Abstract
Traumatic brain injury (TBI) most frequently occurs in pediatric patients and remains a leading cause of childhood death and disability. Mild TBI (mTBI) accounts for nearly 75% of all TBI cases, yet its neuropathophysiology is still poorly understood. While even a single mTBI injury can lead to persistent deficits, repeat injuries increase the severity and duration of both acute symptoms and long-term deficits. In this study, to model pediatric repetitive mTBI (rmTBI) we subjected unrestrained juvenile animals (postnatal day 20) to repeat weight-drop impacts. Animals were anesthetized and subjected to sham injury or rmTBI once per day for 5 days. Magnetic resonance imaging (MRI) performed 14 days after injury revealed marked cortical atrophy and ventriculomegaly in rmTBI animals. Specifically, beneath the impact zone the thickness of the cortex was reduced by up to 46% and the area of the ventricles increased by up to 970%. Immunostaining with the neuron-specific marker NeuN revealed an overall loss of neurons within the motor cortex but no change in neuronal density. Examination of intrinsic and synaptic properties of layer II/III pyramidal neurons revealed no significant difference between sham-injured and rmTBI animals at rest or under convulsant challenge with the potassium channel blocker 4-aminopyridine. Overall, our findings indicate that the neuropathological changes reported after pediatric rmTBI can be effectively modeled by repeat weight drop in juvenile animals. Developing a better understanding of how rmTBI alters the pediatric brain may help improve patient care and direct “return to game” decision making in adolescents.
Keywords: concussion, electrophysiology, MRI, pediatrics, traumatic brain injury
traumatic brain injury (TBI) is a significant health concern that affects more than 1.5 million Americans each year (Faul et al. 2010; Langlois et al. 2006; Rutland-Brown et al. 2006). At present, no single classification system has been developed that encompasses the host of clinical, pathological, behavioral, and cellular changes that occur as a result of TBI. In general, TBI is categorized into mild, moderate, and severe. Mild TBI (mTBI), including concussions, accounts for nearly 75% of all TBI cases (Cassidy et al. 2004; Elder and Cristian 2009; Langlois et al. 2005, 2006; Miniño et al. 2006). mTBI is often called an “invisible wound,” as it results in a minimal loss of consciousness (<30 min) and minimal acute neuropathological findings (Carroll et al. 2004; Morey et al. 2013; Smith et al. 2013). Consequently, mTBI is often difficult to detect and diagnose in the early acute stages after injury and may result in the incidence being underreported. After mTBI patients may experience cognitive and behavioral impairments including confusion, memory and attention deficits, and headaches (Barkhoudarian et al. 2011). These symptoms usually resolve completely within 2–3 wk after a single mTBI (Lovell et al. 2003; McCrea et al. 2003). However, especially with repeat injuries, these symptoms may persist for extended periods of time (Arciniegas et al. 2005; Halstead et al. 2010; Pellman et al. 2003).
Repetitive mTBI (rmTBI) significantly increases symptom severity (Collins et al. 2002), leads to longer-term cognitive and motor deficits (De Beaumont et al. 2007; Guskiewicz 2011; Omalu et al. 2010b), and increases the risk for developing dementia (Guskiewicz et al. 2005) and neurodegenerative disorders (Masel and DeWitt 2010; McKee et al. 2009, 2010; Plassman et al. 2000). Even a single mTBI event places patients at a greater risk for further TBI events and the ensuing consequences of rmTBI (Barkhoudarian et al. 2011; MacGregor et al. 2011; Tremblay et al. 2013; Zemper 2003). In contrast to a single mTBI event, rmTBI induces significant long-term structural changes to the brain including brain atrophy and enlargement of the ventricles (Giza 2006; Huh et al. 2007; Maxwell 2012; Smith et al. 2013; Wang et al. 2014). Currently no effective treatments are available to prevent the adverse complications associated with rmTBI. Development of new therapeutic strategies is contingent on an improved understanding of the underlying pathophysiological processes induced by rmTBI.
Recent public and research attention has focused on understanding rmTBI that occurs in adult athletes and military personnel. However, recent reports indicate that children may be particularly susceptible and sensitive to the effects of TBI (Barlow et al. 2010; Eisenberg et al. 2013; Field et al. 2003; Guskiewicz et al. 2000; Kontos et al. 2013). In children, TBI remains a leading cause of death and disability (Faul et al. 2010), with >10% experiencing a mTBI by the age of 10 (Barlow et al. 2010; Bruns and Hauser 2003). As with adults, the source of TBI varies greatly in children, but it may occur from a combination of events including accidents, abuse (shaken baby syndrome), or adolescent sport concussions. The pediatric brain is different from the adult brain owing to a host of ongoing neurodevelopmental processes including cortical hypertrophy, synaptogenesis, use-dependent pruning, enhanced glucose metabolism, increased neurotrophic factors, and altered excitatory amino acid receptors (Adelson 1999; Chugani et al. 1987; Friedman et al. 1991; Giza 2006; Insel et al. 1990). These processes have often been thought to confer children with an advantage in coping with brain injury, but recent evidence suggests they may be particularly sensitive to the effects of rmTBI (Eisenberg et al. 2013; Field et al. 2003).
Several models of TBI have been developed (Angoa-Pérez et al. 2014; Cernak 2005; Xiong et al. 2013), but many are intended to induce a more severe TBI and do not effectively model mTBI. In this study, we modified a recently published adult weight-drop rmTBI model (Kane et al. 2012) for use in juvenile rats. This method of inducing rmTBI recapitulates the nature of the injury (closed head injury, unrestrained animal, linear and rotational acceleration forces) and was recently shown to produce several of the cognitive and behavioral outcomes associated with clinical mTBI (Kane et al. 2012; Meaney and Smith 2011; Viano et al. 2007). With the use of this method, in juvenile rats a single mTBI was sufficient to induce clinically relevant impairments to executive, motor, and balance functions (Mychasiuk et al. 2014). The durations of these impairments are variable, but they have been reported to persist for up to months after mTBI (McCrea et al. 2003; Petraglia et al. 2014). The purpose of this study was to examine the neuropathological and neurophysiological changes induced early after rmTBI in juvenile rats with magnetic resonance imaging (MRI), immunohistochemical, and electrophysiological approaches. The results validate the use of this repetitive weight-drop method to effectively model pediatric rmTBI. Similar to what has been reported in humans (Halstead et al. 2010; McCrea et al. 2003; Smith et al. 2013), rmTBI in juvenile rats induced marked cortical atrophy (i.e., decreased cortical thickness) and enlarged ventricles that were most pronounced beneath the impact site. Despite significant cortical atrophy, no intrinsic or synaptic electrophysiological changes were evident in layer II/III neurons recorded from rmTBI animals 14 days after injury. These findings are in contrast to the recent report from adult rodents (Kane et al. 2012) and after single mTBI (Mychasiuk et al. 2014) and suggest that impact number, severity, and age are critical determinants of the pathophysiological changes following rmTBI.
MATERIALS AND METHODS
All procedures were performed under protocols approved by the University of Arizona Institutional Animal Care and Use Committee.
Repetitive mild traumatic brain injury.
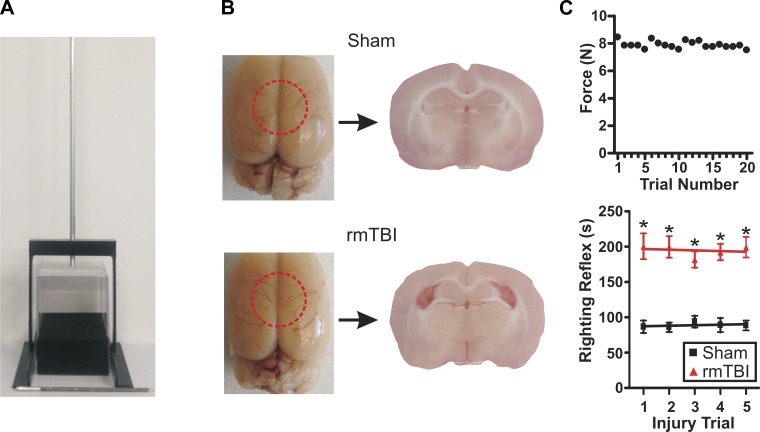
To experimentally model rmTBI we modified a recently developed model by Kane et al. (2012). This model replicates many of the clinical characteristics and mechanics of a mTBI injury including low impact force, low incidence of skull fracture and subdural hematoma, no immediate or early seizures, and no gross cavitation at the impact site. This model has been shown to effectively induce and model rmTBI in adult mice and was here modified to model pediatric rmTBI in juvenile rats. In brief, 20-day-old (P20) male Sprague-Dawley rats were subjected to a single mTBI once per day for 5 consecutive days (Fig. 1). Rats were lightly sedated via isoflurane inhalation and immediately placed ventral side down on a tightly stretched Kimwipe secured to a Plexiglas stage (Fig. 2A). The animal's head was then carefully centered under the vertical aluminum guide tube. As the animal's skull and skin remained completely intact, the animal's position was carefully adjusted using external anatomical landmarks (i.e., ear canals, eyes) so that impacts occurred between the bregma and lambda sutures. The impact weight (92 g, 9-mm diameter) was then positioned at the top of the aluminum tube so that the bottom of the weight was precisely 865 mm above the animal's head and was allowed to fall freely down the aluminum guide tube. The guide tube is threaded to allow for careful adjustment between animals to ensure accurate positioning of the guide tube and impact location (Fig. 2A). The force of the impact caused the animal to break through the stretched Kimwipe, rotate 180°, and land dorsally on the foam pad below. The rat falls away from the impact weight, and no secondary impacts were observed. The animal's movement after impact is not mechanically constrained, allowing simulation of the rotational and linear acceleration and deceleration forces most often associated with this type of injury. The animal was then placed in a supine position and monitored for righting reflex time. Righting reflex was defined as the animal's ability to right itself from a supine to a prone position. Once righted and ambulatory the animal was placed back into its home cage and monitored daily. In the rmTBI animal group, this procedure was repeated once per day for a total of five impacts. Age-matched sham-injured animals were given anesthesia, underwent mock impacts, and were placed in a supine position to test righting reflex times. After the fifth sham injury or rmTBI, animals were again monitored daily but left for 14 days to recover before further experimentation. All “postinjury day” (PID) descriptions were calculated as days between last impact and day of death.
Fig. 1.

Experimental timeline. Overview of the timeline used to model repetitive mild traumatic brain injury (rmTBI). Arrowheads represent time of single impact repeated once daily for 5 days. Control animals were given anesthesia only. Postinjury day (PID) indicates number of days after the 5th rmTBI injury.
Fig. 2.
Experimental model of rmTBI. A: photograph of rmTBI device and impact weight. B, left: photographs of brains acutely prepared 14 days after sham injury or rmTBI (1 impact/day for 5 days) in juvenile (P20) rats. Red dashed circle indicates approximate site of impact. Right: photograph of coronal brain slices taken from respective sham-injured or rmTBI brains. Note the presence of enlarged ventricles and cortical thinning after rmTBI. C, top: scatterplot of impact force measurements taken across 20 trials (weight 92 g, drop height 865 mm). Bottom: line graph of average righting reflex time in sham-injured (n = 38) and rmTBI (n = 42) animals across the 5 injury trial days. *P < 0.01.
Brain fixation and tissue processing.
At 14 days after injury (PID 14) the animals were deeply anesthetized with isoflurane and perfused transcardially with cold 0.9% saline followed by a fixative containing 4% paraformaldehyde. The brains were then removed and fixed in paraformaldehyde overnight. The following day brains were cryoprotected in two stages: 15% sucrose for 24 h followed by 30% sucrose for 24 h. Brains selected for MRI were then washed in PBS for 48 h. For immunofluorescence, 30-μm-thick sections were cut serially with a cryostat (Leica Biosystems) and stored at −20°C. Sections were then stained with the mature neuronal marker NeuN (Abcam, Cambridge, MA). In brief, the sections were first washed in PBS (2 × 15 min) before being permeabilized with 0.3% Triton X for 1 h (Abcam). Nonspecific binding was blocked with CAS-Block (Life Technologies). Finally, the sections were moved into the primary NeuN antibody diluted to 1:2,000 and incubated overnight on an orbital shaker at 4°C. The sections were then washed repeatedly in PBS and incubated with a Cy3 secondary antibody (1:1,000, Jackson Immunoresearch) in the dark at 4°C overnight. Images were taken of both control and rmTBI animals with an epifluorescent or confocal microscope.
Magnetic resonance imaging.
MRI was performed on PID 14 brains that had been previously perfusion fixed. Imaging was performed on a Bruker Biospec 7.0-T small-animal MR scanner (Bruker Medizintechnik, Karlsruhe, Germany) with a 72-mm transmitter coil and a rat brain surface receiver coil. 3D RARE sequence was used to acquire coronal a T2-weighted image (TE: 60 ms, TR: 3,000 ms, RARE factor: 8, resolution: 0.1 mm × 0.1 mm × 0.1 mm, matrix: 192 × 192 × 192, FOV: 19.2 mm × 19.2 mm × 19.2 mm, total acquisition time: 3 h 50 min) covering the posterior cerebellum to the frontal lobe. MRI data were analyzed with ImageJ software.
Electrophysiological slice preparation.
Coronal brain slices were made as described previously (Anderson et al. 2005, 2010; Iremonger et al. 2006). In brief, male Sprague-Dawley rats aged 38–45 days (PID 14–21) were deeply sedated via isoflurane inhalation and decapitated. Brains were quickly removed and placed in ice-cold (4°C) carboxygenated (95% O2-5% CO2) high-sucrose solution composed of (in mM) 234 sucrose, 11 glucose, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4·H2O, 10 MgSO4·7H2O, 0.5 CaCl2·2H2O. The tissue was kept in this solution while 350-μm-thick coronal slices were taken with a vibratome (VT 1200; Leica, Nussloch, Germany). Brain slices were harvested from beneath the site of impact in rmTBI animals or from the corresponding area in sham-injured animals. Slices were incubated for 1 h in a water bath-warmed (32°C) container filled with carboxygenated artificial cerebrospinal fluid (aCSF) composed of (in mM) 126 NaCl, 26 NaHCO3, 2.5 KCl, 10 glucose, 1.25 NaH2PO4·H2O, 1 MgSO4·7H2O, 2 CaCl2·2H2O, pH 7.4. After the 1-h incubation, the slices were returned to room temperature before the tissue was moved to a recording chamber for whole cell patch-clamp recording.
Whole cell patch-clamp recording.
Coronal brain slices prepared from rmTBI and sham-injured animals were placed in the recording chamber and immersed in carboxygenated aCSF maintained at a temperature of 32°C. Initial visualization and identification of cortical layers was done under ×4 brightfield magnification. Recordings were made from layer II/III neurons of motor cortex within the impact zone for rmTBI animals or the corresponding region in sham-injured animals. An upright microscope (Axioexaminer, Carl Zeiss) equipped with infrared differential interference contrast optics was used to acquire whole cell patch-clamp recordings from regular-spiking (RS) cortical pyramidal neurons. Current-clamp firing behavior was used to identify RS pyramidal neurons as previously described (Connors et al. 1982; Guatteo et al. 1994). Electrode capacitance and bridge circuit were appropriately adjusted. Neurons chosen for analysis had a stable membrane resistance (Rm) that was less than 20% of the input resistance (RI), a resting membrane potential less than −55 mV, and overshooting action potentials. All current- and voltage-clamp recordings were obtained with a Multiclamp 700A patch-clamp amplifier (Axon Instruments, Union City, CA). Borosilicate glass microelectrodes (tip resistance 2.5–3.5 MΩ) were produced by a Sutter P-97 automated pipette puller (Sutter Instrument, Novato, CA) and used for patch-clamp recordings. For recording excitatory events, pipettes were filled with intracellular solution (in mM: 135 K gluconate, 4 KCl, 2 NaCl, 10 HEPES, 4 EGTA, 4 MgATP, 0.3 Na Tris). For recording inhibitory events, pipettes were filled with intracellular solution (in mM: 70 K gluconate, 70 KCl, 2 NaCl, 10 HEPES, 4 EGTA, 4 MgATP, 0.3 GTP) with a calculated reversal potential of Cl− (ECl−) of −16 mV, resulting in inward GABAA currents at a holding potential (Vhold) of −70 mV. This internal solution has been previously demonstrated (Anderson et al. 2010; Sun et al. 2006) to facilitate detection of inhibitory events.
Data analysis.
Data were analyzed with pCLAMP (Axon Instruments), Prism (GraphPad), ImageJ (National Institutes of Health), and Mini Analysis (Synaptosoft) software and are presented as means ± SE. For immunohistochemical analysis of NeuN staining a region of interest (ROI) of the motor cortex was created with ImageJ software, and NeuN-positive cells within the ROI were manually counted. Cell count and density values are presented as the average cell count for three serial sections from each animal normalized to the width or area of the ROI, respectively. Electrophysiologically recorded spontaneous synaptic events were detected as previously described with automated threshold detection and manual verification (Nichols et al. 2015). RI was calculated from the voltage response to the input of a current step (1 s, 50 mV). The adaptation index was calculated based on the ratio of the last interspike interval (FLast) divided by the second (F2) as per the equation 100 × (1 − FLast/F2). Pyramidal neurons often displayed a highly variable first interspike interval, and consequently F2 was chosen for analysis. Firing frequency was calculated as the number of action potentials induced by a 1-s, 250-pA current step. Rheobase current was determined as the minimum current step (50-ms duration) that produced an action potential. Action potential threshold was calculated as the voltage at the maximum slope of the rheobase voltage recording (Nichols et al. 2015). Statistical significance was determined with an unpaired t-test, one-way ANOVA, or Kolmogorov-Smirnov (K-S) test, and differences were determined to be significant if P < 0.05.
RESULTS
rmTBI is effectively modeled by repetitive weight drop.
To model rmTBI in pediatric patients, we modified the weight-drop method recently published by Kane et al. (2012) for use with juvenile rats (Fig. 2A). Animals subjected to rmTBI demonstrated no gross morphological changes, identifiable surface deformations, or tissue loss at the site of the impact (Fig. 2B). The rmTBI procedure resulted in no incidence of scalp lacerations, and no immediate or late seizures were observed. As previously reported, the incidence of skull fractures or intracranial bleeding was low, and any animals displaying either where removed from further study (Kane et al. 2012). At PID 14–21 rat brains were removed for further experimentation. Acute slices prepared from rmTBI brains revealed marked structural changes including cortical thinning and ventriculomegaly (Fig. 2B). To determine the reproducibility of the rmTBI method, we tested the consistency of the impact force across 20 trials. A force meter (Chatillon DFM-10, Ametek Instruments) was placed at the base of the guide tube, and the peak impact force was measured across 20 trials. We found the average impact force with a 92-g weight to be highly consistent across trials with an average force of 7.890 ± 0.06 N and a maximum variation of <1 N (Fig. 2C, top).
In humans, the duration of loss of consciousness (LOC) is an important criterion in assessing the severity of a brain injury. While brain injury may occur in the absence of LOC, it is generally accepted that “mild” TBIs induce LOC between a few seconds and <30 min (Carroll et al. 2004; Smith et al. 2013). Assessing LOC in rats is difficult, but it has been indirectly evaluated by measuring the righting reflex time as an indicator of neurological restoration (Kane et al. 2012; Zecharia et al. 2012). Righting reflex time was measured after each sham or rmTBI impact as the time for an animal to recover from the supine to the prone position. Compared with sham-injured animals the righting reflex time was significantly increased across all 5 days (Fig. 2C, bottom). However, this increase in righting reflex time was not exacerbated by repeat sham (day 1 86.92 ± 8.8 s vs. day 5 88.59 ± 6.9 s) or rmTBI (day 1 200.60 ± 18.2 s vs. day 5 199.30 ± 14.6 s) injuries (P > 0.05 for both). Averaged across all five impact trials the righting reflex time remained significantly increased between in rmTBI (193.1 ± 6.7 s) vs. sham-injured (92.31 ± 4.0 s) animals.
MRI of rmTBI reveals significant ventriculomegaly and cortical thinning.
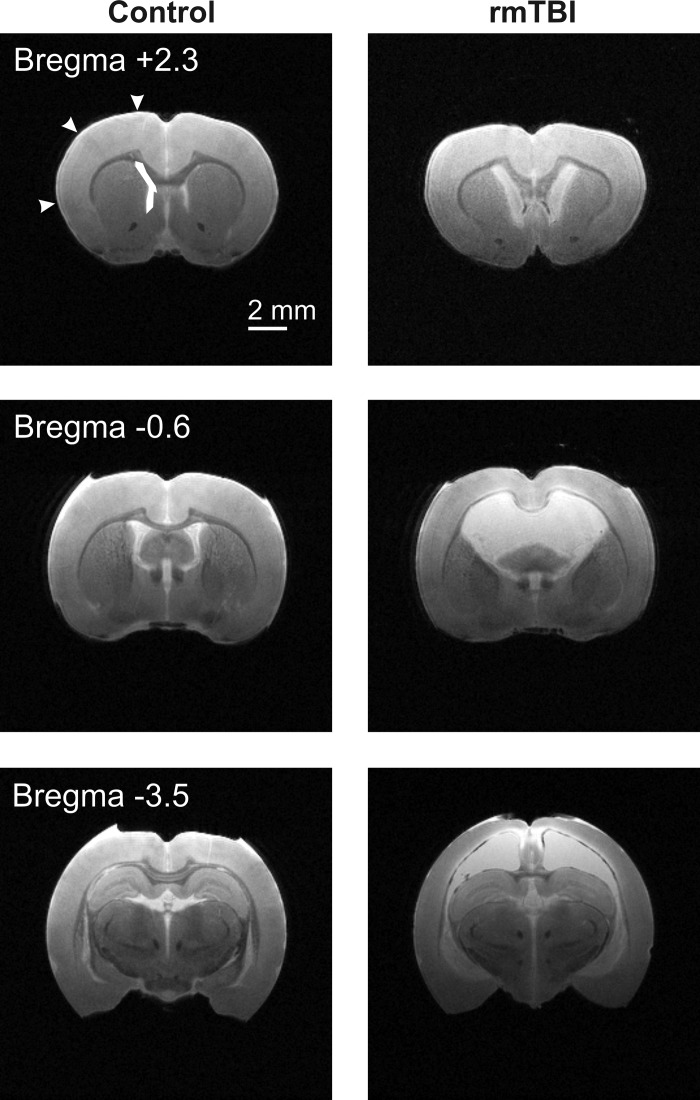
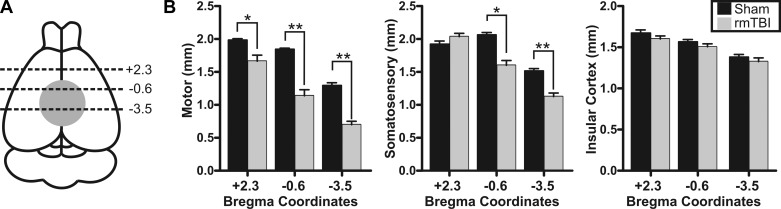
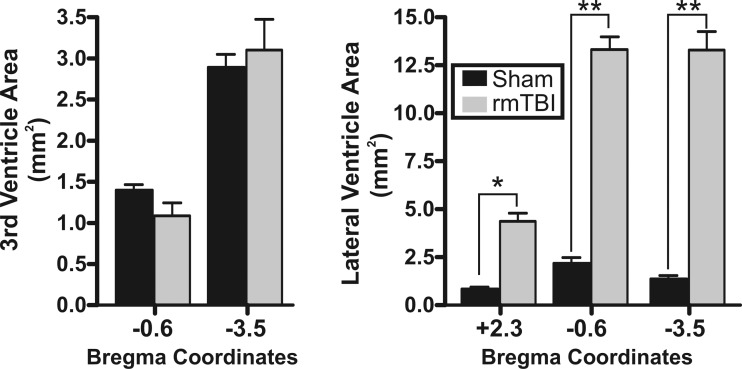
To better assess the anatomical and structural changes to the brain following rmTBI, we performed T2-weighted MRI. Brains were perfusion fixed on PID 14 and ex vivo MRI imaging performed on control (n = 4) or rmTBI (n = 3) brains (Fig. 3). MRI imaging was performed from the frontal cortex to posterior cerebellum. To determine changes in cortical thinning, we measured the depth of the motor, somatosensory, and insular cortex across three regions—one region outside (bregma +2.3) and two regions within (bregma −0.6 and −3.5) the direct impact zone (Fig. 4A). The rmTBI was delivered by a 9-mm impact rod that spanned the region between bregma and lambda sutures in the rat. As imaging was performed ex vivo, we utilized anatomical landmarks to approximate the image location relative to the impact zone and published stereotaxic coordinates (i.e., bregma +2.3 mm, −0.6 mm, or −3.5 mm, respectively) (Paxinos and Watson 2007). In this way, we assessed changes in cortical depth across brain regions in the anterior-posterior as well as medial-lateral directions in both control and rmTBI animals. Substantial cortical thinning was observed in the motor cortex in all three brain regions, with up to a 46% decrease in cortical depth within the impact zone (Fig. 4B). Similarly, the depth of the somatosensory cortex was significantly reduced by over 25%, but this reduction was restricted to directly within the impact zone (i.e., bregma −0.6 mm and bregma −3.5 mm). Measurement of the depth of the insular cortex revealed no significant difference across all three brain regions examined (P > 0.05). We next performed similar measurements on the area of the third and lateral ventricles. While no significant difference in the area of the third ventricle was observed (P > 0.05), the lateral ventricle area increased up to 970% after rmTBI (Fig. 5). Within the impact zone (bregma −0.6 and −3.5), the lateral ventricle maximally increased from 1.37 ± 0.2 mm2 to 13.30 ± 1.0 mm2 (P < 0.0001). Outside of the direct impact zone (bregma +2.3), the lateral ventricles were again significantly increased from 0.84 ± 0.1 mm2 to 4.40 ± 0.4 mm2 (P < 0.0001). Collectively, the data reveal that rmTBI induces rapid and significant reduction in the depth of the cortex and ventriculomegaly that is most substantial at the site of impact.
Fig. 3.
Magnetic resonance imaging (MRI) reveals significant structural changes after rmTBI. Coronal T2-weighted MRI images were obtained with a 7-T MRI scanner from perfusion fixed brains 14 days after sham injury or rmTBI. Representative images from sham injury (Control, left) or rmTBI (right) are presented. Approximate anatomical position of images are referenced relative to bregma. Arrowheads and box represent regions where cortical depth and lateral ventricle area measurements were taken. Similar respective measurements were made across all sham injury and rmTBI images. In T2-weighted images water and edema are bright, while gray and white matter appear darker. Note the significant cortical thinning and ventriculomegaly evident in rmTBI brains.
Fig. 4.
rmTBI induces cortical thinning. A: schematic indicating site of TBI impact (gray filled circle) and relative MRI image locations (black dashed lines) where cortical depth was measured. Numerical values are approximate bregma coordinates. B: bar charts of average cortical depth measured in MRI images at the listed bregma coordinates. Measurements of motor (left), somatosensory (center), or insular (right) cortical depth were made for each stereotaxic position (i.e., +2.3 mm, −0.6 mm, and −3.5 mm). Average values for sham injury and rmTBI are presented for each cortical region and location. No statistical difference was observed between sham injury and rmTBI for somatosensory cortex at +2.3 (P > 0.05) or for any insular cortex measurement (P > 0.05). *P < 0.05, **P < 0.01.
Fig. 5.
rmTBI induces lateral ventriculomegaly: average ventricle area measured in MRI images from the 3rd ventricle (left) or lateral ventricle (right). Corresponding sham injury and rmTBI values are presented for regions in the anterior, middle, and posterior positions in the brain. Positions in the brain are identified relative to approximated stereotaxic coordinates from bregma (i.e., +2.3 mm, −0.6 mm, and −3.5 mm). No statistical difference was observed in the area of the 3rd ventricle between sham injury and rmTBI. *P < 0.05, **P < 0.01.
rmTBI induces no change in neuronal density or gross tissue damage.
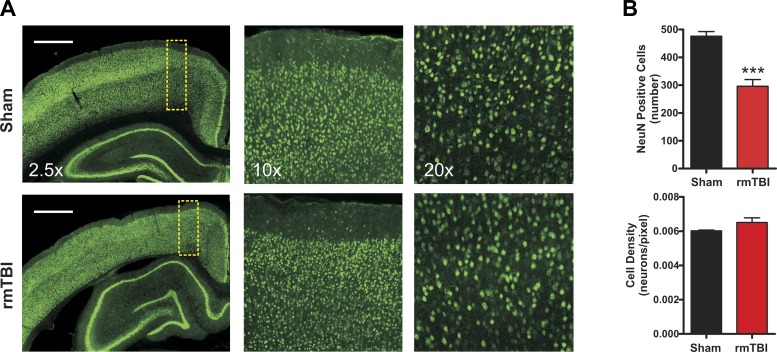
To determine whether rmTBI altered the total number or density of neurons within the cortex, we performed immunohistochemical analysis with the neuron-specific marker NeuN (Fig. 6A). As the amount of rmTBI-induced cortical thinning was most pronounced in the motor cortex, we focused the analysis on this region. The decrease in cortical thickness resulted in an overall decrease in total NeuN-positive cells between sham-injured (476.1 ± 17) and rmTBI (296.1 ± 24) animals (P < 0.001). However, analysis of the density of neurons (i.e., total neurons/area) revealed no significant change between sham-injured and rmTBI animals (P = 0.21) (Fig. 6B). Therefore, the data suggest that rmTBI induces a significant reduction in the volume of the cortex, but the cortex that remains is of similar neuronal density as that in sham-injured animals.
Fig. 6.
Effect of rmTBI on NeuN staining. A: representative epifluorescence and confocal images taken from sham injury (n = 8) or rmTBI (n = 6) stained with the neuron-specific marker NeuN (green). Scale bars, 1 mm. Images are at ×2.5, ×10, and ×20 magnification. B: bar graphs of average neuronal number (top) and density (bottom) within the motor cortex. Cell counts were made of NeuN-positive cells within standardized regions of interest (yellow dashed boxes in A). Note the substantial reduction of NeuN-positive cells after rmTBI but absence of neuronal density changes. ***P < 0.0001.
rmTBI does not significantly alter electrophysiological properties of layer II/III motor neurons.
Structurally, this study has revealed that rmTBI induces a significant reduction in the depth of the cortex that is most widespread and profound within the region of the motor cortex. To determine whether these structural changes result in functional changes to the intrinsic and synaptic properties of neurons within the motor cortex we performed electrophysiological experiments. Specifically, we recorded from layer II/III motor cortex pyramidal neurons within the injury zone of rmTBI animals or from the corresponding area in age-matched sham-injured animals.
Intrinsic excitability.
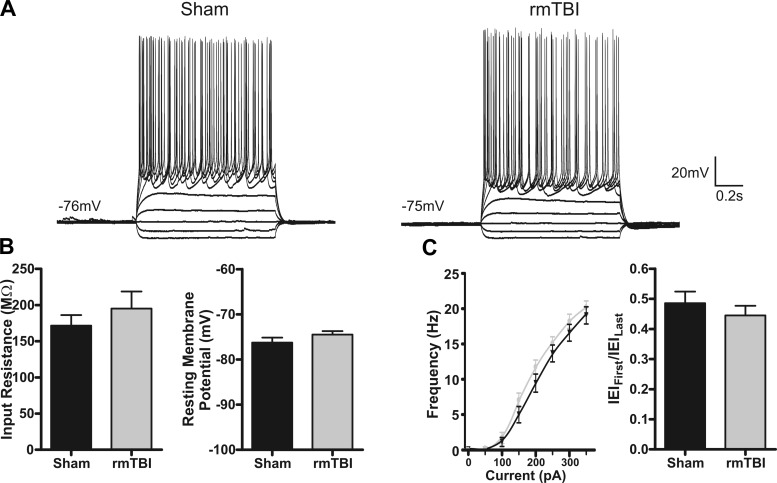
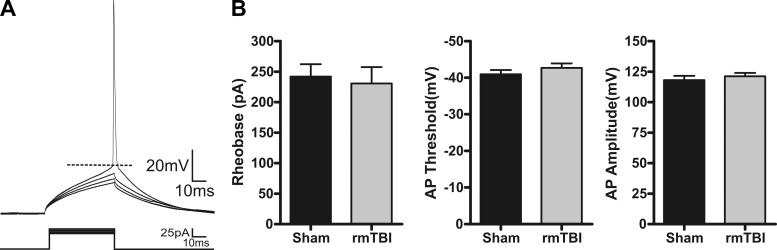
Intrinsic excitability refers to the propensity of a neuron to fire an action potential and is governed by the membrane properties, currents, and channels expressed by a neuron. Alterations to intrinsic excitability have been shown in numerous models of CNS disorders (Willmore 1990; Yang et al. 2007) and may contribute to the pathophysiology of rmTBI. To examine for changes in intrinsic excitability induced by rmTBI, we recorded under current clamp the response of sham-injured (n = 10) or rmTBI (n = 14) neurons to a series of hyperpolarizing and depolarizing steps (−100 pA to 350 pA, 50-pA steps). Analysis revealed no statistical difference in RI (P = 0.38), resting membrane potential (P = 0.77), or accommodation index (P = 0.82) between sham-injured and rmTBI neurons (Fig. 7). Using a rheobase protocol (50 ms, 5-pA steps), we performed a more detailed analysis of action potential properties but again found no statistical difference in rheobase current (P = 0.73), action potential threshold (P = 0.52), or amplitude (P = 0.31) (Fig. 8).
Fig. 7.
Intrinsic membrane properties are not altered by rmTBI. A: representative current-clamp recordings in response to intracellular current steps (−100 pA to 350 pA, 1 s) in layer II/III pyramidal neurons from sham-injured (n = 10) or rmTBI (n = 14) animals. Note the similarity in the intrinsic cellular response. B: average intrinsic membrane properties. No significant difference was found for input resistance (P = 0.38) or resting membrane potential (P = 0.77). C: comparison of firing properties of a sham-injured and an rmTBI animal. Left: plot of average firing frequency vs. current (f-I curve). Right: adaptation index [first interevent interval (IEIFirst) between action potentials/last interevent interval (IEILast)].
Fig. 8.
Action potential properties are not altered by rmTBI. A: representative whole cell current-clamp recording in response to a series of 50-ms injection (5-pA steps). B: average values for sham injury (n = 15) or rmTBI (n = 18). Rheobase was calculated as the minimum current that produced an action potential (AP). Threshold was measured at the greatest change in calculated slope. Amplitude was measured as the difference between threshold and the peak of the action potential. No statistically significant differences were found between control and rmTBI animals for rheobase (P = 0.73), action potential amplitude (P = 0.52), or threshold (P = 0.31).
Spontaneous activity.
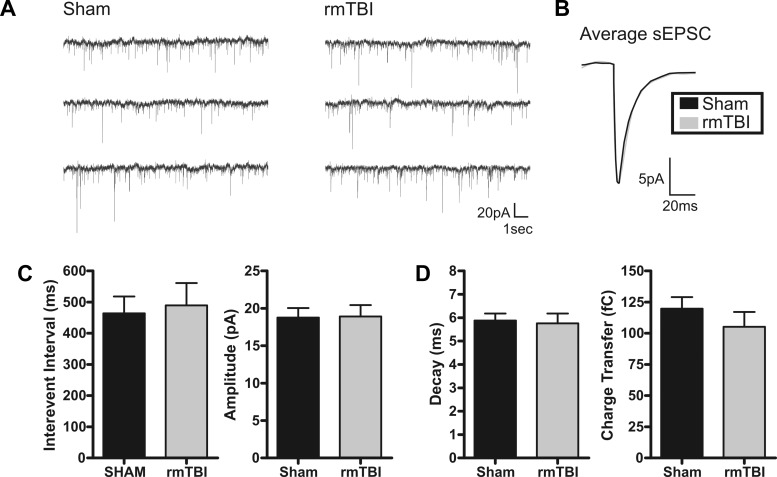
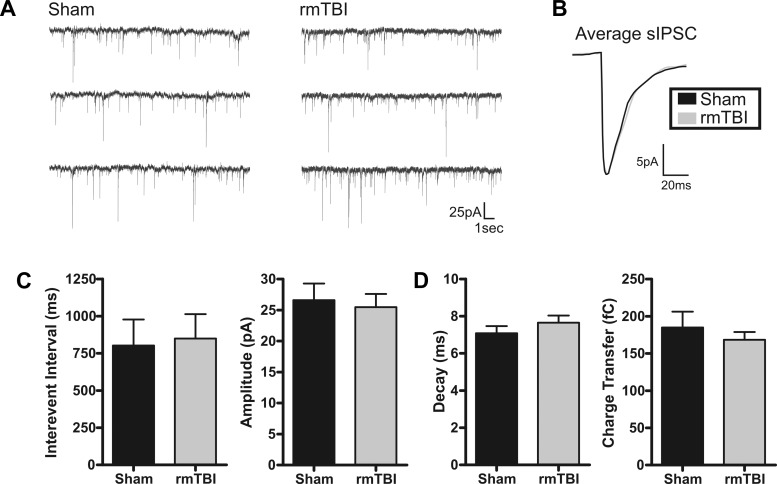
The frequency of activity and strength of synaptic connections between neurons are fundamental to the way the brain processes and relays information. To investigate whether rmTBI disrupts or alters cortical synaptic excitability we again recorded from layer II/III pyramidal neurons in the motor cortex of sham-injured or rmTBI animals. First, under voltage clamp (Vhold = −70 mV), we examined for rmTBI-induced changes to spontaneous excitatory postsynaptic currents (sEPSCs). To minimize detection of inhibitory events, neurons were held near and positive of the ECl− (Vhold = −70 mV, calculated ECl− = −80 mV) and only inward synaptic events were detected. Pharmacological isolation of glutamatergic events was avoided, as the resultant synaptic disinhibition may mask rmTBI-induced changes to network excitability. In neurons from rmTBI animals, there were no significant changes in the average interevent interval (P = 0.77), amplitude (P = 0.94), decay time (P = 0.82), or charge transfer (0.34) of sEPSCs (Fig. 9). Next, we similarly examined for changes in spontaneous inhibitory postsynaptic currents (sIPSCs). Inhibitory events were pharmacologically isolated with bath application of the glutamate receptor antagonist kynurenate (2 mM). To enhance detection fidelity of inhibitory synaptic events, a modified high-intracellular Cl− internal solution was used as previously described (Anderson et al. 2010; Sun et al. 2006). Again, no significant change was observed in sIPSC properties including interevent interval (P = 0.90), amplitude (P = 0.74), decay time (P = 0.33), and charge transfer (P = 0.46). Representative traces and summary of these results are shown in Fig. 10.
Fig. 9.
Excitatory spontaneous synaptic activity is not altered by rmTBI. A: voltage-clamp recordings of spontaneous excitatory postsynaptic currents (sEPSCs) in sham-injured (n = 19) or rmTBI (n = 14) animals. B: overlay of sham-injured and rmTBI scaled average sEPSC. C: bar charts of average sEPSC interevent interval (IEI) and amplitude for sham injury and rmTBI. No significant difference was determined for IEI (P = 0.77) or amplitude (P = 0.94). D: average sEPSC kinetic properties. No significant difference was detected between sham injury and rmTBI for sEPSC decay time (P = 0.82) or charge transfer (P = 0.34). Holding potential (Vhold) = −70 mV.
Fig. 10.
Inhibitory spontaneous synaptic activity is not altered by rmTBI. A: voltage-clamp recordings of spontaneous inhibitory postsynaptic currents (sIPSCs) in sham-injured (n = 15) or rmTBI (n = 18) animals. B: overlay of sham-injured and rmTBI scaled average sIPSC. C: average sIPSC IEI and amplitude for sham and rmTBI. No significant difference was determined for IEI (P = 0.90) or amplitude (P = 0.74). D: average sIPSC kinetic properties. No significant difference was detected between sham injury and rmTBI for sEPSC decay time (P = 0.33) or charge transfer (P = 0.46). Vhold = −70 mV.
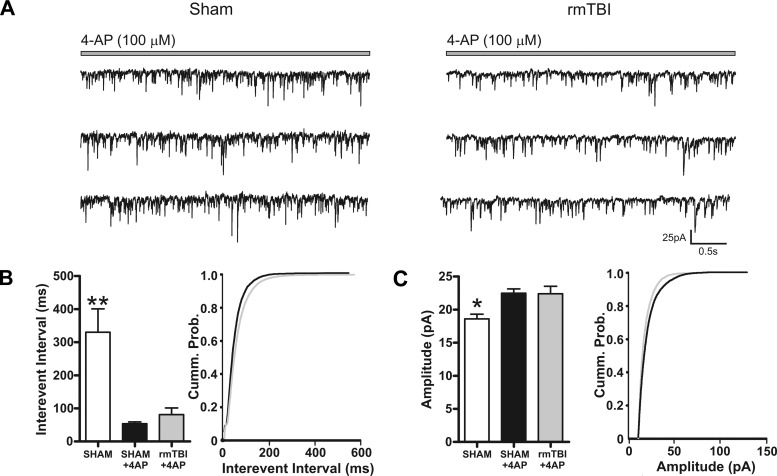
Finally, the effects of rmTBI in humans are often subtle and may not be reflected in changes to baseline synaptic activity but only become evident during periods of high activity or demand. The observed reduction of cortical depth and neuronal number in rmTBI animals relative to sham-injured animals may result in loss of peak network or synaptic activity. To test these possibilities we challenged pyramidal neurons from sham-injured (n = 15) or rmTBI (n = 18) animals with the convulsant 4-aminopyridine (4-AP, 100 μM). Bath application of 4-AP for 15 min induced a rapid decrease in interevent interval of sEPSCs recorded in neurons from both sham-injured (53.59 ± 5.6 ms) and rmTBI (81.21 ± 20.0 ms) animals (Fig. 11A). The amplitude of sEPSCs was similarly increased by 4-AP in neurons from both sham-injured (22.47 ± 0.6 pA) and rmTBI (22.40 ± 1.1 pA) animals (Fig. 11B). However, neither the interevent interval nor amplitude during application of 4-AP was statistically different between neurons recorded from sham-injured and rmTBI animals (P > 0.05). Overall, this suggests that despite a significant loss of the depth of the motor cortex in rmTBI animals, the injury fails to alter excitatory or inhibitory synaptic properties or the potential peak state of synaptic excitability.
Fig. 11.
rmTBI does not enhance the response to the convulsant 4-aminopyridine (4-AP). A: voltage-clamp recordings of sEPSCs from sham-injured (n = 14) or rmTBI (n = 12) animals during bath application of 4-AP (100 μM). B and C: bar chart and cumulative probability curves of sham injury, sham injury during 4-AP, and rmTBI during 4-AP for IEI (B) or amplitude (C). Bath application of 4-AP induced a significant decrease in IEI and amplitude of sEPSCs. However, the effects of 4-AP on sEPSC IEI and amplitude were not statistically different between sham injury and rmTBI. Vhold = −70 mV. *P < 0.05, **P < 0.01.
DISCUSSION
In the pediatric population, TBI remains a significant health concern that is known to place patients at risk for adverse long-term cognitive and behavioral changes. TBI may vary in severity, but >75% of all TBI is classified as mild (Cassidy et al. 2004; Elder and Cristian 2009; Langlois et al. 2005; Miniño et al. 2006). In this study, we sought to determine how rmTBI affects the pediatric brain. To effectively model human rmTBI, we modified a recently developed method for inducing rmTBI in adult animals (Kane et al. 2012) for use in juveniles. This rmTBI weight-drop method produced highly consistent impact forces across trials. The impacts occurred in a nonrestrained animal and have been shown to effectively model the direct, acceleration, and deceleration forces determined to be important to human TBI (Gennarelli and Thibault 1982; Holbourn 1943; Kane et al. 2012; Ommaya et al. 1967; Panzer et al. 2014). After mTBI, animals exhibited a significant increase in righting reflex time that suggests a brief injury-induced period of sensory and/or motor dysfunction. In contrast to what has generally been reported after single mTBI (Mychasiuk et al. 2014) or rmTBI in adult animals (Kane et al. 2012), rmTBI in juvenile animals induced significant structural changes to the brain including cortical atrophy and ventriculomegaly. This is supported by recent evidence that indicates that children may be more prone to the effects of repeat concussions (Eisenberg et al. 2013; Field et al. 2003). Neuronal specific immunostaining revealed that the cortical atrophy was accompanied by a loss of total cortical neurons. However, this overall neuronal loss was not due to a specific reduction in cortical density. The cortical atrophy was most pronounced in the motor cortex, with up to a 46% decrease in cortical thickness beneath the site of injury in rmTBI animals. At PID 14 the significant structural changes to the motor cortex were not accompanied by significant changes in the intrinsic or synaptic properties of layer II/III pyramidal neurons at rest or under convulsant challenge. Overall, our results indicate the effectiveness of this new weight-drop method for reliably inducing a clinically relevant rmTBI. The select changes induced by rmTBI in juvenile rats suggest a potentially unique pathophysiological response to TBI in children.
Modeling repetitive mild traumatic brain injury.
Recent attention by patients, families, researchers, and the media has highlighted the significant short- and long-term consequences of rmTBI (Creeley et al. 2004; Longhi et al. 2005; Shitaka et al. 2011). Critical to understanding the pathophysiological mechanisms that drive rmTBI has been the development of new, clinically relevant models. Effective modeling of rmTBI requires an induced injury that reflects the type of impact and forces known to occur in mTBI and that results in neuropathological and clinically relevant outcomes. mTBI is characterized as occurring in a closed skull with minimal skull fractures and minimal tissue loss after a single mTBI. The impact of the mTBI induces direct force to the skull that translates into acceleration, deceleration, and shearing forces in the brain that are thought to be important to the injury process (Duhaime et al. 2012). Several models of TBI exist, including controlled cortical impact and fluid percussion (Xiong et al. 2013), but these require a craniotomy and/or a fixed skull that inadequately models these forces. Limited data exist on the exact biomechanical forces that would be classified as “mild” or concussion inducing, but the most comprehensive data have been obtained from head impact telemetry devices placed within athletes' helmets. An in-depth review of the combined telemetry impact studies revealed that concussion is correlated with g-forces above 100g (Beckwith et al. 2013). In our study, calculated impact forces were on average 26.8g and well within the “mild” range [i.e., g-force = (F = ma)/9.8 m/s2; F = 7.89 N, m = 30g (P20-25 rat)]. The method used in this study overcomes these limitations and effectively models both the biomechanical forces of the impact and has been shown to induce clinically relevant cognitive and behavioral changes (Gennarelli and Thibault 1982; Kane et al. 2012; Meaney and Smith 2011; Mychasiuk et al. 2014; Panzer et al. 2014).
Repetitive mild traumatic brain injury induces significant neuropathology.
A single mTBI often resolves quickly and has generally not been associated with any significant neuroimaging abnormalities (Belanger et al. 2007; Petchprapai and Winkelman 2007; Morey et al. 2013). As a result, mTBI is often referred to as an “invisible wound” and is difficult to diagnose. Whether a single mTBI induces long-term deficits is currently a source of significant debate (Carroll et al. 2004; Klein et al. 1996; Konrad et al. 2011; Vanderploeg et al. 2005; Vasterling et al. 2012; Yuh et al. 2014). It is clear, however, that when a patient receives multiple mTBIs within a short period of time it results in more severe symptoms, a longer recovery period, and increased risk for serious long-term consequences (Guskiewicz et al. 2000, 2003). In contrast to a single mTBI event, rmTBI patients show clear neuropathological findings including enlarged ventricles (ventriculomegaly) and cortical atrophy (Huh et al. 2007; Smith et al. 2013). These findings are supported by the results of this study, which indicate that after rmTBI the lateral ventricles may be increased up to 970% while the thickness of the cortex may be reduced by up to 46%. The interplay and timing of the enlarged ventricles and cortical atrophy remain to be determined. However, the cortex appears to not be simply compressed by the enlarged ventricles, as no change in cortical neuronal density was observed. These changes were not observed after rmTBI in adult animals (Kane et al. 2012), suggesting a potentially unique response to TBI in juvenile animals. While the impact force used in this study was “mild,” the neuropathological findings in rmTBI animals are more significant and may highlight the deleterious effects of receiving multiple mTBIs.
In humans, rmTBI can induce a neurodegenerative disease termed chronic traumatic encephalopathy (CTE) (Gavett et al. 2011; Smith et al. 2013) that has been most commonly found in professional athletes (McKee et al. 2009; Omalu et al. 2010a, 2010b) or soldiers exposed to blast or concussive injury (Goldstein et al. 2012). CTE can currently only be diagnosed on autopsy but results in degeneration of brain tissue (i.e., cortical atrophy) and ventriculomegaly similar to what was observed in this study. Additional characteristics of CTE include tau accumulation, cognitive impairments, memory loss, confusion, and depression (McKee et al. 2009, 2010; Miller 1966). Further work examining these characteristics will be required to determine whether the neuropathological outcome of rmTBI in this study is indicative of underlying CTE.
Cortical excitability is not altered early after repetitive mild traumatic brain injury.
Structurally, this study revealed extensive thinning of the cortex that was most pronounced beneath the site of injury in the motor cortex. Immunohistochemical staining revealed that rmTBI reduced the total number of cortical neurons, but this was not accompanied by a decrease in neuronal density. The significant loss of motor cortex is supported by several studies that have indicated persistent motor dysfunction and abnormalities in the motor cortex after mTBI (De Beaumont et al. 2011, 2012; Tremblay et al. 2014). In addition, many of the behavioral deficits associated with rmTBI such as balance, reaction time, and visual memory involve high levels of integration across cortical regions (Covassin et al. 2008; Khurana and Kaye 2012; Slobounov et al. 2007) that are thought to be governed by input and output from layer II/III cortex (Douglas and Martin 2004; Kamper et al. 2013). This agrees with a recent study that found mTBI induces specific dendritic degeneration and synaptic reduction in cortical layer II/III pyramidal neurons (Gao and Chen 2011). As such, in this study we began by examining for rmTBI-induced changes in the intrinsic and synaptic properties of layer II/III pyramidal neurons within the motor cortex.
A neuron's intrinsic excitability determines the probability it will fire an action potential, and the output pattern of that firing has been shown to contribute to the pathophysiology of several other neurological disorders (Bush et al. 1999; Prince and Connors 1986; Prinz et al. 2013; van Zundert et al. 2012). However, at PID 14 we investigated several possible measures of intrinsic excitability and found no significant differences between our rmTBI and sham-injured groups. This finding is supported by recent work from our lab where even severe TBI in juvenile rats failed to alter the intrinsic properties of cortical pyramidal neurons (Nichols et al. 2015). At a synaptic level, again at PID 14 no significant changes were found in the strength, frequency, or kinetics of either excitatory or inhibitory synaptic neurotransmission following rmTBI. To our knowledge this is the first study to investigate detailed intracellular electrophysiological changes following rmTBI.
In humans, the use of transcranial magnetic stimulation from 72 h to 2 mo after mTBI has shown increases in intracortical inhibition (Miller et al. 2014). Young athletes who have sustained multiple concussions have also been reported to have abnormal intracortical inhibition (De Beaumont et al. 2007, 2011; Tremblay et al. 2011). While no change in inhibition onto pyramidal neurons was observed in this study, future examination of the impact of rmTBI directly on other cortical layers and inhibitory interneurons may reveal distinct changes. Given the significant neuropathological changes following rmTBI, it is surprising to find no accompanying electrophysiological changes. The lack of synaptic excitability changes observed after rmTBI in this study contrast with recent findings after severe TBI from our lab in juvenile rats (Nichols et al. 2015) and from previous reports in adult animals (Cantu et al. 2014). As this study only examined animals at 14 days after injury it will be important to examine changes that may occur in the acute and more chronic time points after rmTBI. The data suggest that juvenile rats have a unique injury phenotype after rmTBI that may be in part due to high levels of plasticity in the juvenile brain (Akbik et al. 2013; Grutzendler et al. 2002; Li and Asante 2011; Selemon 2013), ongoing development (Kolb and Gibb 2011), and/or potential trauma-induced postnatal neurogenesis (Gregg et al. 2001; Kolb et al. 2007).
The effects of mTBI may often be subtle and only evident when the cortex is challenged with a high-demand task (Abdel et al. 2009). With the clear loss of mature neurons and significant cortical atrophy, we hypothesized that rmTBI animals may have a reduced upper limit of synaptic activity that would be evident only when the cortex was put under “stress.” To test this, we examined the synaptic properties of sham-injured and rmTBI animals during application of 4-AP, a potassium channel blocker and known convulsant. 4-AP has been shown to increase synaptic excitability (Boudkkazi et al. 2011; Buckle and Haas 1982) and to affect cortical pyramidal neuron intrinsic excitability (Higgs and Spain 2011; Shu et al. 2007). As expected, both the frequency and amplitude of spontaneous excitatory activity were increased from control periods by bath application of 4-AP. However, the effects of 4-AP were not statistically different between sham-injured and rmTBI animals. Therefore, even when cortical excitability is pharmacologically increased, rmTBI animals remain equally responsive and able to enhance synaptic activity compared with sham-injured animals. However, in this study we only tested the response of a saturating dose of 4-AP that produces a near-maximal level of synaptic activity. The use of a dose-response protocol may reveal subtler changes in network excitability or 4-AP sensitivity after rmTBI.
In conclusion, rmTBI has been associated with serious clinical consequences including chronic traumatic encephalopathy and an increased risk for the development of dementia and neurodegenerative diseases (McKee et al. 2009, 2010; Omalu et al. 2010a, 2010b). In this study, we found that rmTBI can be effectively modeled in young animals with a modified weight-drop method. The impacts can be consistently delivered and replicate clinically relevant impact forces and structural changes including cortical atrophy and ventriculomegaly. This method of inducing mTBI has also recently been shown in juvenile (Mychasiuk et al. 2014) and adult (Kane et al. 2012) animals to induce clinically relevant changes to cognition and behavior. At present, the findings from this study suggest that the pathophysiology of rmTBI may be unique when occurring in pediatric patients. An improved understanding of how the pediatric brain responds to rmTBI may help identify novel therapeutic targets, influence pediatric treatment, and improve “return to game” decision making in adolescents.
GRANTS
Support for this project was provided by the University of Arizona College of Medicine-Phoenix.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.G., J.N., and C.W. performed experiments; C.G., J.N., C.W., and T.A. analyzed data; C.G., J.N., C.W., and T.A. interpreted results of experiments; C.G., C.W., and T.A. prepared figures; C.G. and T.A. drafted manuscript; C.G., J.N., C.W., and T.A. edited and revised manuscript; C.G., C.W., and T.A. approved final version of manuscript; T.A. conception and design of research.
REFERENCES
- Abdel B, Samah G, Kao HY, Kelemen E, Fenton AA, Bergold PJ. A hierarchy of neurobehavioral tasks discriminates between mild and moderate brain injury in rats. Brain Res 1280: 98–106, 2009. [DOI] [PubMed] [Google Scholar]
- Adelson PD. Animal models of traumatic brain injury in the immature: a review. Exp Toxicol Pathol 51: 130–136, 1999. [DOI] [PubMed] [Google Scholar]
- Akbik FV, Bhagat SM, Patel PR, Cafferty WB, Strittmatter SM. Anatomical plasticity of adult brain is titrated by Nogo Receptor 1. Neuron 77: 859–866, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GV, Gerami D, Esser MJ. Conditioning effects of repetitive mild neurotrauma on motor function in an animal model of focal brain injury. Neuroscience 99: 93–105, 2000. [DOI] [PubMed] [Google Scholar]
- Anderson TR, Huguenard JR, Prince DA. Differential effects of Na+-K+ ATPase blockade on cortical layer V neurons. J Physiol 588: 4401–4414, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TR, Jarvis CR, Biedermann AJ, Molnar C, Andrew RD. Blocking the anoxic depolarization protects without functional compromise following simulated stroke in cortical brain slices. J Neurophysiol 93: 963–979, 2005. [DOI] [PubMed] [Google Scholar]
- Angoa-Pérez M, Kane MJ, Briggs DI, Herrera-Mundo N, Viano DC, Kuhn DM. Animal models of sports-related head injury: bridging the gap between pre-clinical research and clinical reality. J Neurochem 129: 916–931, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciniegas DB, Anderson CA, Topkoff J, McAllister TW. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat 1: 311–327, 2005. [PMC free article] [PubMed] [Google Scholar]
- Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med 30: 33–48, 2011. [DOI] [PubMed] [Google Scholar]
- Barlow KM, Crawford S, Stevenson A, Sandhu SS, Belanger F, Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics 126: e374–e381, 2010. [DOI] [PubMed] [Google Scholar]
- Beckwith JG, Greenwald RM, Chu JJ, Crisco JJ, Rowson S, Duma SM, Broglio SP, McAllister TW, Guskiewicz KM, Mihalik JP, Anderson S, Schnebel B, Brolinson PG, Collins MW. Head impact exposure sustained by football players on days of diagnosed concussion. Med Sci Sports Exerc 45: 737–746, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger HG, Vanderploeg RD, Curtiss G, Warden DL. Recent neuroimaging techniques in mild traumatic brain injury. J Neuropsychiatry Clin Neurosci 19: 5–20, 2007. [DOI] [PubMed] [Google Scholar]
- Boudkkazi S, Fronzaroli-Molinieres L, Debanne D. Presynaptic action potential waveform determines cortical synaptic latency. J Physiol 589: 1117–1131, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns J, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia 44, Suppl 10: 2–10, 2003. [DOI] [PubMed] [Google Scholar]
- Buckle PJ, Haas HL. Enhancement of synaptic transmission by 4-aminopyridine in hippocampal slices of the rat. J Physiol 326: 109–122, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush PC, Prince DA, Miller KD. Increased pyramidal excitability and NMDA conductance can explain posttraumatic epileptogenesis without disinhibition: a model. J Neurophysiol 82: 1748–1758, 1999. [DOI] [PubMed] [Google Scholar]
- Cantu D, Walker K, Andresen L, Taylor-Weiner A, Hampton D, Tesco G, Dulla CG. Traumatic brain injury increases cortical glutamate network activity by compromising GABAergic control. Cereb Cortex (March 7, 2014). doi: 10.1093/cercor/bhu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Holm L, Kraus J, Coronado VG, WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 43, Suppl: 113–125, 2004. [DOI] [PubMed] [Google Scholar]
- Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L, Kraus J, Coronado VG, WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 43, Suppl: 28–60, 2004. [DOI] [PubMed] [Google Scholar]
- Cernak I. Animal models of head trauma. NeuroRx 2: 410–422, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol 22: 487–497, 1987. [DOI] [PubMed] [Google Scholar]
- Collins MW, Lovell MR, Iverson GL, Cantu RC, Maroon JC, Field M. Cumulative effects of concussion in high school athletes. Neurosurgery 51: 1175–1179, 2002. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ, Prince DA. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol 48: 1302–1320, 1982. [DOI] [PubMed] [Google Scholar]
- Covassin T, Stearne D, Elbin R. Concussion history and postconcussion neurocognitive performance and symptoms in collegiate athletes. J Athletic Training 43: 119–124, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeley CE, Wozniak DF, Bayly PV, Olney JW, Lewis LM. Multiple episodes of mild traumatic brain injury result in impaired cognitive performance in mice. Acad Emerg Med 11: 809–819, 2004. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Lassonde M, Leclerc S, Théoret H. Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery 61: 329–336, 2007. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Mongeon D, Tremblay S, Messier J, Prince F, Leclerc S, Lassonde M, Théoret H. Persistent motor system abnormalities in formerly concussed athletes. J Athletic Training 46: 234–240, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaumont L, Tremblay S, Poirier J, Lassonde M, Théoret H. Altered bidirectional plasticity and reduced implicit motor learning in concussed athletes. Cereb Cortex 22: 112–121, 2012. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci 27: 419–451, 2004. [DOI] [PubMed] [Google Scholar]
- Duhaime AC, Beckwith JG, Maerlender AC, McAllister TW, Crisco JJ, Duma SM, Brolinson PG, Rowson S, Flashman LA, Chu JJ, Greenwald RM. Spectrum of acute clinical characteristics of diagnosed concussions in college athletes wearing instrumented helmets: clinical article. J Neurosurg 117: 1092–1099, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg MA, Andrea J, Meehan W, Mannix R. Time interval between concussions and symptom duration. Pediatrics 132: 8–17, 2013. [DOI] [PubMed] [Google Scholar]
- Elder GA, Cristian A. Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. Mt Sinai J Med 76: 111–118, 2009. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, 2010. [Google Scholar]
- Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr 142: 546–553, 2003. [DOI] [PubMed] [Google Scholar]
- Friedman WJ, Olson L, Persson H. Cells that express brain-derived neurotrophic factor mRNA in the developing postnatal rat brain. Eur J Neurosci 3: 688–697, 1991. [DOI] [PubMed] [Google Scholar]
- Friess SH, Ichord RN, Ralston J, Ryall K, Helfaer MA, Smith C, Margulies SS. Repeated traumatic brain injury affects composite cognitive function in piglets. J Neurotrauma 26: 1111–1121, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Chen J. Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J Neuropathol Exp Neurol 70: 183–191, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med 30: 179–88, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarelli TA, Thibault LE. Biomechanics of acute subdural hematoma. J Trauma 22: 680–686, 1982. [DOI] [PubMed] [Google Scholar]
- Giza C. Lasting effects of pediatric traumatic brain injury. Indian J Neurotrauma 3: 19–26, 2006. [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, McKee AC. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 4: 134ra60, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg CT, Shingo T, Weiss S. Neural stem cells of the mammalian forebrain. Symp Soc Exp Biol 53: 1–19, 2001. [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature 420: 812–816, 2002. [DOI] [PubMed] [Google Scholar]
- Guatteo E, Bacci A, Franceschetti S, Avanzini G, Wanke E. Neurons dissociated from neocortex fire with “burst” and “regular” trains of spikes. Neurosci Lett 175: 117–120, 1994. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM. Balance assessment in the management of sport-related concussion. Clin Sports Med 30: 89–102, 2011. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 57: 719–726, 2005. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, Onate JA, Kelly JP. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA 290: 2549–2555, 2003. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Weaver NL, Padua DA, Garrett WE. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med 28: 643–650, 2000. [DOI] [PubMed] [Google Scholar]
- Halstead ME, Walter KD, Council on Sports Medicine and Fitness. American Academy of Pediatrics Clinical Report—sport-related concussion in children and adolescents. Pediatrics 126: 597–615, 2010. [DOI] [PubMed] [Google Scholar]
- Higgs MH, Spain WJ. Kv1 channels control spike threshold dynamics and spike timing in cortical pyramidal neurones. J Physiol 589: 5125–5142, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbourn AH. The mechanics of head injuries. Lancet 2: 438–441, 1943. [Google Scholar]
- Huh JW, Widing AG, Raghupathi R. Basic science; repetitive mild non-contusive brain trauma in immature rats exacerbates traumatic axonal injury and axonal calpain activation: a preliminary report. J Neurotrauma 24: 15–27, 2007. [DOI] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain. I. N-methyl-d-aspartate and quisqualate receptors. Neuroscience 35: 31–43, 1990. [DOI] [PubMed] [Google Scholar]
- Iremonger KJ, Anderson TR, Hu B, Kiss ZH. Cellular mechanisms preventing sustained activation of cortex during subcortical high-frequency stimulation. J Neurophysiol 96: 613–621, 2006. [DOI] [PubMed] [Google Scholar]
- Kamper JE, Pop V, Fukuda AM, Ajao DO, Hartman RE, Badaut J. Juvenile traumatic brain injury evolves into a chronic brain disorder: behavioral and histological changes over 6 months. Exp Neurol 250: 8–19, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Angoa-Pérez M, Briggs DI, Viano DC, Kreipke CW, Kuhn DM. A mouse model of human repetitive mild traumatic brain injury. J Neurosci Methods 203: 41–49, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana VG, Kaye AH. An overview of concussion in sport. J Clin Neurosci 19: 1–11, 2012. [DOI] [PubMed] [Google Scholar]
- Klein M, Houx PJ, Jolles J. Long-term persisting cognitive sequelae of traumatic brain injury and the effect of age. J Nerv Ment Dis 184: 459–467, 1996. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R. Brain plasticity and behaviour in the developing brain. J Can Acad Child Adolesc Psychiatry 20: 265–276, 2011. [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Morshead C, Gonzalez C, Kim M, Gregg C, Shingo T, Weiss S. Growth factor-stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. J Cereb Blood Flow Metab 27: 983–997, 2007. [DOI] [PubMed] [Google Scholar]
- Konrad C, Geburek AJ, Rist F, Blumenroth H, Fischer B, Husstedt I, Arolt V, Schiffbauer H, Lohmann H. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol Med 41: 1197–1211, 2011. [DOI] [PubMed] [Google Scholar]
- Kontos R, Elbin RJ, Fazio-Sumrock VC, Burkhart S, Swindell H, Maroon J, Collins MW. Incidence of sports-related concussion among youth football players aged 8–12 years. J Pediatr 163: 717–720, 2013. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil 20: 229–238, 2005. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MW. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 21: 375–378, 2006. [DOI] [PubMed] [Google Scholar]
- Li HL, Asante CO. Developmental plasticity of descending motor pathways. J Neurophysiol 105: 1963–1965, 2011. [DOI] [PubMed] [Google Scholar]
- Longhi L, Saatman KE, Fujimoto S, Raghupathi R, Meaney DF, Davis J, McMillan A, Conte V, Laurer HL, Stein S, Stocchetti N, McIntosh TK. Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery 56: 364–374, 2005. [DOI] [PubMed] [Google Scholar]
- Lovell MR, Collins MW, Iverson GL, Field M, Maroon JC, Cantu R, Podell K, Powell JW, Belza M, Fu FH. Recovery from mild concussion in high school athletes. J Neurosurg 98: 296–301, 2003. [DOI] [PubMed] [Google Scholar]
- MacGregor AJ, Doughert AL, Morrison RH, Quinn KH, Galarneau MR. Repeated concussion among U.S. military personnel during Operation Iraqi Freedom. J Rehabil Res Dev 48: 1269–1278, 2011. [DOI] [PubMed] [Google Scholar]
- Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma 27: 1529–1540, 2010. [DOI] [PubMed] [Google Scholar]
- Maxwell WL. Traumatic brain injury in the neonate, child and adolescent human: an overview of pathology. Int J Dev Neurosci 30: 167–183, 2012. [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, Kelly JP. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA 290: 2556–2563, 2003. [DOI] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68: 709–735, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, Perl DP, Hedley-Whyte ET, Price B, Sullivan C, Morin P, Lee HS, Kubilus CA, Daneshvar DH, Wulff M, Budson AE. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 69: 918–929, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney DF, Smith DH. Biomechanics of concussion. Clin Sports Med 30: 19–31, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. Mental after-effects of head injury. Proc R Soc Med 59: 257–261, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NR, Yasen AL, Maynard LF, Chou LS, Howell DR, Christie AD. Acute and longitudinal changes in motor cortex function following mild traumatic brain injury. Brain Inj 28: 1270–1276, 2014. [DOI] [PubMed] [Google Scholar]
- Miniño AM, Anderson RN, Fingerhut LA, Boudreault MA, Warner M. Deaths: injuries, 2002. Natl Vital Stat Rep 54: 1–124, 2006. [PubMed] [Google Scholar]
- Morey RA, Haswell CC, Selgrade ES, Massoglia D, Liu C, Weiner J, Marx CE, MIRECC Work Group, Cernak I, McCarthy G. Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afghanistan war veterans. Hum Brain Mapp 34: 2986–2999, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Farran A, Esser MJ. Assessment of an experimental rodent model of pediatric mild traumatic brain injury. J Neurotrauma 31: 749–757, 2014. [DOI] [PubMed] [Google Scholar]
- Nichols J, Perez R, Wu C, Adelson PD, Anderson TR. Traumatic brain injury induces rapid enhancement of cortical excitability in juvenile rats. CNS Neurosci Ther 21: 193–203, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omalu BI, Fitzsimmons RP, Hammers J, Bailes J. Chronic traumatic encephalopathy in a professional American wrestler. J Forensic Nurs 6: 130–136, 2010a. [DOI] [PubMed] [Google Scholar]
- Omalu BI, Hamilton RL, Kamboh MI, DeKosky ST, Bailes J. Chronic traumatic encephalopathy (CTE) in a National Football League player: case report and emerging medicolegal practice questions. J Forensic Nurs 6: 40–46, 2010b. [DOI] [PubMed] [Google Scholar]
- Ommaya AK, Yarnell P, Hirsch AE. Scaling of experimental data on cerebral concussion in subhuman primates to concussion threshold for man. In: Proceedings of the 11th Stapp Car Crash Conference, 73–80, 1967. [Google Scholar]
- Panzer MB, Wood GW, Bass CR. Scaling in neurotrauma: how do we apply animal experiments to people? Exp Neurol 261C: 120–126, 2014. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (6th ed). Amsterdam: Academic, 2007. [Google Scholar]
- Pellman EJ, Viano DC, Tucker AM, Casson IR, Waeckerle JF. Concussion in professional football: reconstruction of game impacts and injuries. Neurosurgery 53: 799–812, 2003. [DOI] [PubMed] [Google Scholar]
- Petchprapai N, Winkelman C. Mild traumatic brain injury: determinants and subsequent quality of life. A review of the literature. J Neurosci Nurs 39: 260–272, 2007. [PubMed] [Google Scholar]
- Petraglia AL, Plog BA, Dayawansa S, Chen M, Dashnaw ML, Czerniecka K, Walker CT. The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J Neurotrauma 31: 1211–1224, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 55: 1158–1166, 2000. [DOI] [PubMed] [Google Scholar]
- Prince DA, Connors BW. Mechanisms of interictal epileptogenesis. Adv Neurol 44: 275–299, 1986. [PubMed] [Google Scholar]
- Prinz A, Selesnew LM, Liss B, Roeper J, Carlsson T. Increased excitability in serotonin neurons in the dorsal raphe nucleus in the 6-OHDA mouse model of Parkinson's disease. Exp Neurol 248: 236–245, 2013. [DOI] [PubMed] [Google Scholar]
- Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil 21: 544–548, 2006. [DOI] [PubMed] [Google Scholar]
- Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry 3: e238, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitaka Y, Tran HT, Bennett RE, Sanchez L, Levy MA, Dikranian K, Brody DL. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol 70: 551–567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Yu Y, Yang J, McCormick DA. Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proc Natl Acad Sci USA 104: 11453–11458, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov S, Slobounov E, Sebastianelli W, Cao C, Newell K. Differential rate of recovery in athletes after first and second concussion episodes. Neurosurgery 61: 338–344, 2007. [DOI] [PubMed] [Google Scholar]
- Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 9: 211–221, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci 26: 1219–1230, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Beaulé V, Proulx S, Tremblay S, Marjańska M, Doyon J, Lassonde M, Théoret H. Multimodal assessment of primary motor cortex integrity following sport concussion in asymptomatic athletes. Clin Neurophysiol 125: 1371–1379, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, De Beaumont L, Henry LC, Boulanger Y, Evans AC, Bourgouin P, Poirier J, Théoret H, Lassonde M. Sports concussions and aging: a neuroimaging investigation. Cereb Cortex 23: 1159–1166, 2013. [DOI] [PubMed] [Google Scholar]
- Tremblay S, De Beaumont L, Lassonde M, Théoret H. Evidence for the specificity of intracortical inhibitory dysfunction in asymptomatic concussed athletes. J Neurotrauma 28: 493–502, 2011. [DOI] [PubMed] [Google Scholar]
- Vanderploeg RD, Curtiss G, Belanger HG. Long-term neuropsychological outcomes following mild traumatic brain injury. J Int Neuropsychol Soc 11: 228–236, 2005. [DOI] [PubMed] [Google Scholar]
- Van Zundert B, Izaurieta P, Fritz E, Alvarez FJ. Early pathogenesis in the adult-onset neurodegenerative disease amyotrophic lateral sclerosis. J Cell Biochem 113: 3301–3312, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Proctor SP, Kane R, Heeren T, Franz M. Neuropsychological outcomes of mild traumatic brain injury, post-traumatic stress disorder and depression in Iraq-deployed US Army soldiers. Br J Psychiatry 201: 186–192, 2012. [DOI] [PubMed] [Google Scholar]
- Viano DC, Casson IR, Pellman EJ. Concussion in professional football: biomechanics of the struck player—Part 14. Neurosurgery 61: 313–327, 2007. [DOI] [PubMed] [Google Scholar]
- Wang X, Xie H, Cotton AS, Tamburrino MB, Brickman KR, Lewis TJ, McLean SA, Liberzon L. Early cortical thickness changes after mild traumatic brain injury following motor vehicle collision. J Neurotrauma 32: 455–463, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmore LJ. Post-traumatic epilepsy: cellular mechanisms and implications for treatment. Epilepsia 31, Suppl 3: S67–S73, 1990. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci 14: 128–142, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Benardo LS, Valsamis H, Ling DS. Acute injury to superficial cortex leads to a decrease in synaptic inhibition and increase in excitation in neocortical layer V pyramidal cells. J Neurophysiol 97: 178–187, 2007. [DOI] [PubMed] [Google Scholar]
- Yuh EL, Hawryluk GW, Manley GT. Imaging concussion: a review. Neurosurgery 75, Suppl 4: S50–S63, 2014. [DOI] [PubMed] [Google Scholar]
- Zecharia AY, Yu X, Götz T, Ye Z, Carr DR, Wulff P, Bettler B, Vyssotski AL, Brickley SG, Franks NP, Wisden W. GABAergic inhibition of histaminergic neurons regulates active waking but not the sleep-wake switch or propofol-induced loss of consciousness. J Neurosci 32: 13062–13075, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemper ED. Two-year prospective study of relative risk of a second cerebral concussion. Am J Phys Med Rehabil 82: 653–659, 2003. [DOI] [PubMed] [Google Scholar]