the output stage of the polarization-vision network (Fig. 13). Here, anticipation of near-future E-vector angles additionally promotes compass orientation.
Together, the findings outlined above support the concept of the central complex as a substrate of the contextualization of sensory information for locomotor control in goal-driven behaviors, demonstrated in flies (Strauss 2002; Triphan et al. 2010) and cockroaches (Bender et al. 2010; Guo and Ritzmann 2013). To this end, robust sensory inputs to the network may become contextualized by certain response features such as SSA as well as via modulation by state indicators to be integrated in the formation of motor programs or not, depending on current behavioral goals. A recent study of the encoding of food odor value in the Drosophila brain suggests that this preliminary model on central-complex function might hold across species and sensory modalities (Beshel and Zhong 2013). The activity of neurons that invade the fan-shaped body, the fly's homolog of the CBU, reflected the behaviorally indicated attractiveness of food odors as a function of the animal's feeding state. It thus exceeds the role of a mere sensory representation of the odors by adding a context-dependent weighing to it, providing a signaling of the acute value of a respective food odor that is predictive of a locomotor approach response to the odor source.
Parallels to vertebrates.
Our findings point to three ways in which polarization signaling in the locust brain parallels higher sensory processing in vertebrate brains: the coshaping of responses by OA, the specific adaptation to constant stimuli, and the prediction of upcoming stimuli from recent stimulus history.
In addition to the relationship between responsiveness and the level of OA in CPU neurons, a modulatory role for OA is also suggested by the fact that during polarizer rotation, spike rates at neutral E-vector angles closely matched those of OA, in particular in TB1 and CPU neurons. This may point to the observed response as resulting from superimposition of an ideal E-vector response with acute levels of OA. In vertebrates, an integration of prestimulus OA with an ideal representation of a visual stimulus was shown to explain the large variability in V1 responses (Arieli et al. 1996). Several studies confirmed that variations in prestimulus OA can actually predict stimulus detection rate in monkey vision (Supèr et al. 2003) as well as perceptual decisions on ambiguous stimuli in both human vision (Hesselmann et al. 2008) and human somatosensation (Boly et al. 2007). In those studies, states of OA were referred to as brain states related to attention or vigilance.
Responses to constant stimuli (i.e., polarized light with stationary E-vector angle) were marked by SSA in all cell types downstream of TL2 neurons. In vertebrates, SSA is a prominent feature of higher-stage auditory processing and a presumed correlate of behavioral habituation (Netser et al. 2011), as we hypothesize here for orientation responses in locusts. Work in progress suggests that SSA in the locust central complex extends to other types of visual stimuli as well.
Previous work (Heinze and Homberg 2007) has drawn an analogy between polarization-sensitive compass neurons in the locust central complex and vertebrate head-direction (HD) cells, discovered in rats by Ranck (1984) and studied in detail by Taube and colleagues (Clark and Taube 2012; Taube 2007). Rat HD cells refer to visual landmarks that signal heading direction in local sceneries; they are low in OA and fire to their preferred stationary HD (Φmax,stationary) with a Gaussian-shaped tuning covering ∼90° centered to Φmax,stationary. Our current observations extend the analogy between vertebrate HD cells and polarization-sensitive neurons in the locust central complex to anticipatory signaling under rotatory stimulation. However, the pronounced asymmetry of spiking around Φmax (i.e., the difference between Φmax and the angle at peak firing) that occurred in polarization-sensitive neurons has not been reported for HD cells. Its presence could be confined to rotation velocities below those applied in the rat studies. In fact, the shift of angular tuning in HD cells is a function of rotation velocity: it is adjusted as to result in anticipation of the preferred angle by an invariant, cell-type-specific time interval of about 25–75 ms. Future studies should aim to elucidate whether the anticipatory shift in locust polarization-sensitive neurons is also invariant in time period or, alternatively, constant in the angular domain or a function of rotation velocity in both respects. In HD cells, anticipation is believed to depend on idiothetic indicators of head motion, such as vestibular signals, proprioceptive signals, and motor-efference copies. By contrast, the predictive signaling by polarization-sensitive neurons we observed apparently does not require idiothetic indication of head movements, as locusts were mounted to a holder with their heads immobilized. Rather, it might be controlled by a mechanism that includes information on the velocity and direction of (apparent) rotatory movements inferred from the stimulus history per se (i.e., from the E-vector angles encountered in the near past and the corresponding time intervals).
Adaptation to constant head orientation has not been reported in rat HD cells. In fact, they show sustained activity during periods that lack visual cueing, a feature beneficial for tasks of path integration (dead reckoning), such as keeping track of changes in orientation in the dark (see Taube 2007 for review). This holds for HD cells encountered at various processing stages and might point to a difference between the navigational strategies applied by rats and locusts. Rats typically explore their environment by collecting visual cues and turning the head while locomoting about the local scenery. Spatial learning based on this strategy may strongly depend on exact and ongoing path integration. Locusts could refer to sky compass signals for the simpler purpose of locomotion in a straight direction, independent from how this direction may relate to specific features of the present local scenery (Mappes and Homberg 2004).
Methodological considerations on the interpretation of the novel response measures.
We have characterized E-vector responses with measures of CS (quantified by r2) and VS (quantified by |r|). Below, we provide some reflection on the adequacy and informative value of these measures as a basis for future discussions of E-vector responses within this framework. The rationale for which we refer to VS as an indirect measure of modulation depth (h) rather than to calculate h directly (as the ratio of modulation amplitude to carrier amplitude) is that estimating a carrier amplitude for the E-vector response from activity during rotation of the polarizer is not trivial, albeit the recent results show that one might approximate it in higher-stage neurons by the overall mean or the spike rate at a rather neutral angle such as Φmax ±45°.
Although the statistical interpretation of r2 and |r| alone is straight, their numerical relation is not trivial (see Fig. 7), and their respective relevance may depend on physiological concepts. A perfect correlation between spiking and E-vector angle (r2 = 1) may concur with minimum modulation in spike rate (|r| ≪1). An increase in |r| does not necessitate an increase in r2 and may well concur with a decrease in it. If different levels of uncorrelated, constant amplitude offsets are added to the same artificial spike-rate signal (i.e., if its carrier amplitude is varied while its absolute modulation amplitude, peak width, and period remain unchanged), |r| behaves positively related to relative h, but r2 does not change. For the characterization of responses to a rotating polarizer, the criterion for principle responsiveness to E-vectors should be the statistical significance of the correlation between spiking and E-vector angle (the P value of r2 at α = 0.05 and β = 0.2).
For physiological interpretation, we suggest considering CS and VS in a processing-stage-dependent manner. At the input level, high VS may reflect sharp E-vector tuning within peripheral polarotopic channels, a prerequisite for enhanced CS in higher-stage responses integrated across channels, whereas within-channel CS may be comparatively low. Near the output stage of the central-complex network, enhanced CS could help to bundle the activity of the polarotopic population of neurons into a premotor output, which is meaningful over the entire range of E-vectors even if its overall VS is lower compared with the input stage, corresponding to the CS-VS tradeoff discussed above. Still, VS may serve to quantify differences in amplitude between higher-stage responses that have equal CS but are superimposed by different levels of uncorrelated OA. In case of complete masking of an E-vector dependency by superimposition of uncorrelated OA, very low VS is accompanied by nonsignificant and numerically small r2.
Conclusion.
Studies of insect sensory systems and behavior have shown that (and in part explained how) higher goal-driven behavior can be controlled by minute brains. For instance, visual processing low on the phylogenetic scale can mediate spatial orientation across a range of scales (obstacle avoidance and navigation) and crucial event-related behavior (escape predators and catch prey) most precisely and efficiently despite lacking the conscious perceptual organization of visual landscapes that we readily experience during comparable tasks (e.g., see Milner and Goodale 1995). We have described dynamics of compass signaling in an insect brain suited to mediate the control of locomotion in a navigation-like fashion, in particular by relating instantaneous compass signaling to stimulus history via anticipation and adaptation. Current work addresses the additional modulation of this compass signaling by events in the visual object-background scenery.
GRANTS
Support for this study was provided by Deutsche Forschungsgemeinschaft Grants HO 950/16-3, HO 950/21-1, and HO 950/23-1 to U. Homberg.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.B. and U.H. conception and design of research; T.B. performed experiments; T.B. analyzed data; T.B. and U.H. interpreted results of experiments; T.B. prepared figures; T.B. drafted manuscript; T.B. and U.H. edited and revised manuscript; T.B. and U.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Keram Pfeiffer for feedback on data analyses, Arseny Finkelstein for alluding to the information-theoretic approaches by Skaggs and colleagues, Joss von Hadeln for the reconstruction of the TLU1 neuron, Milosz Krala and Stefan Ries for participation in some experiments, and Dr. Erich Buchner for supplying anti-synapsin antibodies.
REFERENCES
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273: 1868–1871, 1996. [DOI] [PubMed] [Google Scholar]
- Ashida G, Wagner H, Carr CE. Processing of phase-locked spikes and periodic signals. In: Analysis of Parallel Spike Trains, edited by Grün S, Rotter S. New York: Springer, 2010, p. 59–74. [Google Scholar]
- Bech M, Homberg U, Pfeiffer K. Receptive fields of locust brain neurons are matched to polarization patterns of the sky. Curr Biol 24: 2124–2129, 2014. [DOI] [PubMed] [Google Scholar]
- Bender JA, Pollak AJ, Ritzmann RE. Neural activity in the central complex of the insect brain is linked to locomotor changes. Curr Biol 20: 921–926, 2010. [DOI] [PubMed] [Google Scholar]
- Berens P. CircStat: a MATLAB toolbox for circular statistics. J Stat Softw 31: 2009. [Google Scholar]
- Beshel J, Zhong Y. Graded encoding of food odor value in the Drosophila brain. J Neurosci 33: 15693–15704, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquett P, Laureys S. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA 104: 12187–12192, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D, Labhart T. Behavioural evidence for polarization vision in crickets. Physiol Entomol 12: 1–10, 1987. [Google Scholar]
- Clark BJ, Taube JS. Vestibular and attractor network basis of the head direction cell signal in subcortical circuits. Front Neural Circuits 6: 7, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AN, May TE. Studies on locust neuromuscular physiology in relation to glutamic acid. J Exp Biol 60: 673–705, 1974. [DOI] [PubMed] [Google Scholar]
- el Jundi B, Heinze S, Lenschow C, Kurylas A, Rohlfing T, Homberg U. The locust standard brain: a 3D standard of the central complex as a platform for neural network analysis. Front Syst Neurosci 3: 21, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Jundi B, Pfeiffer K, Heinze S, Homberg U. Integration of polarization and chromatic cues in the insect sky compass. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 200: 575–589, 2014. [DOI] [PubMed] [Google Scholar]
- el Jundi B, Pfeiffer K, Homberg U. A distinct layer of the medulla integrates sky compass signals in the brain of an insect. PLoS One 6: e27855, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher NI. Statistical Analysis of Circular Data (revised ed.). Cambridge, UK: Cambridge Univ. Press, 1995. [Google Scholar]
- Frost BJ, Mouritsen H. The neural mechanisms of long distance animal navigation. Curr Opin Neurobiol 16: 481–488, 2006. [DOI] [PubMed] [Google Scholar]
- Guo P, Ritzmann RE. Neural activity in the central complex of the cockroach brain is linked to turning behaviors. J Exp Biol 216: 992–1002, 2013. [DOI] [PubMed] [Google Scholar]
- Heinze S, Gotthardt S, Homberg U. Transformation of polarized light information in the central complex of the locust. J Neurosci 29: 11783–11793, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Linking the input to the output: new sets of neurons complement the polarization vision network in the locust central complex. J Neurosci 29: 4911–4921, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Maplike representation of celestial E-vector orientations in the brain of an insect. Science 315: 995–997, 2007. [DOI] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Neuroarchitecture of the central complex of the desert locust: intrinsic and columnar neurons. J Comp Neurol 511: 454–478, 2008. [DOI] [PubMed] [Google Scholar]
- Heinze S, Reppert SM. Sun compass integration of skylight cues in migratory monarch butterflies. Neuron 69: 345–358, 2011. [DOI] [PubMed] [Google Scholar]
- Hesselmann G, Kell CA, Eger E, Kleinschmidt A. Spontaneous local variations in ongoing neural activity bias perceptual decisions. Proc Natl Acad Sci USA 105: 10984–10989, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg U. Flight-correlated activity changes in neurons of the lateral accessory lobes in the brain of the locust Schistocerca gregaria. J Comp Physiol A 175: 597–610, 1994. [Google Scholar]
- Homberg U. In search of the sky compass in the insect brain. Naturwissenschaften 91: 199–208, 2004. [DOI] [PubMed] [Google Scholar]
- Homberg U, Heinze S, Pfeiffer K, Kinoshita M, el Jundi B. Central neural coding of sky polarization in insects. Philos Trans R Soc Lond B Biol Sci 366: 680–687, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg U, Vitzthum H, Müller M, Binkle U. Immunocytochemistry of GABA in the central complex of the locust Schistocerca gregaria: identification of immunoreactive neurons and colocalization with neuropeptides. J Comp Neurol 409: 495–507, 1999. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Pfeiffer K, Homberg U. Spectral properties of identified polarized-light sensitive interneurons in the brain of the desert locust Schistocerca gregaria. J Exp Biol 210: 1350–1361, 2007. [DOI] [PubMed] [Google Scholar]
- Kreuz T, Chicharro D, Greschner M, Andrzejak RG. Time-resolved and time-scale adaptive measures of spike train synchrony. J Neurosci Methods 195: 92–106, 2011. [DOI] [PubMed] [Google Scholar]
- Mappes M, Homberg U. Behavioral analysis of polarization vision in tethered flying locusts. J Comp Physiol A 190: 61–68, 2004. [DOI] [PubMed] [Google Scholar]
- Massey FJ. The Kolmogorov-Smirnov test for goodness of fit. J Am Stat Assoc 46: 68–78, 1951. [Google Scholar]
- Merlin C, Heinze S, Reppert SM. Unraveling navigational strategies in migratory insects. Curr Opin Neurobiol 22: 1–9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. The Visual Brain in Action. Oxford, UK: Oxford Univ. Press, 1995. [Google Scholar]
- Mouritsen H. Navigation in birds and other animals. Image Vision Comput 19: 713–731, 2001. [Google Scholar]
- Müller M, Homberg U, Kühn A. Neuroarchitecture of the lower division of the central body in the brain of the locust (Schistocerca gregaria). Cell Tissue Res 288: 159–176, 1997. [DOI] [PubMed] [Google Scholar]
- Netser S, Zahar Y, Gutfreund Y. Stimulus-specific adaptation: can it be a neural correlate of behavioral habituation? J Neurosci 31: 17811–17820, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuser K, Triphan T, Mronz M, Poeck B, Strauss R. Analysis of a spatial orientation memory in Drosophila. Nature 453: 1244–1247, 2008. [DOI] [PubMed] [Google Scholar]
- Ofstad TA, Zuker CS, Reiser MB. Visual place learning in Drosophila melanogaster. Nature 474: 204–207, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer K, Homberg U. Coding of azimuthal directions via time-compensated combination of celestial compass cues. Curr Biol 17: 960–965, 2007. [DOI] [PubMed] [Google Scholar]
- Pfeiffer K, Homberg U. Organization and functional roles of the central complex in the insect brain. Annu Rev Entomol 59: 165–184, 2014. [DOI] [PubMed] [Google Scholar]
- Ranck JB., Jr Head-direction cells in the deep cell layers of dorsal presubiculum in freely moving rats. Soc Neurosci Abstr 10: 599, 1984. [Google Scholar]
- Ritzmann RE, Harley CM, Daltorio KA, Tietz BR, Pollak AJ, Bender JA, Guo P, Horomanski AL, Kathman ND, Nieuwoudt C, Brown AE, Quinn RD. Deciding which way to go: how do insects alter movements to negotiate barriers? Front Neurosci 6: 97, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Gothard KM, Markus EJ. An information-theoretic approach to deciphering the hippocampal code. Adv Neural Inf Process Syst 5: 1030–1037, 1993. [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6: 149–172, 1996. [DOI] [PubMed] [Google Scholar]
- Strauss R. The central complex and the genetic dissection of locomotor behavior. Curr Opin Neurobiol 12: 633–638, 2002. [DOI] [PubMed] [Google Scholar]
- Strutt JW. On the light from the sky, its polarization and colour. Philos Mag 41: 107–120, 274–279, 1871a. [Google Scholar]
- Strutt JW. On the scattering of light by small particles. Philos Mag 41: 447–454, 1871b. [Google Scholar]
- Supèr H, van der Togt C, Spekreijse H, Lamme VA. Internal state of monkey primary visual cortex (V1) predicts figure-ground perception. J Neurosci 23: 3407–3414, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci 30: 181–207, 2007. [DOI] [PubMed] [Google Scholar]
- Träger U, Homberg U. Polarization-sensitive descending neurons in the locust: connecting the brain to thoracic ganglia. J Neurosci 31: 2238–2247, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triphan T, Poeck B, Neuser K, Strauss R. Visual targeting of motor actions in climbing Drosophila. Curr Biol 20: 663–668, 2010. [DOI] [PubMed] [Google Scholar]
- Vitzthum H, Müller M, Homberg U. Neurons of the central complex of the locust Schistocerca gregaria are sensitive to polarized light. J Neurosci 22: 1114–1125, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis (4th ed). Upper Saddle River, NJ: Prentice Hall, 1999. [Google Scholar]