Abstract
The benefit of preoperative chemotherapy in resectable gastroesophageal adenocarcinomas was not observed in signet ring cell subtype. However, the potential interest of taxane-based preoperative chemotherapy on this subtype is still an unresolved issue. Nineteen patients with localized signet ring cell adenocarcinomas received taxane-based regimens, and 17 patients underwent surgery. Complete resection was achieved in 80 %, and median overall survival was 40.8 months (95 % confidence interval (CI), 20.2—not reached). Even though one patient achieved a complete pathological response, seven patients had an upstaging of their tumors at surgery. The potential benefits of taxane-based chemotherapy seem to be limited to a reduced number of patients.
Electronic supplementary material
The online version of this article (doi:10.1186/s13045-015-0148-y) contains supplementary material, which is available to authorized users.
Keywords: Signet ring cell, Gastroesophageal cancer, Gastric cancer, Preoperative, Neoadjuvant, Taxane, Docetaxel, Paclitaxel
Findings
Background
Signet ring cell (SRC) adenocarcinoma is a particular histological subtype of gastroesophageal adenocarcinomas (GEA) displaying a worse prognosis [1]. Even though the perioperative chemotherapy (PCT) in resectable GEA demonstrated a significant benefit in terms of overall survival (OS) compared to surgery alone [2, 3], this benefit seems to be limited to non-SRC histology [4]. This observation prompted physicians to perform surgery without preoperative chemotherapy in SRC GEA patients with a resectable disease.
Taxanes are potent microtubule-stabilizing agents with demonstrated antitumor activity in advanced GEA and with encouraging results in resectable GEA, as reported in several phase II trials [5–10]. The potential interest of taxane-based PCT on SRC GEA is still an unresolved issue.
Results
Between January 2005 and December 2012, 19 patients with localized SRC GEA received taxane-based PCT from six French hospitals. (Additional file 1) Patients’ median age was 64 years (range, 41–81 years). The majority of tumors (58 %) were located in the stomach and were predominantly stage III (42 %) and II (42 %) (Table 1).
Table 1.
Patient and tumors’ characteristics
| Patient and tumors’ characteristics before surgery | ||
|---|---|---|
| N = 19 | ||
| Age, years (range) | 64 | 41–81 |
| Gender | No | % |
| Male | 14 | 74 |
| Female | 5 | 26 |
| ECOG-PS | No | % |
| 0 | 11 | 58 |
| 1 | 5 | 26 |
| 2 | 3 | 16 |
| Location of tumor | No | % |
| Distal esophagus or GEJ | 8 | 42 |
| Stomach | 11 | 58 |
| Clinical TNM stage | No | % |
| Stage I | 3 | 16 |
| Stage II | 8 | 42 |
| Stage III | 8 | 42 |
| Stage IV | 0 | 0 |
| Neoadjuvant chemotherapy | No | % |
| DCF | 7 | 37 |
| PET | 7 | 37 |
| TFOX | 3 | 16 |
| Docetaxel-Cisplatin | 1 | 5 |
| Cisplatin-Paclitaxel-Doxorubicin | 1 | 5 |
| Surgery and postoperative variables | ||
| N = 17 | ||
| Type of surgery, No (%) | No | % |
| Total gastrectomy | 8 | 47 |
| Subtotal gastrectomy | 2 | 12 |
| Lewis–Santi esophagectomy* | 7 | 41 |
| Lymphadenectomy extend | No | % |
| D1 | 4 | 31 |
| Modified D2 | 6 | 46 |
| D2 | 3 | 23 |
| Missing | 4 | – |
| Resection | No | % |
| R0 | 12 | 80 |
| R1 | 3 | 20 |
| R2 | 0 | 0 |
| Missing | 2 | – |
| Pathological tumor classification | No | % |
| pT0 | 1 | 6 |
| pT1 | 2 | 13 |
| pT2 | 3 | 19 |
| pT3 | 5 | 31 |
| pT4 | 5 | 31 |
| Missing | 1 | – |
| Pathologic nodal classification | No | % |
| pN0 | 2 | 13 |
| pN1 | 6 | 38 |
| pN2 | 3 | 19 |
| pN3 | 5 | 31 |
| Missing | 1 | – |
| Pathologic metastatic stage | No | % |
| pM0 | 15 | 88 |
| pM1 | 2 | 12 |
| Adjuvant treatment | No | % |
| DCF | 3 | 18 |
| Docetaxel-Cisplatin | 1 | 6 |
| TFOX | 1 | 6 |
| mFOLFOX6 | 6 | 35 |
| EOX | 2 | 12 |
| Chemoradiotherapy | 2 | 12 |
| No adjuvant treatment | 2 | 12 |
ECOG-PS Eastern Cooperative Oncology Group—performance status, GEJ gastroesophageal junction, DCF 3 cycles of docetaxel (75 mg/m2 d1), cisplatin (75 mg/m2 d1), and 5-fluorouracil (750 mg/m2/d on continuous perfusion on days 1 to 5), every 3 weeks, PET 8 cycles of cisplatin (30 mg/m2 d1), epirubicin (50 mg/m2 d1), and paclitaxel (80 mg/m2 d1), every week, TFOX docetaxel (50 mg/m2), oxaliplatin (85 mg/m2), leucovorin (400 mg/m2) and 5FU continuous infusion 48 h (2400 mg/m2), S surgery alone, D1 lymphadenectomy limited to regional lymph nodes, modified D2 extended lymph node dissection without pancreatectomy and splenectomy, D2 extended lymph node dissection with pancreatectomy and/or splenectomy, mFOLFOX6 oxaliplatin, leucovorin, 5FU bolus and 5FU continuous infusion 48 h, EOX epirubicin, oxaliplatin and capecitabine
*Oesophagectomy via abdominal and right thoracic approaches
Most frequent neoadjuvant chemotherapy regimens were DCF regimen in seven patients (37 %), as described in TAX-325 trial, and PET regimen in other seven patients (37 %) as previously published [5, 10]. Three patients (16 %) received TFOX regimen (Table 1).
Seventeen patients (89 %) underwent surgery. One patient presented an unexpected death (cardiac failure) after three DCF cycles and before surgery, and another patient refused surgery after eight PET cycles. Total gastrectomy was performed in eight patients (47 %) and esophagogastrectomy via abdominal and right thoracic approaches (Lewis–Santi) in seven patients (41 %). Postoperative adverse events were observed in three patients with favorable recovery (Table 1).
All 17 patients who underwent surgery had a curative-intent resection. Pathological information about surgical margins was available in 15 patients, and the pathological complete resection (R0) was achieved in 12 patients (80 %). One patient presented a complete pathological response (pCR). This patient had a T2 disease with lymph node enlargement at diagnosis. In seven patients, more advanced disease was found at surgery compared to initial staging. Two patients presented intraoperative peritoneal metastases, and five patients had T4 disease (Table 1).
After a median follow up of 26.2 months (15.5–71.5), eight patients died and nine patients progressed. The median OS was 40.8 months (95 % confidence interval (CI), 20.2—not reached), and the median PFS was 36.8 months (95 % CI, 10.0—not reached). Five-year OS and PFS rates were 30.4 % and 29.3 %, respectively (Fig. 1).
Fig. 1.
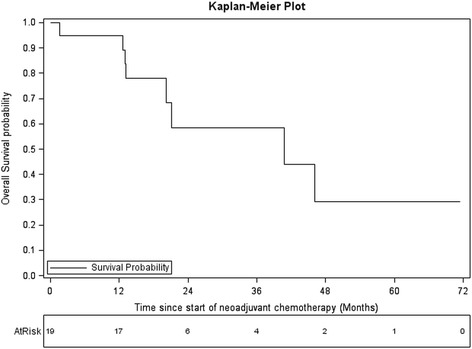
OS estimated using Kaplan Meier method
Conclusion
Even though our study has obvious limitations as a retrospective analysis and regarding the limited number of patients, this is the largest cohort of SRC GEA patients treated with preoperative taxane-based chemotherapy published so far. The potential benefits of taxane-based PCT seem to be limited to a reduced number of patients with SRC GEA. The high number of patients with pathological upstaging reinforces the results of Messager et al. and the recommendation to perform front line surgery in resectable SRC GEA without PCT [4]. Future efforts should be focused on developing predictive biomarkers to identify SRC GEA patients potentially sensitive to taxanes.
Future perspectives
Targeted agents have shown promising results in advanced GEA. [11] Among them, trastuzumab (in HER2 positive patients) and ramucirumab have been approved in advanced GEA. However, most SRC GEAs are HER2 negative, and ramucirumab, an antiangiogenic mAb selectively targeting VEGFR2, will hardly be developed in perioperative setting due to negative experience of bevacizumab in gastrointestinal adenocarcinomas [12, 13]. Among novel molecules in development in GEA, checkpoint inhibitors are probably the most promising. Pembrolizumab, an antiPD1 mAb was administered as monotherapy in 39 GEA patients with PD-L1 expression. Most patients have received ≥2 prior chemotherapies. An encouraging overall response rate of 22 % and the 6-month OS rate of 69 % were observed [14]. The expression of PD-L1 in SRC GEA is present in about 23 %, and a growing body of evidence suggests that taxane induces immunogenic cell death sustaining the potential interest to combine taxane and antiPD1 in clinical trials including SRC GEA patients [15, 16].
Pembrolizumab and other checkpoint inhibitors should be evaluated in prospective preoperative trials in GEA patients including SRC histology, probably in association with taxane-based chemotherapy. Future exhaustive molecular analysis in SRC GEA is needed to find targets for novel molecules in this chemorefractory disease.
Acknowledgments
The authors would like to thank Guadalupe Tizon for English writing assistance.
Abbreviations
- SRC
Signet ring cell
- GEA
Gastroesophageal adenocarcinomas
- PCT
Preoperative chemotherapy
- OS
Overall survival
- DCF
3 cycles of docetaxel, cisplatin, and 5-fluorouracil, every 3 weeks
- PET
8 cycles cisplatin, epirubicin, paclitaxel, weekly
- TFOX
6 cycles of docetaxel, oxaliplatin, leucovorin, and 5FU, every 2 weeks
- R0
Complete resection
- pCR
Pathological complete response
- PFS
Progression free survival
- HER2
Human epidermal growth factor receptor 2
- mAb
Monoclonal antibody
- VEGFR2
Vascular endothelial growth factor receptor 2
- PD1
Programmed cell death protein 1
- PD-L1
Programmed cell death-ligand 1
Additional file
Methods.
Footnotes
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
SK conceived the study, participated in its design, acquisition and interpretation of data, and coordination, and helped to draft the manuscript. FFi participated in study design, acquisition of data and statistical analysis, and helped to draft the manuscript. SPB participated in statistical analysis and helped to draft manuscript. FG participated in acquisition. ZL participated in acquisition of data. MJ participated in acquisition of data. FFe participated in acquisition of data. FB participated in its design and statistical analysis and helped to draft the manuscript. CM participated in acquisition and interpretation of data and helped to draft the manuscript. CB conceived the study, participated in its design and interpretation of data, and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Stefano Kim, Email: chkim@chu-besancon.fr.
Frederic Fiteni, Email: fredericfiteni@gmail.com.
Sophie Paget-Bailly, Email: spaget@chu-besancon.fr.
François Ghiringhelli, Email: FGhiringhelli@cgfl.fr.
Zaher Lakkis, Email: zaher.lakkis@gmail.com.
Marine Jary, Email: jary.marine@yahoo.fr.
Francine Fein, Email: ffein@chu-besancon.fr.
Franck Bonnetain, Email: franck.bonnetain@univ-fcomte.fr.
Christophe Mariette, Email: Christophe.mariette@chru-lille.fr.
Christophe Borg, Email: Christophe.borg@efs.sante.fr.
References
- 1.Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250:878–87. doi: 10.1097/SLA.0b013e3181b21c7b. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, Thompson JN, van de Velde CJH, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 3.Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–21. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 4.Messager M, Lefevre JH, Pichot-Delahaye V, Souadka A, Piessen G, Mariette C, et al. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma. Ann Surg. 2011;254:684–93. doi: 10.1097/SLA.0b013e3182352647. [DOI] [PubMed] [Google Scholar]
- 5.Jary M, Ghiringhelli F, Jacquin M, Fein F, Nguyen T, Cleau D, et al. Phase II multicentre study of efficacy and feasibility of dose-intensified preoperative weekly cisplatin, epirubicin, and paclitaxel (PET) in resectable gastroesophageal cancer. Cancer Chemother Pharmacol. 2014;74:141–50. doi: 10.1007/s00280-014-2482-0. [DOI] [PubMed] [Google Scholar]
- 6.Ferri LE, Ades S, Alcindor T, Chasen M, Marcus V, Hickeson M, et al. Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann Oncol. 2012;23:1512–7. doi: 10.1093/annonc/mdr465. [DOI] [PubMed] [Google Scholar]
- 7.Thuss-Patience PC, Hofheinz RD, Arnold D, Florschütz A, Daum S, Kretzschmar A, et al. Perioperative chemotherapy with docetaxel, cisplatin and capecitabine (DCX) in gastro-oesophageal adenocarcinoma: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Ann Oncol. 2012;23:2827–34. doi: 10.1093/annonc/mds129. [DOI] [PubMed] [Google Scholar]
- 8.Park I, Ryu M-H, Choi YH, Kang HJ, Yook JH, Park YS, et al. A phase II study of neoadjuvant docetaxel, oxaliplatin, and S-1 (DOS) chemotherapy followed by surgery and adjuvant S-1 chemotherapy in potentially resectable gastric or gastroesophageal junction adenocarcinoma. Cancer Chemother Pharmacol. 2013;72:815–23. doi: 10.1007/s00280-013-2257-z. [DOI] [PubMed] [Google Scholar]
- 9.Schulz C, Kullmann F, Kunzmann V, Fuchs M, Geissler M, Vehling-Kaiser U, et al. NeoFLOT: multicenter phase II study of perioperative chemotherapy in resectable adenocarcinoma of the gastroesophageal junction or gastric adenocarcinoma-Very good response predominantly in patients with intestinal type tumors. Int J Cancer. 2014 doi: 10.1002/ijc.29403. [DOI] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Wu N, Li J. Novel targeted agents for gastric cancer. J Hematol Oncol. 2012;5:31. doi: 10.1186/1756-8722-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KC, Koh YW, Chang H-M, Kim TH, Yook JH, Kim BS, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18:2833–40. doi: 10.1245/s10434-011-1695-2. [DOI] [PubMed] [Google Scholar]
- 13.Idelevich E, Kashtan H, Klein Y, Buevich V, Baruch NB, Dinerman M, et al. Prospective phase II study of neoadjuvant therapy with cisplatin, 5-FU, and bevacizumab for locally advanced resectable esophageal cancer. Onkologie. 2012;35:427–31. doi: 10.1159/000340072. [DOI] [PubMed] [Google Scholar]
- 14.Muro K, Bang Y-J, Shankaran V, Geva R, Thomas DV, Gupta S, et al. Relationship between PD-L1 expression and clinical outcomes in patients with advanced gastric cancer treated with the anti-PD-1 monoclonal antibody pembrolizumab in KEYNOTE-012. J Clin Oncol. 2015;33(Suppl 3):abstr 3. [Google Scholar]
- 15.Qing Y, Li Q, Ren T, Xia W, Peng Y, Liu GL, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. DDDT. 2015;9:901–9. doi: 10.2147/DDDT.S75152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337:1678–84. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]


