SUMMARY
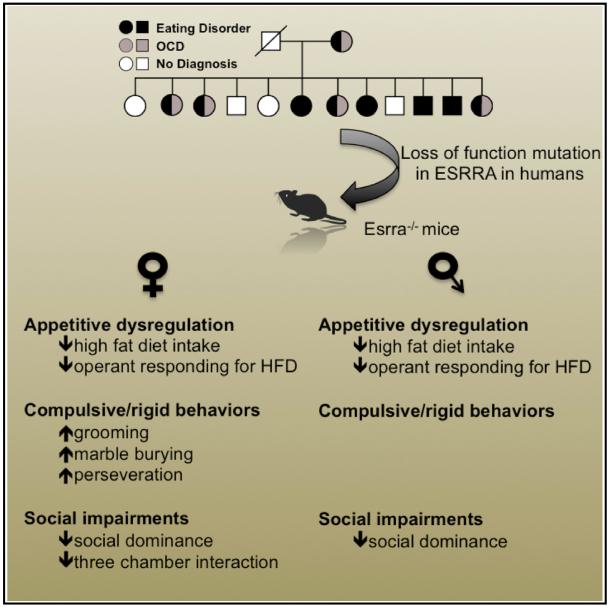
Eating disorders, such as anorexia nervosa and bulimia nervosa, are common and severe mental illnesses of unknown etiology. Recently, we identified a rare missense mutation in the transcription factor estrogen-related receptor alpha (ESRRA) that is associated with the development of eating disorders. However, little is known about ESRRA function in the brain. Here, we report that Esrra is expressed in the mouse brain and demonstrate that Esrra levels are regulated by energy reserves. Esrra-null female mice display a reduced operant response to a high-fat diet, compulsivity/behavioral rigidity, and social deficits. Selective Esrra knockdown in the prefrontal and orbitofrontal cortices of adult female mice recapitulates reduced operant response and increased compulsivity, respectively. These results indicate that Esrra deficiency in the mouse brain impairs behavioral responses in multiple functional domains.
Graphical Abstract
INTRODUCTION
While eating disorders (EDs) are known to result from a complex interaction of environmental stress and genetic vulnerability (Kaye et al., 2013), the specific neural substrates that mediate development of EDs remain poorly understood. To elucidate molecular pathways that lead to the development of EDs, we previously conducted next-generation sequencing of two large families with multiple members affected by EDs (Cui et al., 2013). In that study, we identified rare missense mutations in the estrogen-related receptor alpha (ESRRA) and histone deacetylase 4 (HDAC4) genes that significantly increase the risk of having an ED (Cui et al., 2013). Specifically, the ESRRAR188Q mutation reduces induction of known ESRRA-target genes in vitro, while the HDAC4A786T mutation increases transcriptional repression of ESRRA by HDAC4. As both mutations result in decreased ESRRA transcriptional activity, we hypothesized that loss of ESRRA activity confers specific susceptibility to developing an ED.
While ESRRA is known to be expressed in the CNS, relatively little is known about its function in neurons. Esrra is also required for induction of mitochondrial biogenesis in the brain, in response to the peroxisome proliferator-activated receptor gamma agonist rosiglitazone (Strum et al., 2007). Furthermore, parkin, a protein associated with the development of Parkinson’s disease, regulates Esrra levels through ubiquitination and degradation of Esrra by the proteasome, which decreases expression of the Esrra-target genes monoamine oxidase A and B (Ren et al., 2011). Recently, Esrra has also been implicated in mediating increased expression of the Fdnc5 and Bdnf genes in mouse hippocampus following endurance exercise (Wrann et al., 2013). Taken together, these findings suggest that tight control of Esrra levels in the CNS may regulate multiple metabolic processes critical to neuronal function. In the present study, we examine Esrra function in mouse brain by using genetic methods to examine the behavioral consequences of loss of Esrra expression.
RESULTS
To study the role of Esrra in the brain, we performed immunohistochemistry to determine its expression pattern in the whole mouse brain. Esrra is abundantly expressed in several cortical regions, including orbitofrontal cortex, medial prefrontal cortex (mPFC), and cingulate cortex (Figures 1A–1C). Additionally, Esrra is highly expressed in the primary and secondary somato-sensory cortex, piriform cortex, retrosplenial granular cortex, hippocampus, thalamus, mammillary nucleus, cerebellum, and several brainstem nuclei involved in transmission of sensory information (Figures S1A–S1C). Moderate levels of expression are observed in dorsolateral aspects of the striatum, septum, global pallidus, lateral hypothalamic area, and some midbrain structures including tegmentum and substantia nigra. Very limited expression was observed in the bed nuclei of stria terminalis, amygdala, and the remainder of the hypothalamus, regions that have been traditionally associated with a role in feeding regulation. Double-immunohistochemistry staining of Esrra and glial fibrillary acid protein failed to reveal colocalization of Esrra in glia (data not shown).
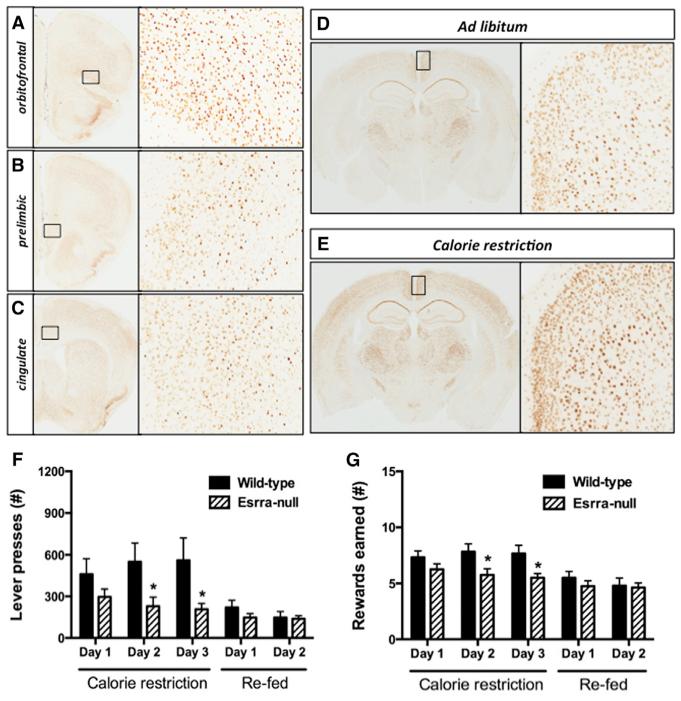
Figure 1. Esrra-Null Mice Display Impaired Behavioral Responses to Calorie Restriction.
(A–C) Expression of Esrra in 14-week-old C57BL6/J mice in lateral orbitofrontal, mPFC, and cingulate cortex.
(D and E) 60% calorie restriction for 10 days increases Esrra expression in mouse brain. Magnification shows retrosplenial region of cingulate cortex.
(F and G) Wild-type (n = 7) and Esrra-null (n = 6) littermate mice were trained to lever press for a HFD pellet on a fixed ratio and then tested for total lever presses (F) and reward earned (G) on a progressive ratio under calorie restriction and ad lib feeding conditions.
Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 indicate significant differences between groups (Student’s t test). See also Figure S1.
Upregulation of Esrra expression has been observed in peripheral tissues following calorie restriction (Ranhotra, 2009). Thus, we examined whether Esrra expression in brain would also be increased in this same condition. Ten days of 60% calorie restriction significantly increased the intensity of Esrra staining throughout the brain, including the cortex (Figures 1D, 1E, S1D, and S1E), indicating that Esrra activity may be involved in the response to energy balance. To quantify this effect, we performed quantitative real-time PCR to measure the relative abundance of Esrra mRNA in the cortex. Consistent with immunohistochemical analysis, 60% calorie restriction induced a significant increase in Esrra mRNA abundance in the cortex compared to that in mice housed under ad lib feeding conditions (2.48 ± 0.59 versus 4.93 ± 0.50, n = 6/group, p = 0.0098).
After determining Esrra expression in several mouse brain regions and that Esrra expression is regulated by metabolic status, we examined the behavioral consequences of Esrra loss. A previous study reported that chow-fed male and female Esrra-null mice were ~15% smaller than wild-type littermates and displayed reduced weight gain on a high-fat diet (HFD), which is consistent with the clinical diagnosis of anorexia nervosa where body weight is maintained at or below 85% of ideal body weight (Luo et al., 2003). We also verified that Esrra-null mice were ~15% smaller than wild-type littermates and consumed less chow and HFD (data not shown); however, these phenotypes were identified in animals on an ad libitum diet. As the magnitude of ad libitum food intake primarily reflects intra-meal satiety independent of any component of behavioral effort (Perello et al., 2010), we utilized instrumental responding as a more sensitive measure of motivation to obtain a HFD.
Following a fixed-ratio training session, wild-type and Esrra-null mice were advanced to a progressive-ratio schedule in which obtaining each successive HFD pellet required an increased number of lever presses. Effortful responding for a HFD was then assessed during both caloric restriction and ad libitum feeding conditions by measuring the total number of lever presses and total number of pellets earned during the 2-hr session. During caloric restriction, Esrra-null mice exhibited reduced total lever presses and number of reward obtained compared to wild-type littermates (Figures 1F and 1G). Following re-feeding, total lever presses and reward obtained decreased for both groups as expected, and no significant differences were noted between treatment groups. At the end of testing, we measured fasting glucose and body composition to control for differences in energy reserves that might account for the decreased responding in Esrra-null mice. Fasting glucose levels (Figure S1F) and body composition (Figure S1G) were not different for both groups, indicating that reduced effortful responding observed in Esrra-null mice was not secondary to differences in energy reserves.
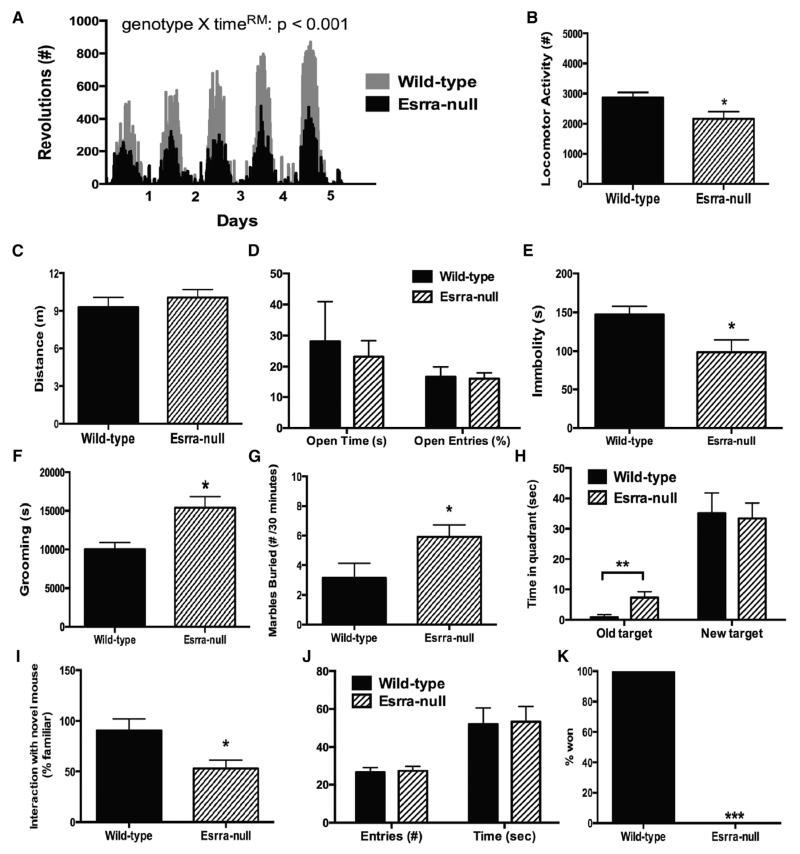
In separate cohorts of female mice, we performed a battery of behavioral tasks relevant to mental illness including locomotor activity, depression/anxiety, compulsivity/behavioral rigidity, and impairments in social functioning. Esrra-null mice have recently been reported to have reduced locomotion in both treadmill exercise and voluntary activity in a metabolic cage assessment (Perry et al., 2014). As locomotor activity varies greatly depending on the context, we measured activity under three conditions: voluntary wheel running, home-cage activity, and exploration of a novel environment. When given free access to a running wheel for 5 days, Esrra-null mice exhibited normal circadian patterns of activity but also a reduction in total revolutions (Figure 2A). Using the Laboras system, we assessed home-cage activity and found a modest reduction in locomotion in Esrra-null mice compared to wild-type littermates (Figure 2B). Finally, we evaluated exploration of a novel environment by measuring distance traveled in the elevate-plus-maze model of anxiety-like behavior and found no significant difference between the groups (Figure 2C). This finding is important as the majority of behavioral tests that we performed involved activity within a novel environment.
Figure 2. Behavioral Deficits in Esrra-Null Mice.
(A) Voluntary wheel-running activity for female wild-type (WT) and Esrra-null mice for 5 days (n = 3 WT, 6 Esrra null).
(B) Time spent active in home cage as measured by vibration plate (n = 17 WT, 5 Esrra null).
(C and D) Distance moved and time spent on the open arm of the elevated-plus-maze (n = 8 WT, 14 null).
(E) Time immobile in the forced-swim test (n = 8 WT, 14 null).
(F) Home-cage grooming as measured by vibration plate (n = 17 WT, 5 Esrra null).
(G) Number of marbles buried within 30 min (n = 7 WT, 12 Esrra null).
(H) Time spent near old target and new target in Barnes maze (n = 5 WT, 6 Esrra null).
(I) Time spent with novel mouse in three-chamber test (n = 8 WT, 10 Esrra null).
(J) Time spent with novel object (n = 9 WT, 11 Esrra null).
(K) Percentage of victories in the social dominance test (n = 24 unique pairings).
Data presented as mean ± SEM with *p < 0.05, **p < 0.01 indicating significant differences between groups (two-way ANOVA [A] or Student’s t test [B–I and K]), except for (J), which is presented as a percentage of total victories with ***p < 0.001 indicating significant differences between groups (chi-square test).
We assessed anxiety- and depression-like behaviors using the elevated-plus-maze and forced-swim test models. Female Esrra-null mice do not display any differences in overall activity or time on the open arm in the elevated-plus-maze (Figures 2C and 2D); however, they did exhibit reduced immobility in the forced-swim test (Figure 2E). Several tasks modeling aspects of perseveration and behavioral rigidity in mice have been used to study related neuropsychiatric disorders, such as autism and obsessive-compulsive disorder, including compulsive grooming (Shmelkov et al., 2010; Welch et al., 2007; Xu et al., 2013), marble burying (Angoa-Pérez et al., 2013), and reversal learning (Kim et al., 2011). Using a vibration-plate based system to quantify 24-hr home-cage grooming, we observed that loss of Esrra increases grooming in female mice (Figure 2F). The fact that chronic fluoxetine treatment has been shown to reduce repetitive grooming in several other genetic models of compulsivity (Shmelkov et al., 2010; Welch et al., 2007) led us to assess whether this treatment could also ameliorate the increased repetitive grooming in female Esrra-null mice. Separate groups of Esrra-null female mice were tested for baseline grooming and then randomized into two equal groups that received either fluoxetine or saline vehicle. Following 1 week of treatment, no effect of fluoxetine was noted (saline 88.2% ± 12.1% versus fluoxetine 93.0% ± 8.9%, n = 6/group, p = 0.71). We also assessed behavioral compulsivity in Esrra-null mice using the marble-burying test. Consistent with the compulsive grooming results, female Esrra-null mice showed a significantly greater degree of marble-burying behaviors after 30 min than wild-type littermates (Figure 2G). Mice were trained for 4 days to find an escape hole in the Barnes maze to assess hippocampal-dependent memory formation. On the fifth day, a probe trial was performed showing that both wild-type and Esrra-null mice spent more time in the area of the escape hole without a significant difference noted between the groups. Following this initial training, mice were next subjected to an additional 4 days of training in which the escape hole was relocated to the opposite position. Another probe trial was then performed, and time spent at the old and new target was measured. While both groups learned the location of the new escape hole, Esrra-null mice spent significantly more time at the original target (Figure 3H), an indication of behavioral rigidity.
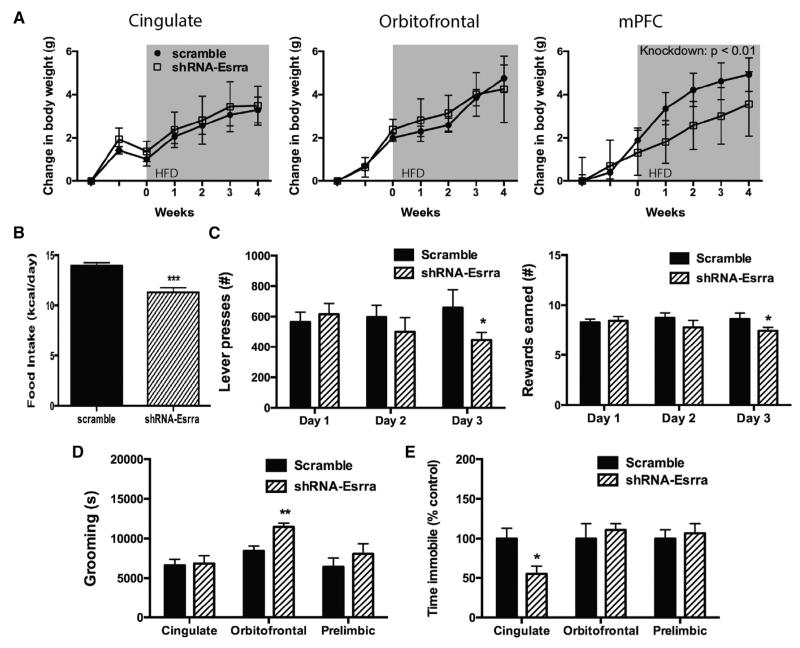
Figure 3. Knockdown of Esrra Expression by shRNA in Cortex Produces Region-Specific Behavioral Deficits.
(A) Body-weight gain in female C57BL6/J mice on a HFD after AAV delivery into cingulate (n = 10 scramble and 9 shRNA-Esrra), orbitofrontal (n = 9 scramble and 10 shRNA-Esrra), or mPFC (n = 9 scramble and 11 shRNA-Esrra).
(B) Intake of a HFD after Esrra knockdown in mPFC.
(C) A separate cohort of mice received AAV into mPFC and was tested for total lever presses and reward earned.
(D and E) Mice from (A) were tested for home-cage grooming (D) and immobility in the forced-swim test (E).
Data presented as mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 indicate significant differences between groups (ANOVA [A] or Student’s t test [B–E]). See also Figure S2.
Several tasks modeling social dysfunction in mice have been designed, including the three-chamber social interaction, social dominance, and novel object recognition. Using this battery of tasks, we also found that Esrra-null mice displayed reduced preference for interaction with a novel social target (Figure 2I), increased social subordination (Figure 2J), and no differences in novel object recognition as compared with wild-type littermate mice. Together these behavioral findings indicate that Esrra-null mice exhibit behavioral deficits in multiple domains including decreased motivation for HFD, perseveration/ behavioral rigidity, and impaired social functioning.
We next interrogated the neural circuits underlying the behavioral phenotypes exhibited by Esrra-null mice by abrogating expression of this gene in a region-specific manner in wild-type female mice. Using viral-mediated gene transfer to selectively knockdown expression of Esrra by short hairpin RNA (shRNA)-mediated silencing (Figures S2A–S2D), we selected several cortical regions implicated in the development of EDs and ED-related behaviors, including cingulate cortex, orbitofrontal cortex, and medial prefrontal cortex (mPFC) (Frank, 2013). While knockdown of Esrra in the cingulate and orbitofrontal cortex had no effect on HFD-induced weight gain in female mice, knockdown in the mPFC reduced weight gain (Figure 3A) via reduced intake of a HFD (Figure 3B). We tested a separate cohort of mice to assess whether knockdown of Esrra in the mPFC disrupted operant responding for HFD. Consistent with our observations in Esrra-null mice, knockdown of Esrra in mPFC was sufficient to reduce total lever presses and reward earned (Figure 3C). Subsequent analyses also examined the capacity of knockdown of Esrra within selected cortical fields to reproduce other behavioral phenotypes observed in null mutants. Increased grooming was observed only after knockdown of Esrra in orbitofrontal cortex, whereas no differences in grooming were observed following either cingulate or mPFC knockdown (Figure 3D). Reduced immobility was observed only after knockdown of Esrra in the cingulate cortex (Figure 3E). Together, these findings identify specific neural substrates accounting for the behavioral alterations observed in Esrra-null mice.
Finally, we determined whether there was a sexual dimorphism in any of the changes we observed in female Esrra-null mice by conducting all of the testing on male mice as well. Table 1 summarizes the differences in behavioral phenotypes in female and male Esrra-null mice. Similar to female mice, male Esrra-null mice display reduced locomotor activity, decreased operant responding, and reduced immobility in the forced-swim test, but do not exhibit deficits in the compulsive/behavioral rigidity battery of tasks or the three chamber social interaction test.
Table 1. Summary of Behavioral and Electrophysiological Phenotypes in Female and Male Esrra-Null Mice.
| Femalea | Maleb | |
|---|---|---|
| Operant responding | ||
| Lever presses | decrease | decrease |
| Rewards | decrease | decrease |
| Wheel running | decrease | no difference |
| Home-cage activity | decrease | decrease |
| Elevated plus maze | ||
| Distance traveled | no difference | no difference |
| Time on open arm | no difference | no difference |
| Forced-swim test | decrease | decrease |
| Grooming | increase | no difference |
| Marble burying | increase | no difference |
| Reversal learning | decrease | no difference |
| Three chamber test | decrease | no difference |
| Social dominance | decrease | decrease |
| Novel object | no difference | no difference |
DISCUSSION
In the current study, we examine the forebrain distribution and function of Esrra, a gene identified in human genetic studies of EDs. We report that Esrra-null mice display behavioral impairments relevant, including maintaining body weight at 15% below normal, impaired behavioral responses to caloric restriction, compulsivity/behavioral rigidity, and impaired social functioning. Furthermore, knockdown of Esrra expression within selected cortical subfields distinguished regional specificity for circuitry underlying behavioral deficits identified in Esrra-null mutants. These findings show that Esrra dysfunction in select forebrain regions impairs behaviors relevant to psychiatric disorders and offer insight into the potential role of Esrra dysfunction in the development of eating disorders, such as anorexia nervosa.
Esrra expression in brain is increased during calorie restriction and loss of Esrra by conventional knockout or shRNA-mediated knockdown in the mPFC results in decreased consumption of a HFD and decreased operant responding for a HFD. These results suggest that increased expression of Esrra is important for mediating behavioral responses to negative energy balance. As the ESRRAR188Q mutation that was found to be associated with an increased risk of developing anorexia nervosa results in decreased transcriptional activity (Cui et al., 2013), our results suggest that loss of ESRRA activity in humans may predispose to the development of anorexia nervosa by impairing behavioral responses to calorie restriction.
Identifying the neural circuit that mediates the reduced effortful responding for HFD after fasting is another question of great clinical relevance. Esrra activity in the mPFC seems to be at least partially required for this effect, as viral-mediated knockdown of Esrra expression in the mPFC produces a similar reduction in responding (Figure 3C). Stimulation of glutamatergic neurons in the mPFC by pharmacological (Mena et al., 2013) or optogenetic (Land et al., 2014) approaches potently induces food intake in rodents. However, the site of this action is unclear as mPFC neurons project to multiple sites, including the amygdala, lateral-perifornical hypothalamic area, and nucleus accumbens that have been implicated in this behavioral response (Land et al., 2014; Mena et al., 2013). Recently developed chemogenetic and optogenetic approaches to the study of feeding may ultimately be required to deconstruct these circuits.
Additionally, the behavioral deficits we observe in Esrra-null mice might have important implications for other symptoms associated with the development of eating disorders. Neuropsychological testing has consistently found that patients with EDs display increased perseveration and behavioral rigidity (Roberts et al., 2010). Patients with EDs display deficits in social functioning as well including insecure attachment, reduced emotional awareness, increased avoidance of emotion, and increase sense of social inferiority (Caglar-Nazali et al., 2014).
Our findings may also be relevant to the basis of sex differences observed in neuropsychiatric disorders. Many psychiatric disorders, including EDs, major depression, and most anxiety disorders, predominantly affect women although the neural basis of this sexual dimorphism remains unclear. Our data suggest that females may be more susceptible to the loss of Esrra function, as female knockout mice exhibited more behavioral impairments than male mice. The mechanism for the observed sex differences remains unclear. There is an estrogen response element in the promoter of the Esrra gene, and previous studies have indicated that estrogen receptor signaling affects expression of Esrra in peripheral tissues (Bonnelye et al., 2002; Liu et al., 2003), although we did not observe any change in Esrra mRNA levels in mPFC 4 weeks after ovariectomy in young adult female mice (data not shown). Alternatively, estrogen receptors alpha and beta have been localized to dendritic spines in the hippocampus and may have a direct, non-genomic signaling effect on synaptic plasticity (Sala and Segal, 2014). Delineating the roles of Esrra and estrogen receptor activity will have important implications in understanding sex-based differences in mental illness.
Several limitations of the current study should be noted. Esrra-null mice have reduced locomotor activity, which can confound the interpretation of certain behavioral tasks like operant responding and the forced-swim test. This hypolocomotor phenotype appears to be most severe for endurance activity, such as treadmill exercise (Perry et al., 2014) or voluntary wheel running (Figure 2A). The phenotype is less severe for home-cage activity (Figure 2B) and absent in the exploration of a novel environment (Figure 2C), which represents the majority of our tasks. Also, we have tried to select a balanced set of tasks to control for differences in locomotor activity. For instance, the increased activity of Esrra-null mice in the forced-swim test, marble-burying test, and grooming assay is in the opposite direction of an expected hypolocomotor effect.
Finally, there are limitations with the two methods used to decrease Esrra activity. We selected the Esrra-null mouse because the ESRRAR188Q mutation that predisposes to the development of eating disorders in human populations decreases transcriptional activity of Esrra in vitro (Cui et al., 2013). While we believe that the Esrra-null mouse is a reasonable first approach to testing our hypothesis that reduced Esrra activity increases the risk of developing ED-related behaviors, we cannot be certain that the R188Q mutation does not induce an unanticipated change in the activity of the Esrra protein. Additionally, the use of shRNA-mediated gene knockdown has been questioned for possible off-target effects (Baek et al., 2014). While this concern is offset by our limited use of shRNA in the context of mapping behavioral deficits originally identified in Esrra-null mice, the use of more targeted genetic models, such as conditional deletion of Esrra expression with a Cre-lox approach, will further expand our understanding of the genetic necessity of Esrra within discrete neural circuits.
In conclusion, we show that loss of the transcription factor Esrra results in behavioral deficits in multiple functional domains, including maintenance of a low body weight, reduced effortful responding for HFD after calorie restriction, compulsivity/behavioral rigidity, and impaired social functioning. These findings offer insight into the neurobiological function of Esrra and have potential implications for understanding the neurobiological basis of eating disorders.
EXPERIMENTAL PROCEDURES
Animals
Esrra-null and C57BL/6J mice were obtained from Jackson Laboratory and backcrossed more than five generations onto C57BL/6J. All animal procedures were performed in accordance with University of Iowa Institutional Animal Care and Use Committee guidelines. Mice heterozygous for Esrra+/− alleles were bred to generate wild-type (Esrra+/+) and Esrra-null (Esrra−/−) littermate mice. Mice were weaned and genotyped at 3–4 weeks of age. See Supplemental Experimental Procedures for housing and animal husbandry conditions.
Behavioral Studies
Twelve- to 16-week-old animals were used for behavioral studies. Some mice were used after completing body-weight monitoring and had previously been exposed to a high-fat diet (42.8% calories from fat, TD.88137, Harlan-Teklad). No behavioral differences, however, were noted between chow- and HFD-fed animals and groups (data not shown). Separate cohorts of mice not exposed to a HFD were used for wheel running and operant responding, respectively, so that no mouse included in the other behavioral tasks had been exposed to increase physical activity or calorie restriction. See Supplemental Experimental Procedures for full methodology for behavioral experiments.
Immunohistochemistry
Immunohistochemistry was performed as previously reported (Cui et al., 2012). Rabbit monoclonal anti-Esrra antibody was used (04-1134; Millipore) at 1/1,000 dilution. The specificity of anti-Esrra antibody was confirmed by both immunohistochemistry (Figure S1B) and immunoblotting (Figure S1C) in Esrra-null mice. Quantification of immunohistochemistry was performed as previously described (Yin et al., 2014).
Viral Preparation
An shRNA sequence targeting nucleotides 1203–1223 of mouse Esrra (NM_007953) or scramble control were commercially prepared (Vector Laboratories). See Supplemental Experimental Procedures for full description of viral preparations.
Viral Delivery
Twelve-week-old female C57BL/6J (The Jackson Laboratory, #00664) mice were randomly assigned into two groups to receive either adeno-associated virus 2 (AAV) encoding both mEsrra-shRNA and GFP or AAV encoding scrambled sequence and GFP as control into the following regions: mPFC (AP +1.8 mm, ML +0.8 mm, DV −2.8 mm, 10-degree angle), lateral orbitofrontal (AP +2.8 mm, ML +1.7 mm, DV −2.0 mm, 10-degree angle), and cingulate (AP +0.2 mm, ML +0.6 mm, DV −1.7 mm, 10-degree angle). See Supplemental Experimental Procedures for surgical procedures.
Data Analysis
Data are presented as mean ± SEM. GraphPad Prism 6 software (GraphPad) were used to perform all statistical analyses. For feeding and wheel-running studies, comparisons between groups were made by two-way ANOVA with time as the repeated measure. The chi-square test was used to determine significance in the social dominance test. Student’s t test was used for all other measures. p < 0.05 was considered to be statistically significant.
Supplementary Material
Highlights.
Esrra expression in the mouse brain is regulated by energy status
Loss of Esrra reduces response to a high-fat diet
Esrra-null mice display behavioral compulsivity and social impairments
Esrra knockdown in mPFC and OFC recapitulates feeding and grooming deficits
ACKNOWLEDGMENTS
This material is based upon work supported by the Dylan Tauber Researcher Award from the Brain and Behavior Foundation (M.L.), the Klarman Family Foundation Grants Program in Eating Disorder Research (M.L.), and MH-095972 (J.J.R.).
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, two figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2015.03.032.
REFERENCES
- Angoa-Pérez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. 2013:50978. doi: 10.3791/50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek ST, Kerjan G, Bielas SL, Lee JE, Fenstermaker AG, Novarino G, Gleeson JG. Off-target effect of doublecortin family shRNA on neuronal migration associated with endogenous microRNA dysregulation. Neuron. 2014;82:1255–1262. doi: 10.1016/j.neuron.2014.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelye E, Kung V, Laplace C, Galson DL, Aubin JE. Estrogen receptor-related receptor alpha impinges on the estrogen axis in bone: potential function in osteoporosis. Endocrinology. 2002;143:3658–3670. doi: 10.1210/en.2002-220095. [DOI] [PubMed] [Google Scholar]
- Caglar-Nazali HP, Corfield F, Cardi V, Ambwani S, Leppanen J, Olabintan O, Deriziotis S, Hadjimichalis A, Scognamiglio P, Eshkevari E, et al. A systematic review and meta-analysis of ‘Systems for Social Processes’ in eating disorders. Neurosci. Biobehav. Rev. 2014;42:55–92. doi: 10.1016/j.neubiorev.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Cui H, Sohn JW, Gautron L, Funahashi H, Williams KW, Elmquist JK, Lutter M. Neuroanatomy of melanocortin-4 receptor pathway in the lateral hypothalamic area. J. Comp. Neurol. 2012;520:4168–4183. doi: 10.1002/cne.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Moore J, Ashimi SS, Mason BL, Drawbridge JN, Han S, Hing B, Matthews A, McAdams CJ, Darbro BW, et al. Eating disorder predisposition is associated with ESRRA and HDAC4 mutations. J. Clin. Invest. 2013;123:4706–4713. doi: 10.1172/JCI71400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK. Altered brain reward circuits in eating disorders: chicken or egg? Curr. Psychiatry Rep. 2013;15:396. doi: 10.1007/s11920-013-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci. 2013;36:110–120. doi: 10.1016/j.tins.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Lee HR, Sim SE, Baek J, Yu NK, Choi JH, Ko HG, Lee YS, Park SW, Kwak C, et al. PI3Kγ is required for NMDA receptor-dependent long-term depression and behavioral flexibility. Nat. Neurosci. 2011;14:1447–1454. doi: 10.1038/nn.2937. [DOI] [PubMed] [Google Scholar]
- Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, Sarhan M, Guarnieri DJ, Deisseroth K, Aghajanian GK, DiLeone RJ. Medial prefrontal D1 dopamine neurons control food intake. Nat. Neurosci. 2014;17:248–253. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Zhang Z, Gladwell W, Teng CT. Estrogen stimulates estrogen-related receptor alpha gene expression through conserved hormone response elements. Endocrinology. 2003;144:4894–4904. doi: 10.1210/en.2003-0432. [DOI] [PubMed] [Google Scholar]
- Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguère V. Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor alpha. Mol. Cell. Biol. 2003;23:7947–7956. doi: 10.1128/MCB.23.22.7947-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena JD, Selleck RA, Baldo BA. Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J. Neurosci. 2013;33:18540–18552. doi: 10.1523/JNEUROSCI.3323-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Chuang JC, Scott MM, Lutter M. Translational neuroscience approaches to hyperphagia. J. Neurosci. 2010;30:11549–11554. doi: 10.1523/JNEUROSCI.2578-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MC, Dufour CR, Tam IS, B’chir W, Giguère V. Estrogen-related receptor-α coordinates transcriptional programs essential for exercise tolerance and muscle fitness. Mol. Endocrinol. 2014;28:2060–2071. doi: 10.1210/me.2014-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhotra HS. Up-regulation of orphan nuclear estrogen-related receptor alpha expression during long-term caloric restriction in mice. Mol. Cell. Biochem. 2009;332:59–65. doi: 10.1007/s11010-009-0174-6. [DOI] [PubMed] [Google Scholar]
- Ren Y, Jiang H, Ma D, Nakaso K, Feng J. Parkin degrades estrogen-related receptors to limit the expression of monoamine oxidases. Hum. Mol. Genet. 2011;20:1074–1083. doi: 10.1093/hmg/ddq550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ME, Tchanturia K, Treasure JL. Exploring the neurocognitive signature of poor set-shifting in anorexia and bulimia nervosa. J. Psychiatr. Res. 2010;44:964–970. doi: 10.1016/j.jpsychires.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Sala C, Segal M. Dendritic spines: the locus of structural and functional plasticity. Physiol. Rev. 2014;94:141–188. doi: 10.1152/physrev.00012.2013. [DOI] [PubMed] [Google Scholar]
- Shmelkov SV, Hormigo A, Jing D, Proenca CC, Bath KG, Milde T, Shmelkov E, Kushner JS, Baljevic M, Dincheva I, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat. Med. 2010;16:598–602. doi: 10.1038/nm.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strum JC, Shehee R, Virley D, Richardson J, Mattie M, Selley P, Ghosh S, Nock C, Saunders A, Roses A. Rosiglitazone induces mitochondrial biogenesis in mouse brain. J. Alzheimers Dis. 2007;11:45–51. doi: 10.3233/jad-2007-11108. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Grueter BA, Britt JK, McDaniel L, Huntington PJ, Hodge R, Tran S, Mason BL, Lee C, Vong L, et al. Double deletion of melanocortin 4 receptors and SAPAP3 corrects compulsive behavior and obesity in mice. Proc. Natl. Acad. Sci. USA. 2013;110:10759–10764. doi: 10.1073/pnas.1308195110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin TC, Britt JK, De Jesús-Cortés H, Lu Y, Genova RM, Khan MZ, Voorhees JR, Shao J, Katzman AC, Huntington PJ, et al. P7C3 neuroprotective chemicals block axonal degeneration and preserve function after traumatic brain injury. Cell Rep. 2014;8:1731–1740. doi: 10.1016/j.celrep.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.