Abstract
Importance
The period prevalence of depression among women is 21.9% during the first postpartum year; however, questions remain about the value of screening for depression.
Objectives
To screen for depression in postpartum women and evaluate positive screen findings to determine the timing of episode onset, rate and intensity of self-harm ideation, and primary and secondary DSM-IV disorders to inform treatment and policy decisions.
Design
Sequential case series of women who recently gave birth.
Setting
Urban academic women’s hospital.
Participants
During the maternity hospitalization, women were offered screening at 4 to 6 weeks post parturn by telephone. Screen-positive women were invited to undergo psychiatric evaluations in their homes.
Main Outcomes and Measures
A positive screen finding was an Edinburgh Postnatal Depression Scale (EPDS) score of 10 or higher. Self-harm ideation was assessed on EPDS item 10: “The thought of harming myself has occurred to me” (yes, quite often; sometimes; hardly ever; never). Screen-positive women underwent evaluation with the Structured Clinical Interview for DSM-IV for Axis I primary and secondary diagnoses.
Results
Ten thousand mothers underwent screening, with positive findings in 1396 (14.0%); of these, 826 (59.2%) completed the home visits and 147 (10.5%) completed a telephone diagnostic interview. Screen-positive women were more likely to be younger, African American, publicly insured, single, and less well educated. More episodes began post partum (40.1%), followed by during pregnancy (33.4%) and before pregnancy (26.5%). In this population, 19.3% had self-harm ideation. All mothers with the highest intensity of self-harm ideation were identified with the EPDS score of 10 or higher. The most common primary diagnoses were unipolar depressive disorders (68.5%), and almost two-thirds had co-morbid anxiety disorders. A striking 22.6% had bipolar disorders.
Conclusions and Relevance
The most common diagnosis in screen-positive women was major depressive disorder with comorbid generalized anxiety disorder. Strategies to differentiate women with bipolar from unipolar disorders are needed.
Trial Registration
clinicaltrials.gov Identifier: NCT00282776
With a period prevalence of 21.9% the year after birth,1 depression is a frequent complication of childbearing. However, recognition and treatment rates are even lower in pregnant and postpartum women (14%) than in the general population (26%),2,3 Low treatment rates are juxtaposed against mounting evidence that antenatal and postpartum depression (PPD) increase the risk for multiple adverse outcomes among women and their offspring. Maternal depression interferes with child development and increases the rates of insecure attachment and poor cognitive performance.4,6 Suicide accounts for about 20% of postpartum deaths7 and is the second most common cause of mortality in postpartum women.7
Childbearing is an opportune time for intervention because women have contact with health care professionals, have access to health insurance, and are motivated toward positive behaviors to invest in their offspring’s welfare.8 Identification of PPD through universal screening has been recommended (and is mandated in several states9); however, screening without system enhancements, such as diagnostic evaluation with intervention implementation, is not currently justifiable10 or cost-effective11 and may incur ethical and liability concerns.12
Recommendations have reflected this conundrum13 by concluding that existing data were insufficient to support a firm recommendation for universal perinatal screening but that such screening could benefit women and their families and should be strongly considered. Milgrom et al14 commented that abandoning PPD screening altogether invokes a sense of “throwing the baby out with the bathwater.” With the aim of reducing the burden of maternal morbidity and mortality, diagnostic characterization is key information for mental health practitioners and policy decision makers14 because optimal treatment derives from accurate diagnostic formulation.
The most frequently used PPD screening tool is the Edinburgh Postnatal Depression Scale (EPDS).13 Since publication of the EPDS in 1987, a substantial literature has accrued.16,17 The sensitivity and specificity are equivalent to screens that are used in primary care settings.1 The utility of the EPDS is enhanced by its free availability, ease of administration, and acceptance by patients.17 Any screening measure identifies only the risk for having a disorder and must be followed by a diagnostic assessment. Data from larger, non-treatment-seeking samples with complete psychiatric characterization (ie, primary diagnosis and comorbidities) are needed to define the heterogeneity inherent in the diagnostic yield from screening for “depression” in postpartum women. A number of key questions related to PPD screening are controversial, and studies in large racially and demographically diverse samples are needed.1 In women who undergo postpartum screening, what proportion has postpartum episodes (by the DSM-IV definition of onset within 4 weeks18), onset during pregnancy, or chronic episodes predating the index pregnancy? What are the psychiatric diagnoses identified in women in whom the screen findings are positive for depression (screen-positive findings)? Rowe and colleagues19 suggested that the term PPD has become an umbrella term that includes a range of disorders in addition to depression. Interest in postpartum anxiety disorders has increased20; in fact, lifetime anxiety disorders were more common (36.4%) than depression (24.9%) in women in the National Comorbidity Survey.21 Finally, how frequent is self-harm ideation in postpartum women? Howard et al22 found that 9% of more than 4000 women who completed the EPDS endorsed suicidal ideation.
The goals of this investigation were to (1) determine the proportions of women undergoing postpartum screening with episode onset post partum, during pregnancy, or predating pregnancy; (2) evaluate the rate of self-harm ideation for women with screen-positive EPDS findings; and (3) define primary and secondary D5M-IV Axis I disorders associated with positive screens. To our knowledge, no similar large-scale PPD screening study with complete DSM-IV diagnostic characterization from a nonclinical sample of women who recently gave birth has been published.
METHODS
SCREENING PROCEDURES
We conducted a screening program for PPD at an urban obstetrical hospital (Magee-Womens Hospital, University of Pittsburgh) as the initial component of a comprehensive case identification, diagnosis, and intervention project. The intervention, care management vs usual care for women with depressive disorders, is being analyzed for a separate article. The screening measure was the EPDS,15 which was selected because it is brief (10 items), scored by simple addition, free, available in 23 languages, used with a variety of socioeconomic and ethnic groups, and the most commonly used PPD screening tool worldwide.17 Previous work from our institution suggested that the EPDS is a favorable measure from a patient acceptability and psychometric standpoint in our setting.23 The developers of the EPDS suggested 2 cut points based on the screening site’s resources to perform follow-up assessment for screen-positive women.24 A lower cut point of 10 or higher was recommended for settings with the capacity to facilitate evaluation among women with positive screen findings and a cutoff of 13 or higher for settings with limited resources.24 Item 10 of the EPDS includes the prompt: “The thought of harming myself has occurred to me,” with 4 response options consisting of yes, quite often; sometimes; hardly ever; and never. The time frame is the past 7 days.
Women who delivered a live infant at Magee-Womens Hospital were visited by a nurse or social worker on the maternity ward and provided information about PPD. The mothers were offered screening by telephone at 4 to 6 weeks post partum. Exclusion criteria included being non-English speaking, younger than 18 years, or unable to provide consent and having no telephone available. Eligible women signed a waiver approved by the University of Pittsburgh institutional review board, which allowed collection of contact information and later telephone screening. The 4- to 6-week period after birth was chosen because women typically have their obstetrical evaluation then, and we emphasized mental health as a component of postpartum well-being. This time frame also includes the postpartum peak in psychiatric contact (0–19 days)25 and captures women with rapid-onset, postpartum episodes of mental illness.
We implemented a centralized PPD screening program within our women’s mental health research center to tap the efficiency of volume and the streamlined computerized database. The telephone screeners were college students or graduates trained to deliver the EPDS and supervised by experienced master’s-level psychiatric clinicians (M.C.M., D.M.R., R.A.Z., C.L.H., and M.L.C). From 4 to 6 weeks, an intense effort was made to reach the participants, with day and evening calls. If the woman was not reached after 3 days, a postcard encouraging her to contact our team was sent and the calls continued. If she was not reached by 6 weeks, she was removed from the call list and no further contact was attempted.
All women with screen-positive findings (defined as an EPDS score ≥10) were offered a home-visit evaluation for psychiatric diagnostic assessment. Women who declined the home visit were offered a telephone screen to determine the presence or absence of major depressive disorder (MDD). The goal for timing of the home visiting was within 2 weeks of the screen. Any woman who had a very high screening score (EPDS score ≥20) or endorsed any response other than “none” on the EPDS self-harm question was immediately interviewed by the supervising clinician for safety assessment and intervention planning.
DIAGNOSTIC ASSESSMENT
The women provided written informed consent (approved by the University of Pittsburgh institutional review board) at the home-visit evaluation. The complete Structured Clinical Interview for DSM-IV (SCID)26 was administered in the women’s homes by master’s-level clinicians (M.C.M., D.M.R., R.A.Z., C.L.H., and M.T.C.) (with child care provided by a research assistant as needed). The interviews typically lasted from 2 to 3 hours. The SCID interviewers were trained by viewing 8 standard videotaped diagnostic modules, passing a written examination, and completing reliability ratings with a trained interviewer. Every assessment was reviewed with a board-certified psychiatrist (K.L.W., D.K.Y.S., E.T.M.-K., or C.S.F.) for diagnostic confirmation. If the woman declined the home visit, our office or public settings were suggested as venues. If these were not acceptable, the woman was offered an assessment for PPD by telephone with the SCID criteria for MDD only.
DATA ANALYSIS
Descriptive statistics are presented as mean (SD) for continuous variables and percentages for discrete variables. The comparison of subject characteristics was conducted with a χ2 (or a Fisher exact) test. The diagnoses of screen-positive women were grouped into the following categories: (1) unipolar depressive disorders, (2) bipolar disorders, (3) anxiety disorders, (4) substance use disorders, (5) other disorders, and (6) no diagnosis. The primary disorder was defined as the condition that was chiefly responsible for the symptoms that prompted the home-visit assessment and was the main focus of attention (the DSM-IV “principal diagnosis”).26 Secondary diagnoses were categorized as (1) anxiety disorders, with subtypes included because of the high frequency of these comorbidities; (2) substance use disorders; (3) eating disorders; and (4) depressive disorders. Data management and statistical analyses were performed by staff from the Epidemiology Data Center at the University of Pittsburgh (H.F.E., J.F.L., and S.R.W.).
RESULTS
SUBJECT FLOW AND SAMPLE CHARACTERISTICS
A total of 17601 women were approached and. offered screening. Some women (n = 175) were excluded because they were non-English speaking, had no telephone access, or were unable to provide informed consent. New mothers who were younger than 18 years were not included because of the institutional review board requirement for parental consent for participation in research. The remaining 17426 women (99.0%) agreed to telephone screening. Of these eligible women, 13442 (77.1%) were contacted and 10 000 (74.4%) underwent screening. All 1396 women with screen-positive findings were offered a home visit for diagnostic assessment with the SCID, and 826 (59.2%) accepted. Women who declined the home visit were offered a telephone screen for MDD, and 147 (10.5%) participated (Figure 1).
Figure 1.
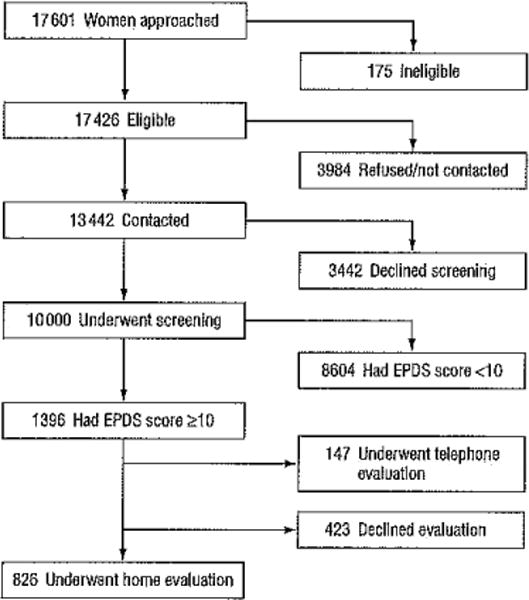
Subject flow. EPDS indicates Edinburgh Postnatal Depression Scale.
The EPDS scores followed the expected right-skewed distribution (Figure 2). The percentages of the 10 000 women with screen-positive findings at the 2 recommended cut points were 14.0% with an EPDS score of 10 or higher and 7.0% with an EPDS score of 13 or higher.
Figure 2.
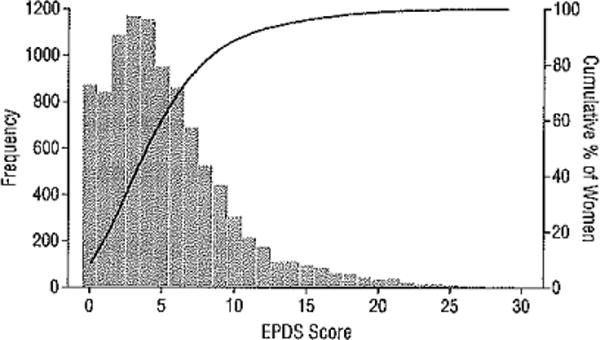
Frequency distribution of the Edinburgh Postnatal Depression Scale (EPDS) scores.
DEMOGRAPHICS
The characteristics of the 1396 screen-positive women are described in the eTable (http://www.jamapsych.com) by the category of follow-up psychiatric evaluation (declined diagnostic assessment after the EPDS screening, accepted a telephone evaluation for MDD only, or completed a home visit). The demographic characteristics of women who chose these 3 pathways after screening differed significantly. Women who accepted home visits were more likely than women undergoing assessment by telephone or who declined assessment to have higher mean EPDS scores (14.3 [3.9] vs 12.3 [3.0] vs 13.3 [3.7], respectively). Women who received a home visit also were more likely than the 2 other groups to be African American, publicly insured or uninsured, and single. Women who received a home visit were more likely than the other 2 groups to be younger, African American, less educated, and single.
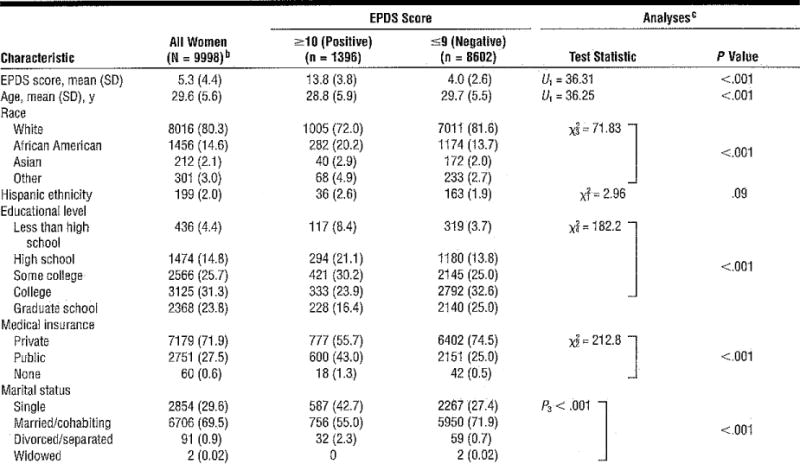
The demographic characteristics of women grouped by their positive or negative EPDS screen results (Table 1) also significantly differed in all characteristics evaluated. As anticipated, women with screen-negative findings had significantly lower average scores (mean EPDS, 4.0 [2.6]) compared with those who had screen-positive findings (mean EPDS, 13.8 [3.8]). Screen-positive women were significantly younger, more likely to be African American or a member of another minority group, less highly educated, more likely to have public insurance, and more likely to be single.
Table 1.
Demographic Characteristics of Women With Positive vs Negative EPDS Findingsa
|
Abbreviations: EPDS, Edinburgh Postnatal Depression Scale; U, Mann-Whitney.
Unless otherwise indicated, data are expressed as the number (percentage) of women. Percentages have been rounded and might not total 100. Numbers for each category sum to less than the totals because of missing data.
Two women had incomplete EPDS data.
Descriptive statistics are based on available data. The test statistic P indicates the Fisher exact test.
TIMING OF EPISODE ONSET
For the 826 women who received home visits, the episode onset was most frequently post partum (within 4 weeks after birth18 for 331 women [40.1%]), followed by during pregnancy (276 [33.4%]) and before pregnancy (219 [26.5%]). Screening at 4 to 6 weeks post partum identified a group of women with psychiatric illnesses with onset times distributed through the prepregnancy, antenatal, and postpartum periods.
SELF-HARM IDEATION
In the sample of 10 000 women who underwent screening, 319 (3.2%) had thoughts of self-harm, including 8 who endorsed “yes, quite often”; 65, “sometimes”; and 246, “hardly ever.” Most women who endorsed self-harm ideation also had screen-positive findings on the EPDS (270 of 319 [84.6%]). The rates of self-harm ideation for women with EPDS scores of 10 or higher (n = 1396) and 13 or higher (n = 703) are shown in Figure 3. At an EPDS score of 10 or higher, the percentage of subjects within each category of response was 80.7% for never and 19.3% for yes, divided as 14.3% for 1 (hardly ever), 4.5% for 2 (sometimes), and 0.6% for 3 (yes, quite often). Women with higher EPDS scores (≥13) had a higher proportion within each category who endorsed thoughts of self-harm, with 70.0% for never and 30.0% for yes, divided as 20.3% for 1, 8.6% for 2, and 11.1% for 3.
Figure 3.
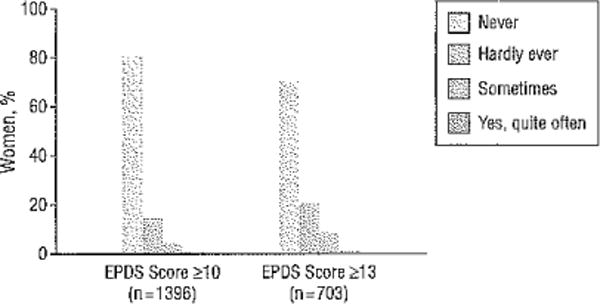
Responses to the Edinburgh Postnatal Depression Scale (EPDS) self-harm item for women with positive EPDS screen findings.
A small number of women endorsing thoughts of self-harm (n = 49) had screen-negative findings. None of these women gave the response of “yes, quite often,” whereas “sometimes” was endorsed by 2 and “hardly ever” by 47. Notably, all the mothers who had the highest level of self-harm ideation were captured with an EPDS of 10 or higher.
DIAGNOSES OF SCREEN-POSITIVE WOMEN
The most common primary diagnoses in home-visited women with EPDS scores of 10 or higher were unipolar depressive disorders (566 women [68.5%], including MDD in 514 [90.8%]), bipolar disorder (187 122.6%]), anxiety disorders (46 [5.6%]), substance use disorders (4 [0.5%]), and other disorders (6 [0.7%]). No diagnosis was found in 17 (2.1%) (Table 2). Most of the women with MDD had developed a recurrent pattern, and few women in this population had other less severe depressive syndromes. Among the women with bipolar disorders, the most common diagnosis was bipolar I disorder (49.7% of the group with bipolar disorders). The polarity of these episodes was depressed, mixed, and manic in an approximately 7:4:1 ratio. About one-third of these women had bipolar II disorder.
Table 2.
Primary and Secondary Diagnoses in Postpartum Women With EPDS Score of 10 or Higher
| Primary Diagnoses (826 Home Visits) | No. (%) | Secondary Diagnoses for Primary Diagnostic Category | No. (%)a |
|---|---|---|---|
| Unipolar depressive disorders | 566 (68.5) | Secondary diagnoses in 374 women with primary depressive disorders | 516 (62.5) |
| Major depression | Anxiety disorders | 428 (82.9) | |
| Recurrent | 368 (65.0) | Generalized anxiety | 224 (52.3) |
| Single episode | 146 (25.8) | Panic | 59 (13.8) |
| Depressive disorder NOS | 38 (6.7) | Social phobia | 53 (12.4) |
| Adjustment disorder with depressed mood | 11 (1.9) | Obsessive-compulsive | 47 (11.0) |
| Mood disorder NOS | 2 (0.4) | Posttraumatic stress | 45 (10.5) |
| Dysthymic disorder | 1 (0.2) | Substance use disorders | 61 (11.8) |
| Eating disorders | 27 (5.2) | ||
| Bipolar disorders | 187 (22.6) | Secondary diagnoses in 136 women with primary bipolar disorder | 223 (27.0) |
| Bipolar II | 58 (31.0) | Anxiety disorders | 189 (84.8) |
| Bipolar I—depressed | 54 (28.9) | Generalized anxiety | 72 (38.1) |
| Bipolar MOS | 35 (18.7) | Panic | 50 (26.5) |
| Bipolar I-mixed episode | 32 (17.1) | Posttraumatic stress | 34 (18.0) |
| Bipolar l-manic episode | 7 (3.7) | Obsessive-compulsive | 33 (17.5) |
| Schizoaffective disorder | 1 (0.5) | Substance use disorders | 27 (12.1) |
| Eating disorders | 7 (3.1) | ||
| Anxiety disorders | 46 (5.6) | Secondary diagnoses in 24 women with primary anxiety disorders | 46 (5.6) |
| Generalized anxiety disorder | 24 (52.2) | Depressive disorders | 23 (50.0) |
| Obsessive-compulsive disorder | 8 (17.4) | Major depression, recurrent | 12 (52.2) |
| Anxiety disorder NOS | 8 (17.4) | Major depression, single episode | 6 (26.1) |
| Adjustment disorder with anxiety | 3 (6.5) | Dysthymic disorder | 5 (21.7) |
| Panic disorder without agoraphobia | 1 (2.2) | Other anxiety disorders | 15 (32.6) |
| Posttraumatic stress disorder | 1 (2.2) | Substance use disorders | 8 (17.4) |
| Specific phobia | 1 (2.2) | ||
| Substance use disorders | 4 (0.5) | ||
| Substance-induced mood disorder | 1 (25.0) | ||
| Alcohol abuse/dependence | 1 (25.0) | ||
| Opioid abuse/dependence | 1 (25.0) | ||
| Polysubstance dependence | 1 (25.0) | ||
| Other disorders | 6 (0.7) | ||
| No diagnosis | 17 (2.1) |
Abbreviation: NOS, not otherwise specified.
The number of secondary diagnoses does not match the number for a primary diagnosis group because some patients have no secondary diagnosis white others present with more than 1 secondary diagnosis. The percentages are the percentages of the total number of secondary diagnoses.
Although anxiety disorders were uncommon as primary diagnoses, most women with unipolar depressive disorders (374 of 566 [66.1%]) had comorbid disorders, with 82.9% being anxiety disorders. The most common was generalized anxiety disorder, which constituted more than half of the secondary anxiety disorder diagnoses. Panic disorder, social phobia, and posttraumatic stress disorder were also represented as secondary anxiety subtypes. Conversely, depressive disorders were the most common comorbid conditions in women with primary anxiety disorders. Secondary diagnoses for bipolar disorder were anxiety disorders and substance use disorders.
The primary diagnoses for the subgroup of 476 home-visited, SCID-assessed women at the higher EPDS cut point of 13 or higher demonstrated that a higher proportion of women had bipolar disorders compared with women with an EPDS score of 10 or higher, including unipolar depressive disorders (324women [68.1%]),bipolar disorders (127 [26.7%]), anxiety disorders (19 [4.0%]), substance abuse (2 [0.4%]), and other (1 [0.2%]). No diagnosis was found in 3 (0.6%). The secondary diagnostic distributions for each primary diagnostic group were similar in the subgroups with EPDS scores of 10 or higher and 13 or higher (data not shown). Of the 147 women who completed the telephone screening for MDD, 25 (17.0%) had the disorder, a rate lower than the rate of MDD in the screen-positive women who participated in a home visit (514 of 1396 [36.8%]).
COMMENT
This sample of 10 000 of women who recently gave birth is the largest American population to undergo screening with the EPDS. The rate of acceptance of postscreening diagnostic evaluation in these women was more favorable (59.2% home visits and 10.5% telephone evaluations) than in many US studies (12%,27 27%,28 and 33%29) and comparable to rates in Australian studies (65%14 and 75%20). Contributing to the acceptance of interviews, which required 2 hours of the mother’s time, was conducting them in the women’s homes30 and providing a $40 gift card. However, our sample of 10 000 was derived from 13 442 women reached by telephone from 17 426 eligible women. According to our institutional review board policy, we were unable to collect demographic data from women who did not undergo EPDS screening at 4 to 6 weeks post partum; therefore, the characteristics of these women are unknown. Anecdotally, the recruitment staff in the maternity hospital reported that some women declined screening because they were receiving mental health treatment. Out-of-service cellular telephones were also a reason for our inability to contact women for screening.
The screen-positive women who completed the home diagnostic interview had higher EPDS scores and were more likely to be African American, publicly insured, younger, and less highly educated than women who declined or elected telephone diagnostic participation only. From a public health standpoint, these more seriously ill, higher-risk women are primary targets for identification and intervention. One-third of all births in the United States occur to women enrolled in Medicaid.31 Elevated rates of MDD have been found in programs serving low-income women,32–34 and use of mental health care resources is particularly limited for minority women with PPD.35 Women with fewer resources and serious functional impairment may be more likely to accept a home-visit evaluation. The study protocol dictated that any woman with an EPDS score of 20 or higher or suicidal ideation during the telephone interview spoke with a clinician who encouraged her to accept the home-visit evaluation, which also increased the likelihood that high-risk women received home visits.
Consistent with epidemiologic studies,36 most of the women (40.1%) identified the onset of their episode as post partum. Onset during pregnancy was described by one-third of the women undergoing screening, whereas chronic illness with onset before pregnancy was true in more than one-quarter. Similar episode onset times were found in a minority population of postpartum women37: 50% of mothers with MDD developed the episode after delivery, 25% developed the episode during pregnancy, and 25% had chronic episodes. These data suggest consideration of screening during pregnancy to identify psychiatric disorders and intervene earlier in the episode course.
Rates of self-harm ideation on the EPDS vary because of population characteristics and the time of postpartum administration. The rate we observed (3.2%) at 4 to 6 weeks post partum is comparable to other studies of new mothers, including 5.4% at 8 weeks in England/0.5% to 3.7% in a multisite study in Canada,7 5.3% at 6 weeks in the United States,7 and 2.7% at 4 weeks in Italy.38 Higher rates (9%) were reported by Howard et al,22 whose population was recruited from socioeconomically deprived areas at 6 to 8 weeks, and by Yonkers et al37 (8.5%) in a minority sample at 3 to 5 weeks post partum. Our finding that the EPDS cut point of 10 or higher identified all women who endorsed the highest intensity of self-harm is notable; conversely, no mothers scoring less than 10 gave this response. Self-harm ideation with high intent is a distal predictor of suicide.7,39 Although the rate of completed suicide is lower in postpartum women than in the general population of women, it is the second leading cause of maternal death40 and is characterized by violent and lethal means (eg, drowning, self-immolation).7 The training of personnel who perform screening must include emergency referral and familiarity with community psychiatric resources.39
A novel contribution of this study is the complete Axis I diagnostic characterization of the subjects. Although an EPDS score of 10 or higher is considered a low screening cut point,24,41 only 2.8% of screen-positive women did not have at least 1 primary DSM-JV Axis I diagnosis. Other investigators have reported rates of EPDS screen-positive women without a psychiatric diagnosis of 6.9%42 at 5 months and 11.3% at 4 months post partum.14 Our low rate of nondiagnosis likely results from administration of a detailed SC1D examination by highly experienced clinicians.
Consistent with epidemiologic studies,20,25,43 most of the screen-positive postpartum women (91.1%) had primary mood disorders. Also consistent is the finding that the most common diagnoses identified were unipolar depressive disorders, with the overwhelming majority being MDD. Similar to MDD outside of childbearing,44 we found that PPD was highly comorbid with anxiety disorders. This observation may explain the reason for the relatively small body of literature on primary anxiety disorders across childbearing.43 Most adults with mood disorders experience an anxiety disorder or significant anxiety symptoms in childhood or adolescence.46 This finding held true in our patients, who had already developed a recurrent pattern of MDD superimposed on an anxiety disorder. Because they increase the likelihood of treatment refractoriness in patients with MDD,47 identification of secondary disorders informs treatment planning and increases the precision of disease management.39
Our diagnostic results can be compared with those of other studies from investigators who conducted post-screening diagnostic assessments. Horowitz et al28 performed telephone screening among women from 2 academic medical centers at 4 to 6 weeks post partum. They invited women who had an EPDS score of 10 or higher to undergo the SCID; 5169 were recruited and 13% (similar to our rate of 14.0%) had positive EPDS screen findings. Major or minor depression (akin to our depressive disorders category) included 77.8% of participants. In the study by Milgrom et al,14 74.4% of women scoring 12 or higher on the EPDS had unipolar depressive disorders. The rates of depressive disorders in these 2 studies may be higher than ours (68.5%) because we classified bipolar disorder (including bipolar depression) separately.
Although the EPDS was developed to screen for depression, a striking finding was that 22.6% of the screen-positive women had bipolar disorder. This figure is likely to be an underestimate of bipolar disorder episode frequency because the EPDS does not screen specifically for the hypomanic/manic phase of the disorder. The postpartum period carries the highest lifetime risk for first-onset and recurrent episodes of bipolar disorder.25,43 Among women known to have bipolar disorder, 50% to 70% have recurrences post partum.25,48 Munk-Olsen and colleagues49 recently reported that 14% of women with a first psychiatric contact during the initial 30 postpartum days had a conversion to a bipolar disorder diagnosis during a 15-year follow-up compared with only 4% with a first contact unrelated to childbirth. Several contributors to this extreme vulnerability for postpartum decompensation have been advanced. Massive withdrawal of gonadal steroid levels contributes to mood instability in these neurobiologically50 and genetically51–53 vulnerable women. Sleep deprivation and interference with circadian rhythms during late pregnancy, labor, and breast-feeding promote mood destabilization.54
Bipolar disorders are common, clinically significant, and underrecognized.55 In an urban general medical care clinic, the rate of positive screen results for lifetime bipolar disorder was nearly 1 of every 10 patients (9.8%).55 Our rate of diagnosed bipolar disorder in an obstetrical sample was even higher for several reasons. First, we conducted our psychiatric evaluations with women who already had been identified with screen-positive EPDS findings. Second, we conducted in-depth SCID interviews for current and lifetime diagnoses. Bipolar disorder is difficult to diagnose because a detailed lifetime history search for hypomania and mixed states must be completed.56 Third, the highly experienced clinicians were specifically trained to differentiate unipolar from bipolar depression.
Recognition of bipolar disorder is the most important prerequisite for adequate treatment.57 Many patients receive treatment for comorbid psychiatric disorders, but lack of recognition of the underlying bipolar disorder results in few receiving appropriate treatment.56 Half of women with “treatment-resistant” PPD actually have bipolar disorder.58 Validated screens for postpartum bipolar disorder or mania are urgently needed.59 Failure to identify mania/hypomania results in the misdiagnosis of bipolar disorder as MDD. Antidepressant monotherapy may increase rapid cycling and the risk for mania or treatment resistance.58 Treatment of the depressed phase of bipolar disorder with a mood stabilizer and an antidepressant does not confer benefit beyond treatment with a mood stabilizer alone.60 Given the critical importance of birth as a life event for families, detection and treatment of bipolar disorder among childbearing women has major public health significance.
This investigation has several strengths, including the large heterogeneous population of non-treatment-seeking women and psychiatric diagnostic interviews with most of the women with positive screen results. Experienced clinicians conducted the interviews, and all diagnostic formulations were reviewed by psychiatrists. Because we were interested in evaluating the yield of diagnoses for screen-positive women, only those with EPDS scores of 10 or higher were offered diagnostic assessments, which limited our capacity to evaluate the sensitivity and specificity of the EPDS from the data. However, an EPDS score of 10 or higher identified 14.0% of the population as at risk for MDD. Lower cut points would yield even more women identified as screen-positive and impose practical limitations owing to a large burden for postscreening assessment.
Although a single screening point is efficient, the timing must balance the accrual of women who develop the disorder post partum against the length of time these women will be ill before identification. Although more than 40% of women in our sample had postpartum-onset disorders, many longer-term psychiatric illnesses began before or during the index pregnancy.61 The elevated risk for psychiatric episodes continues until 3 months post partum,25 which suggests that additional screening points beyond 4 to 6 weeks or rescreening among women with subthreshold scores (such as an EPDS score of ≥8 or ≥9). Finally, despite a comparatively high rate of completed SCID interviews, the diagnostic contribution of the women who declined the home visit or telephone interview for MDD is not known. Women who had a telephone interview or who declined evaluation had lower mean EPDS scores, which implies that diagnostic interviews were more likely to be obtained from the women in the population who were (on average) more ill, which is desirable given the objectives of screening. The demographics also imply that women who had telephone interviews or who declined were single, privately insured working mothers who could not arrange time for an in-home interview.
Although centralized depression screening by telephone as in this study is feasible in the early postpartum period,1 the challenge is to design a therapeutic program to support and retain women through diagnostic evaluation and treatment to maternal recovery and optimal function.61 The diagnostic data demonstrate that the most common episode in postpartum women is recurrent MDD with a comorbid anxiety disorder, typically generalized anxiety disorder, and that strategies for identifying women with bipolar disorder are needed to improve diagnostic precision. A comprehensive screening and diagnostic characterization coupled with diagnosis-specific intervention strategies might reduce maternal disability, improve function, and avert a new generation at risk.8,62
Acknowledgments
Funding Support: This study was supported by grant R01 MH 071825 for Identification and Therapy of Postpartum Depression (Dr Wisner, principal investigator).
Footnotes
Author Contributions: All authors bad full access to all the data in the study; Drs Wisner and Wisniewski take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures: Dr Wisner participated in an advisory board for Eli Lilly Company and received donated estradiol and placebo transdermal patches from Novartis for a National Institute of Mental Health-funded randomized trial, activities that do not involve the work described in this article. Dr Wisniewski has received compensation for consultation to Bristol-Myers Squibb Company (2007–2008), Organon (2007), Case Western Reserve University (2007), Singapore Clinical Research Institute (2009), Dey Pharmaceuticals (2010), and Venebio (2010) and received payment for his board membership for Cyberonic, Inc (2005–2009).
Previous Presentation: Preliminary data for this study were presented at the Marce Society meeting; October 4, 2012; Paris, France.
Additional Contributions: Sarah Scholle, PhD, performed pioneering work in this area while she was at the University of Pittsburgh. The staff at Magee-Womens Hospital, including Terri Redpath, RN, Veta Fanner, MSW, Leah Kelly, MSN, Darla Lane, RN, Rosanne Salt, RN, Jeanne Kingston, RN, Jessica Lott, RN, Lisa Karow, RN, Lisa Stein, RN, and Karen Pennington, RN, offered screening to new mothers. Tracey Capotosto, MBA, arranged the references for this manuscript.
Online-Only Material: The eTable is available at http://www.jamapsych.com.
References
- 1.Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. EvidRep Techno!Assess (Summ) 2005;(119):1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett IM, Marcus SC, Palmer SC, Coyne JC. Pregnancy-related discontinuation of antidepressants and depression care visits among Medicaid recipients. Psychiatr Serv. 2010;61(4):386–391. doi: 10.1176/ps.2010.61.4.386. [DOI] [PubMed] [Google Scholar]
- 3.Vesga-López O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805–815. doi: 10.1001/archpsyc.65.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck CT. The effects of postpartum depression on maternal-infant interaction; a meta-analysis. Nurs Res. 1995;44(5):298–304. [PubMed] [Google Scholar]
- 5.Murray L. The impact of postnatal depression on infant development. J Child Psychol Psychiatry. 1992;33(3):543–561. doi: 10.1111/j.1469-7610.1992.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 6.Whiffen VE, Gotlib IH. Infants of postpartum depressed mothers: temperament and cognitive status. J Abnorm Psychol. 1989;98(3):274–279. doi: 10.1037//0021-843x.98.3.274. [DOI] [PubMed] [Google Scholar]
- 7.Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77–87. doi: 10.1007/s00737-005-0080-1. [DOI] [PubMed] [Google Scholar]
- 8.Misra DP, Guyer B, Allston A. Integrated perinatal health framework: a multiple determinants model with a life span approach. Am J Prev Med. 2003;25(1):65–75. doi: 10.1016/s0749-3797(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 9.Postpartum Support International. US legislative action. 2012 http;//www.postpartum.net/News-and-Events/tegislation.aspx. Accessed February 15, 2013.
- 10.Gilbody S, Sheldon T, House A. Screening and case-finding instruments for depression; a meta-analysis. GMAJ. 2008;178(8):997–1003. doi: 10.1503/cmaj.070281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulden M, Palmer S, Hewitt C, Gilbody S. Screening for postnatal depression in primary care: cost effectiveness analysis. BMJ. 2009;339:5203. doi: 10.1136/bmj.b5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudron LH, Szilagyi PG, Campbell AT, Mounts KO, Mclnerny TK. Legal and ethical considerations: risks and benefits of postpartum depression screening at well-child visits. Pediatrics. 2007;119(1):123–128. doi: 10.1542/peds.2006-2122. [DOI] [PubMed] [Google Scholar]
- 13.American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee opinion No. 453: screening for depression during and after pregnancy. Obstet Gynecol. 2010;115(2, pt 1):394–395. doi: 10.1097/AOG.0b013e3181d035aa. [DOI] [PubMed] [Google Scholar]
- 14.Milgrom J, Holt CJ, Gemmill AW, et al. Treating postnatal depressive symptoms in primary care: a randomised controlled trial of GP management, with and without adjunctive counselling. BMC Psychiatry. 2011;11:95. doi: 10.1186/1471-244X-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1937;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 16.Eberhard-Gran M, Eskild A, Tambs K, Opjordsmoen S, Samuelsen SO. Review of validation studies of the Edinburgh Postnatal Depression Scale. Acta Psychiatr Scand. 2001;104(4):243–249. doi: 10.1034/j.1600-0447.2001.00187.x. [DOI] [PubMed] [Google Scholar]
- 17.Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, Gray R. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119(5):350–364. doi: 10.1111/j.1600-0447.2009.01363.x. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual tor Psychiatric Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 19.Rowe HJ, Fisher JR, Loh WM. The Edinburgh Postnatal Depression Scale detects but does not distinguish anxiety disorders from depression in mothers of infants. Arch Womens Ment Health. 2008;11(2):103–108. doi: 10.1007/s00737-008-0003-z. [DOI] [PubMed] [Google Scholar]
- 20.Austin MP, Hadzi-Pavlovic D, Priest SR, et al. Depressive and anxiety disorders in the postpartum period: how prevalent are they and can we improve their detection? Arch Womens Ment Health. 2010;13(5):395–401. doi: 10.1007/s00737-010-0153-7. [DOI] [PubMed] [Google Scholar]
- 21.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-lll-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 22.Howard L, Flach C, Mehay A, Sharp D, Tylee A. The prevalence of suicidal ideation identified by the Edinburgh Postnatal Depression Scale in postpartum women in primary care: findings from the RESPOND trial. BMC Pregnancy Childbirth. 2011;11:57. doi: 10.1186/1471-2393-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanusa BH, Scholle SH, Hasket RF, Spadaro K, Wisner K. Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt) 2008;17(4):585–596. doi: 10.1089/jwh.2006.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox J, Holden J. Perinatal Mental Health: A Guide to the Edinburgh Postnatal Depression Screening Scale. Glasgow, Scotland: Bell & Bain Ltd; 2003. [Google Scholar]
- 25.Munk-Oisen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: a population-based register study. JAMA. 2006;296(21):2582–2589. doi: 10.1001/jama.296.21.2582. [DOI] [PubMed] [Google Scholar]
- 26.First MB, Spitzer Rt, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition. New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- 27.Horowitz JA, Cousins A. Postpartum depression treatment rates for at-risk women. Nurs Res. 2006;55(2 suppl):S23–S27. doi: 10.1097/00006199-200603001-00005. [DOI] [PubMed] [Google Scholar]
- 28.Horowitz JA, Murphy CA, Gregory KE, Wojcik J. A community-based screening initiative to identify mothers at risk for postpartum depression. J Obstet Gynecol Neonatal Nurs. 2011;40(1):52–61. doi: 10.1111/j.1552-6909.2010.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gjerdingen DK, Yawn BP. Postpartum depression screening: importance, methods, barriers, and recommendations for practice. J Am Board Fam Med. 2007;20(3):280–288. doi: 10.3122/jabfm.2007.03.060171. [DOI] [PubMed] [Google Scholar]
- 30.Flynn HA, Henshaw E, O’Mahen H, Forman J. Patient perspectives on improving the depression referral processes in obstetrics settings: a qualitative study. Gen Hosp Psychiatry. 2010;32(1):9–16. doi: 10.1016/j.genhosppsych.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Center for Health Statistics, Centers for Disease Control and Prevention. Vital Statistics of the United States: 1999: Natality. Atlanta, GA: Centers for Disease Control and Prevention; 2002. p. 1. [PubMed] [Google Scholar]
- 32.Hobfoll SE, Ritter C, Lavin J, Hulsizer MR, Cameron RP. Depression prevalence and incidence among inner-city pregnant and postpartum women. J Consult Clin Psychol. 1995;63(3):445–453. doi: 10.1037//0022-006x.63.3.445. [DOI] [PubMed] [Google Scholar]
- 33.Miranda J, Azocar F, Komaromy M, Golding JM. Unmet mental health needs of women in public-sector gynecologic clinics. Am J Obstet Gynecol. 1998;178(2):212–217. doi: 10.1016/s0002-9378(98)80002-1. [DOI] [PubMed] [Google Scholar]
- 34.Scholle SH, Haskett RF, Hanusa BH, Pincus HA, Kupfer DJ. Addressing depression in obstetrics/gynecology practice. Gen Hosp Psychiatry. 2003;25(2):83–90. doi: 10.1016/s0163-8343(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 35.Boyd RC, Mogul M, Newman D, Coyne JC. Screening and referral for postpartum depression among low-income women: a qualitative perspective from community health workers. Depress Res Treat. 2011;2011:320605. doi: 10.1155/2011/320605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munk-Olsen T, Laursen TM, Mendelson T, Pedersen CB, Mors O, Mortensen PB. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189–195. doi: 10.1001/archgenpsychiatry.2008.528. [DOI] [PubMed] [Google Scholar]
- 37.Yonkers KA, Ramin SM, Rush AJ, et al. Onset and persistence of postpartum depression in an inner-city maternal health clinic system. Am J Psychiatry. 2001;158(11):1856–1863. doi: 10.1176/appi.ajp.158.11.1856. [DOI] [PubMed] [Google Scholar]
- 38.Mauri M, Oppo A, Borri C, Banti S, PND-ReScU group Suicidality in the perinatal period: comparison of two self-report lnstruments: results from PND-ReScU. Arch Womens Ment Health. 2012;15(1):39–47. doi: 10.1007/s00737-011-0246-y. [DOI] [PubMed] [Google Scholar]
- 39.Practice guideline for the assessment and treatment of patients with suicidal behavior. Am J Psychiatry. 2003;16Q(11 suppl):1–60. [PubMed] [Google Scholar]
- 40.Cantwell R, Clutton-Brock T, Cooper G, et al. Saving mothers’ lives; reviewing maternal deaths to make motherhood safer 2006–2008: the Eighth Report on Confidential Enquiries Into Maternal Deaths In the United Kingdom. BJOG. 2011;118(suppl 1):1–203. doi: 10.1111/j.l471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 41.Matthey S, Henshaw C, Elliott S, Barnett B. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale: implications for clinical and research practice. Arch Womens Ment Health. 2006;9(6):309–315. doi: 10.1007/s00737-006-0152-x. [DOI] [PubMed] [Google Scholar]
- 42.Grigoriadis S, de Camps Meschino D, Barrons E, et al. Mood and anxiety disorders In a sample of Canadian perinatal women referred for psychiatric care. Arch Womens Ment Health. 2011;14(4):325–333. doi: 10.1007/s00737-011-0223-5. [DOI] [PubMed] [Google Scholar]
- 43.Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses [published correction appears In Br J Psychiatry. July 1987;151:135] Br J Psychiatry. 1987;150:662–673. doi: 10.1192/bjp.150.5.662. [DOI] [PubMed] [Google Scholar]
- 44.Hettema JM, Prescott CA, Kendler KS. The effects of anxiety, substance use and conduct disorders on risk of major depressive disorder. Psychol Med. 2003;33(8):1423–1432. doi: 10.1017/s0033291703008365. [DOI] [PubMed] [Google Scholar]
- 45.Wisner K, Sit D, Reynolds S, et al. Psychiatric disorders. In: Gabbe S, Niebyl J, Simpson J, et al., editors. Obstetrics: Normal and Problem Pregnancies. 5. Philadelphia, PA: Elsevier; 2007. pp. 1249–1288. [Google Scholar]
- 46.Pine DS. Integrating research on development and fear learning: a vision for clinical neuroscience? Depress Anxiety. 2009;26(9):775–779. doi: 10.1002/da.20595. [DOI] [PubMed] [Google Scholar]
- 47.American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision) Am J Psychiatry. 2000;157(4 suppl):1–45. [PubMed] [Google Scholar]
- 48.Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164(12):1817–1824. doi: 10.1176/appi.ajp.2007.06101639. quiz 1923. [DOI] [PubMed] [Google Scholar]
- 49.Munk-Olsen T, Laursen TM, Meltzer-Brody S, Mortensen PB, Jones I. Psychiatric disorders with postpartum onset: possible early manifestations of bipolar affective disorders. Arch Gen Psychiatry. 2012;69(4):428–434. doi: 10.1001/archgenpsychiatry.2011.157. [DOI] [PubMed] [Google Scholar]
- 50.Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157(6):924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- 51.Binder EB, Jeffrey Newport D, Zach EB, et al. A serotonin transporter gene polymorphism predicts perlpartum depressive symptoms in an at-risk psychiatric cohort. J Psychiatr Res. 2010;44(10):640–646. doi: 10.1016/j.jpsychires.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahon PB, Payne JL, MacKinnon DF, et al. NIMH Genetics Initiative Bipolar Disorder Consortium; BiGS Consortium. Genome-wide linkage and follow-up association study of postpartum mood symptoms. Am J Psychiatry. 2009;166(11):1229–1237. doi: 10.1176/appi.ajp.2009.09030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanjuan J, Martin-Santos R, Garcia-Esteve L, et al. Mood changes after delivery; role of the serotonin transporter gene. Br J Psychiatry. 2008;193(5):383–388. doi: 10.1192/bjp.bp.107.045427. [DOI] [PubMed] [Google Scholar]
- 54.Yonkers KA, Wisner KL, Stowe Z, et al. Management of bipolar disorder during pregnancy and the postpartum period. Am J Psychiatry. 2004;161(4):608–620. doi: 10.1176/appi.ajp.161.4.608. [DOI] [PubMed] [Google Scholar]
- 55.Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293(8):956–963. doi: 10.1001/jama.293.8.956. [DOI] [PubMed] [Google Scholar]
- 56.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arvilommi P, Suominen KS, Mantere OK, Leppämäkl S, Valtonen H, Isometsä ET. Adequacy of treatment received by diagnosed and undiagnosed patients with bipolar I and II disorders. J Clin Psychiatry. 2007;68(1):102–110. doi: 10.4088/jcp.v68n0114. [DOI] [PubMed] [Google Scholar]
- 58.Sharma V, Burt VK, Ritchie HL. Bipolar II postpartum depression: detection, diagnosis, and treatment. Am J Psychiatry. 2009;166(11):1217–1221. doi: 10.1176/appi.ajp.2009.08121902. [DOI] [PubMed] [Google Scholar]
- 59.Chesslck CA, Dimldjian S. Screening for bipolar disorder during pregnancy and the postpartum period. Arch Womens Ment Health. 2010;13(3):233–248. doi: 10.1007/s00737-010-0151-9. [DOI] [PubMed] [Google Scholar]
- 60.Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711–1722. doi: 10.1056/NEJMoa064135. [DOI] [PubMed] [Google Scholar]
- 61.Miller L, Shade M, Vasireddy V. Beyond screening: assessment of perinatal depression in a perinatal care setting. Arch Womens Ment Health. 2009;12(5):329–334. doi: 10.1007/s00737-009-0082-5. [DOI] [PubMed] [Google Scholar]
- 62.Wisner KL, Scholle SH, Stein B. Perinatal disorders: advancing public health opportunities. J Clin Psychiatry. 2008;69(10):1602–1605. doi: 10.4088/jcp.v69n1010. [DOI] [PMC free article] [PubMed] [Google Scholar]


