Abstract
Purpose
The purpose of this study was to describe group and individual characteristics of nighttime sleep-wake patterns among school-age children with cancer receiving inpatient chemotherapy.
Design and Methods
This multiple-case study used wrist actigraphs and sleep diaries to measure sleep for 3 nights in 15 children with cancer.
Results
Nighttime sleep was less than that required for healthy school-age children and was marked by frequent awakenings. Individual variation in sleep characteristics was evident.
Practice Implications
Interventions to promote nighttime sleep in the hospital include system-based and individualized efforts to minimize disruptions and support children’s home sleep routines.
Search terms: Case study research, childhood cancer, school-age children, sleep
Approximately 12,600 children younger than 15 years are diagnosed with cancer each year in the United States (Howlader et al., 2011). Promoting quality of life for children with cancer includes attention to symptom management across the treatment continuum (Hinds et al., 2004). Among the symptoms most commonly reported by children receiving treatment for cancer is disrupted nighttime sleep.
Adequate nighttime sleep is a fundamental aspect of health and well-being for all children (Mindell & Owens, 2010). However, approximately 30% to 45% of children with cancer report disrupted sleep (Baggott et al., 2010; Walker, Gedaly-Duff, Miaskowski, & Nail, 2010). For children with cancer, the nature of their illness, its treatment, and treatment-related effects may compromise sleep quantity and quality both during treatment and following completion of therapy (Baggott et al., 2010; Clanton et al., 2011; Walker, Gedaly-Duff, Miaskowski, & Nail 2010). Children with cancer who require hospitalization for their chemotherapy treatments are particularly vulnerable to disrupted sleep as a result of frequent room entries by staff for medication administration and related supportive cares as well as elevated noise levels (Hinds, Hockenberry, Rai, Zhang, Razzouk, McCarthy et al., 2007; Linder & Christian, 2011). Identifying characteristics and patterns of change of children’s unique nighttime sleep can help guide the development of targeted interventions to improve the quality of sleep for children with cancer.
Sleep-Wake Patterns in School-Age Children
Sleep Physiology
Sleep is a complex, highly regulated and active process involving multiple interactions within the central nervous system. Sleep is divided into two distinct phases: rapid eye movement (REM) sleep and non-REM (NREM). Slow wave sleep (SWS), also regarded as “deep sleep” occurs during NREM sleep and has an important restorative function for physical well-being. REM sleep is associated with cognitive health and development (Mindell & Owens, 2010).
Organization of sleep-wake patterns is a component of normal growth and development. Total sleep needs are greatest during infancy and decrease across childhood. School-age children require 9 to 10 hours of sleep per 24 hours. By the time children are 5 years of age, the sleep cycle has increased to 90 minutes. School-age children typically awaken briefly approximately 4 to 6 times each night at the completion of each sleep cycle (Mindell & Owens, 2010).
Sleep efficiency, a measure of sleep quality, tends to be high for school-age children. Sleep efficiency is defined as the ratio of the actual sleep relative to the time in bed multiplied by 100 (Mindell & Owens, 2010). Ninety percent sleep efficiency is regarded as “acceptable” for children in laboratory conditions, and 95% sleep efficiency represents a good night’s sleep for adults (Berger et al., 2005; Kotagal, 2003).
Consequences of Disrupted Nighttime Sleep
Consequences of disrupted sleep are particularly concerning for children with cancer. During treatment, children must not only recover from the effects of cancer and its treatment-related consequences but also sustain normal physiologic growth. Frequent disruptions in children’s nighttime sleep result in insufficient nighttime sleep and limit the opportunity to achieve adequate SWS and REM sleep, thereby reducing the restorative function of sleep. Natural killer cell activity and cytokine activity are altered in the presence of sleep deprivation, resulting in impaired natural host defenses (Irwin, 2002; Irwin et al., 2006). Cytokine activity can remain impaired even after sleep recovery, suggesting a prolonged impact on immune function. Neuroendocrine processes are vulnerable to the effects of disrupted sleep, including inhibition of growth hormone secretion (Van Cauter & Spiegel, 1999). Decreased growth hormone secretion can impact glucose and insulin regulation and suppress cellular immunity. Behavioral and cognitive consequences of inadequate nighttime sleep include decreased attention, difficulty concentrating, and increased irritability, depression, and impulsivity (Mindell & Owens, 2010).
Nighttime Sleep Characteristics of Children with Cancer
Nighttime sleep quantity and quality vary greatly among children receiving treatment for cancer in both inpatient and ambulatory settings. In a sample of 29 school-age children and adolescents receiving inpatient chemotherapy, sleep duration was greater than 9 hours each night; however, sleep duration ranged from 4.25 to 14.45 hours (Hinds, Hockenberry, Rai, Zhang, Razzouk, Cremer et al., 2007). Thirty-five school-age children receiving cisplatin, doxorubicin, or ifosfamide experienced an average of 8.5 hours of sleep with a range of 6.1 to 12 hours (Hockenberry et al., 2010). Gedaly-Duff, Lee, Nail, Nicholson, and Johnson (2006) identified a mean of 8.03 hours and a range of 6 to 9.5 hours of sleep over a 3-night period among school-age children and adolescents receiving treatment for acute lymphoblastic leukemia (ALL). In a sample of 100 children with ALL, children slept significantly longer while receiving 5 days of dexamethasone compared with the 5 days prior to receipt of dexamethasone (Hinds, Hockenberry, Gattuso et al., 2007). Children’s sleep efficiency, however, was less than 90 percent, suggesting that longer sleep duration does not indicate better sleep quality.
Frequent awakenings contribute significantly to disrupted sleep. In the hospital setting, children’s sleep is marked by as many as 40 awakenings during the night. Frequent room entries by staff, clinical care needs, the presence of other symptoms, and elevated sound levels contribute to nighttime awakenings (Hinds, Hockenberry, Rai, Zhang, Razzouk, McCarthy et al., 2007; Linder & Christian, in press). Frequent awakenings also contribute to increased fatigue levels, which can impair children’s ability to engage in daily age-appropriate activities.
Sleep disturbances persist across the cancer treatment continuum. Children’s and adolescents’ reports of disrupted sleep did not change significantly following a cycle of myelosuppressive chemotherapy compared with reports of disrupted sleep prior to chemotherapy (Baggott et al., 2010). Parents reported sleep disturbances among 42% of school-age children receiving maintenance therapy for ALL. Parents also reported changes in sleep patterns in more than two thirds of these children since the time of diagnosis (Zupanec, Jones, & Stremler, 2010).
Although prior studies have addressed nighttime sleep characteristics among children with cancer, limited research has been conducted to explore changes in sleep characteristics during the course of a hospitalization and individual sources of variation in nighttime sleep characteristics. Understanding individual nighttime sleep characteristics and how these characteristics change during the course of a hospitalization can help guide individualized interventions. Such efforts can lead to improved nighttime sleep and potentially relieve other symptoms, such as fatigue, thereby contributing to improved quality of life.
Evaluation of Sleep and Sleep-Wake Disturbances
The term “sleep-wake disturbances” is not a formal diagnostic term; however, it is a general term describing sleep-related symptoms that may be experienced by an individual (Berger et al., 2005). The range of individual complaints related to sleep-wake disturbances is varied and may include difficulty falling asleep, difficulty staying asleep, or feeling that sleep is inadequate. The sleep parameters addressed in this paper as well as definitions and age-related norms are presented in Table 1.
Table 1.
Parameters Recommended for the Evaluation of Sleep-Wake Disturbances
| Sleep Parameter | Definition | Age-Related Norms for School-Age Children |
|---|---|---|
| Total sleep time | Number of minutes of sleep while in bed | 10 to 11 hours |
| Sleep latency | Number of minutes between going to bed and falling asleep | 19 minutes ± 1.6 minutes |
| Awakenings | Numeric count of awakenings | 4 to 6 awakenings |
| Wake after sleep onset (WASO) | Number of minutes spent awake after sleep onset during the sleep period | Less than 20 minutes for each awakening |
| Sleep efficiency | Number of minutes of sleep divided by number of minutes in bed multiplied by 100 | 90% = “acceptable” for children in laboratory conditions (95% = good night’s sleep for adults; < 80% = poor sleep quality for adults) |
Definitions adapted from: Berger et al. (2005). Sleep/wake disturbances in people with cancer and their caregivers: State of the science. Oncology Nursing Forum, 32, e98–e126.
Purpose
The purpose of this study was to describe group and individual characteristics of nighttime sleep-wake patterns among school-age children with cancer receiving inpatient chemotherapy. Specific aims guiding the study were to:
Describe nighttime sleep characteristics (total sleep minutes, sleep latency, number of awakenings, and sleep efficiency) of school-age children receiving inpatient chemotherapy.
Explore individual differences in nighttime sleep characteristics among school-age children with cancer receiving inpatient chemotherapy.
Methods
Design
The study used a descriptive, multiple-case study design (Yin, 2003). This within-subjects design emphasizes replication across cases and exploration of individual variation rather than identifying differences between groups. A case study design is well-suited to studies using a small sample size and is particularly useful when little is known about the phenomenon under investigation and the study is exploratory or descriptive in nature.
Study Setting and Participants
The study setting was a 24-bed pediatric oncology unit of a tertiary level, free-standing children’s hospital in the Intermountain West. Patient rooms were private and included a private bathroom. Institutional review board approval was granted for the study. Written parental permission (consent) for study participation was obtained for all participants. Written assent was obtained for participants 7 years of age and older.
Inclusion criteria were children diagnosed with cancer (primary or relapsed) between 5 to 12 years of age with an inpatient admission for chemotherapy that was anticipated to last at least 3 nights, and the ability to speak and understand English. Exclusion criteria were surgery during the current admission, central nervous system tumors, significant developmental delays, or previously diagnosed sleep disorders.
The study sample included 10 boys and 5 girls with a mean age of 8.8 years (SD = 2.3; range: 5.4 – 12.3 years). Ten children were being treated for hematologic malignancies (leukemia or lymphoma), and five were being treated for solid tumors. Twelve children were being treated for a primary diagnosis of cancer, and three were being treated for relapsed disease. Fourteen were White/non-Hispanic, and one was African American. No children were receiving corticosteroids, a potential source of disturbed sleep, while participating in the study.
Measures
MicroMini Motionlogger® actigraphs (Ambulatory Monitoring, Inc., 2006) measured sleep-wake states. Actigraphy is recognized as a reliable and valid approach for objectively identifying sleep patterns in infants through adults (Morgenthaler et al., 2007). An actigraph is a device about the size of a wristwatch (face diameter 1–3/8 inches) that contains a piezoelectric sensor that generates a voltage when the actigraph is moved. Sleep/wake states are identified by sampling the individual’s movements several times per second. Data are transformed digitally and stored in 1-minute epochs (Sadeh, 2011; Morgenthaler et al., 2007).
Actigraphs were initialized at the onset of data collection using Act Millennium (Version 3.10.13.1) software (Ambulatory Monitoring, Inc., 2006). Motion in each 1-minute epoch was scored using the Zero Crossing Mode, which counts number of times that the activity signal crosses zero or very near zero (Ambulatory Monitoring, Inc., 2006; Ancoli-Israel et al., 2003; Morgenthaler et al., 2007). At the conclusion of the data collection period, digitized actigraph data were uploaded for scoring and analysis. Action-W Version 2.6 software scored digitized actigraph data files using the Cole-Kripke algorithm (Ambulatory Monitoring Inc., 2006). One case of actigraph failure occurred. As a result, only 14 children had complete actigraph data for the three study nights. Sleep diaries identifying the child’s time to bed and morning waking time were maintained for comparison with actigraph-obtained data.
Data Collection Procedures
Data collection occurred during a scheduled admission for chemotherapy administration and did not interfere with chemotherapy delivery or other routine care. Participants wore the actigraph on their non-dominant wrist continuously during the 3-day and night data collection period. The actigraph was removed during personal activities such as showering to prevent the device from getting wet. Parents and participants completed the sleep diary each day to identify the time that the children went to bed each night and the time that they awakened in the morning.
Data Analysis
Data generated from actigraph files were saved into Excel files and transferred into SPSS version 16 (SPSS, 2006). Individual graphs of sleep variables were constructed for comparison with group means and to identify invididual patterns (Brown, McGuire, Beck, Peterson, & Mooney, 2007). Graphical analysis supports identification of trends within data when fewer data points are available to support more sophisticated statistical analyses.
Results
A description of children’s nighttime sleep characteristics is presented in Table 2. Pearson correlations between nighttime sleep characteristics are presented in Table 3.
Table 2.
Characteristics of Nighttime Sleep Based on Children’s Sleep Period Duration
| Night of Hospitalization | |||
|---|---|---|---|
| Variable | Night 1 (n = 13) | Night 2 (n = 14) | Night 3 (n = 13) |
| Sleep Period Duration (minutes) | |||
| Mean | 655.31 | 583.57 | 597.46 |
| SD | 126.94 | 95.33 | 92.76 |
| Median | 621.00 | 590.50 | 587.00 |
| Range | 526 – 853 | 368 – 733 | 406 – 742 |
| Total Sleep Time (minutes) | |||
| Mean | 572.15 | 504.64 | 506.38 |
| SD | 101.21 | 77.17 | 91.21 |
| Median | 553.00 | 506.50 | 510.00 |
| Range | 449 – 784 | 335 – 648 | 342 – 619 |
| Sleep Latency (minutes) | |||
| Mean | 12.69 | 15.79 | 9.23 |
| SD | 15.73 | 15.51 | 7.76 |
| Median | 7.00 | 10.50 | 7.00 |
| Range | 0 – 58 | 4 – 61 | 4 – 34 |
| Awakenings (number) | |||
| Mean | 13.38 | 11.00 | 12.69 |
| SD | 6.69 | 4.31 | 4.91 |
| Median | 13.00 | 10.50 | 12.00 |
| Range | 5 – 25 | 5 – 17 | 7 – 22 |
| Average Sleep Episode (minutes) | |||
| Mean | 54.03 | 57.12 | 48.95 |
| SD | 25.63 | 26.07 | 24.58 |
| Median | 43.31 | 51.81 | 37.42 |
| Range | 26.36 – 100.80 | 26.27 – 107.25 | 23.32 – 88.43 |
| Longest Sleep Episode (minutes) | |||
| Mean | 129.85 | 139.21 | 112.85 |
| SD | 25.30 | 49.88 | 34.55 |
| Median | 125.00 | 127.00 | 103.00 |
| Range | 88 – 161 | 73 – 260 | 65 – 176 |
| Wake after Sleep Onset (minutes) | |||
| Mean | 76.46 | 64.86 | 83.00 |
| SD | 42.76 | 55.37 | 55.26 |
| Median | 60.00 | 43.00 | 54.00 |
| Range | 17 – 164 | 22 – 201 | 41 – 223 |
| Sleep Efficiency (percentage) | |||
| Mean | 88.51 | 89.11 | 85.94 |
| SD | 4.53 | 8.10 | 8.25 |
| Median | 89.58 | 92.72 | 90.34 |
| Range | 80.68 – 96.74 | 72.01 – 95.98 | 69.58 – 93.79 |
Table 3.
Correlations Between Nighttime Sleep Characteristics
| Total sleep time | Sleep latency | Awakenings | Average sleep episode | Longest sleep episode | Wake after sleep onset | Sleep efficiency | |
|---|---|---|---|---|---|---|---|
| Sleep period duration | .88** | .06 | .60** | −.29 | .11 | .54** | −.31* |
| Total sleep time | .04 | .35* | .01 | .26 | .09 | .15 | |
| Sleep latency | <01 | −.12 | .11 | −.09 | .09 | ||
| Awakenings | −.86** | −.40* | 54** | −.57** | |||
| Average sleep episode | 42** | −.62** | .65** | ||||
| Longest sleep episode | −.23 | .34* | |||||
| Wake after sleep onset | . 96** |
p < .05,
p < .01 (two-tailed)
Bedtime and Time to Awaken
Children’s bedtimes during hospitalization were often delayed past their reported home bedtimes and past bedtimes typical for school-age children. Over two thirds of participants did not fall asleep until after 10:00 pm on each night. On seven nights involving five children, sleep onset occurred after midnight. Likewise, children’s time to awaken was delayed past their reported time to awaken at home. Only three participants reported typical wake times after 8:00 am; however, more than two thirds of children did not awaken until after 8:00 am on each day.
Total Sleep Time
Children’s time in bed exceeded 10 hours only on the first night (M = 10.92 hours). However, their total sleep time did not reach 10 hours on any of the three nights (M = 8.78 hours). A repeated measures analysis of variance did not identify significant differences in total sleep time (F = 2.4, p = .14) across the three nights; however, clinically significant differences were present. A clinically significant decrease in total sleep time for adults, based on an 8-hour period of nighttime sleep is 30 to 60 minutes, representing a decrease of 6.25% to 12.5% (Carskadon & Dement, 1981). Thus, a 6.25% to 12.5% decrease in a school-age child’s 10-hour sleep requirement is a loss of 37.5 to 75 minutes of sleep. Clinically significant decreases in total nighttime sleep minutes were present on the second and third nights relative to both the first night and to age-related norms.
Individual case reviews identified considerable variation in children’s total sleep time. Only one child received greater than the recommended 10 hours of sleep on two nights. Five children failed to achieve 10 hours of sleep or more on any of the three nights. Three patterns of total sleep time were present: 1) total sleep time consistently decreasing across each night (n = 6 children; Fig. 1.a.); 2) total sleep time consistent or increasing each night (n = 4 children; Fig. 1.b.); and 3) variable total sleep time each night (n = 4 children; Fig. 1.c.; actigraph failure occurred with one child). Each of the three children with relapsed disease experienced decreasing minutes of sleep each night. No other commonalities were observed based on age, gender, cancer diagnosis, or treatment protocol.
Fig. 1.
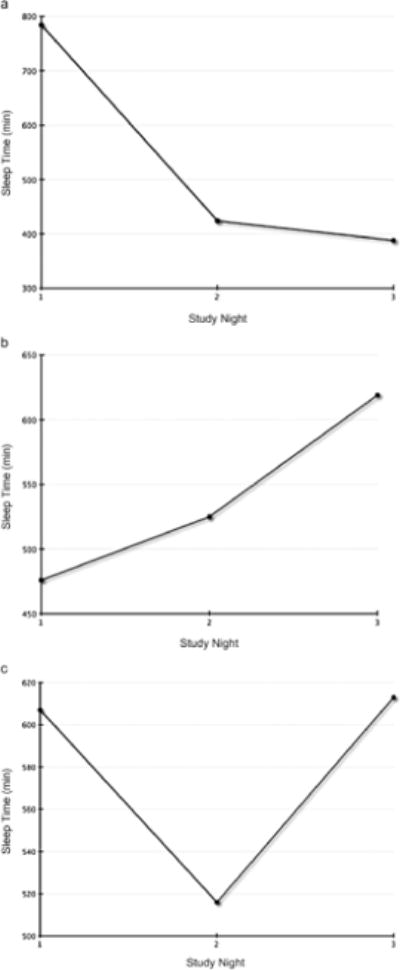
Individual patterns of total sleep time. (a) Decreasing total sleep time. (b) Consistent or increasing total sleep time. (c) Variable total sleep time.
Sleep Latency
Average sleep latency (i.e., time to fall asleep), was less than 16 minutes each night, suggesting that most children did not experience difficulty falling asleep. On seven occasions involving six children, none of whom had relapsed disease, sleep latency was prolonged (median = 33 minutes; range 21 – 61) relative to age-related norms. One child had prolonged sleep latency on the second and third nights. Two children experienced prolonged sleep latency on the first night. The remaining three children experienced prolonged sleep latency on the second night.
Awakenings
Nighttime awakenings exceeded the 4 to 6 brief arousals that are typical for school-age children (Mindell & Owens, 2010). The number of nighttime awakenings was positively associated with the child’s total time in bed (r = .60, p < .01) and the total minutes of nighttime sleep (r = .35, p < .05), suggesting that more time spent in bed did not necessarily indicate better sleep quality. The average length of each awakening was 6.93 minutes (SD = 2.72), suggesting that children were able to fall back to sleep quickly following each awakening.
A repeated measures analysis of variance did not identify significant differences in nighttime awakenings across the three nights (F = 1.90, p = .17). Four patterns of nighttime awakenings were identified: 1) a consistent low number of awakenings (fewer than 10 per night) with low variability (n = 3; Fig. 2.a.); 2) a consistent high number of awakenings (more than 15 per night; n = 4; Fig. 2.b.); 3) a moderate number of awakenings with an increasing trend (n = 4; Fig. 2.c.); and 4) a moderate number of awakenings with a decreasing trend (n = 3; Fig. 2.d.). No commonalities were observed with regard to age, gender, primary versus relapsed disease, diagnosis, or treatment protocol.
Fig. 2.
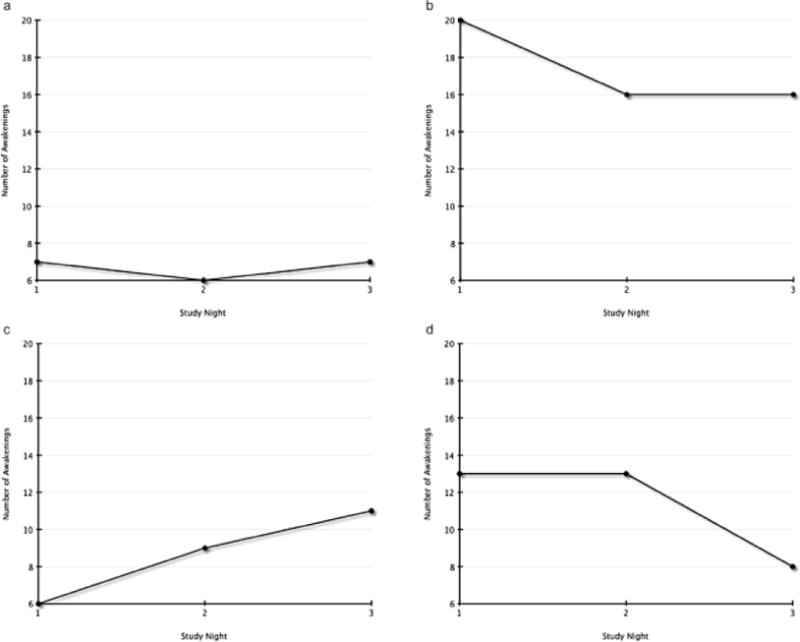
Individual patterns of nighttime awakenings. (a) Low awakenings and low variability. (b) High awakenings. (c) Moderate and increasing awakenings. (d) Moderate and decreasing awakenings.
Frequent nighttime awakenings resulted in fragmented nighttime sleep with average sleep episodes lasting less than 60 minutes. The length of the average sleep episode was strongly negatively associated with both the number of awakenings (r = −.86; p < .01) and the minutes spent awake after initially falling asleep (r = −.62; p < .01).
Nine children experienced at least one sleep episode lasting 90 minutes or longer, (i.e., one full sleep cycle), each of the three nights. On eight occasions, however, children failed to experience at least one 90-minute period of uninterrupted nighttime sleep. Two participants failed to achieve at least one sleep episode of 90 minutes or longer on the second and third nights. Four other children failed to achieve at least one sleep episode of 90 minutes of longer on one of the three nights. All children with relapsed disease had at least one 90-minute or longer sleep episode.
Wake After Sleep Onset (WASO)
The duration of time that children were awake after initially falling asleep (i.e., wake after sleep onset; WASO) was positively associated with the number of awakenings (r = .64; p < .01). This finding indicates that the more awakenings children experienced, the longer they were awake during the night. WASO also was positively associated with the duration of time spent in bed (r = .54; p < .01), indicating that longer time in bed did not indicate better sleep quality.
Sleep Efficiency
Mean sleep efficiency was less than 90% for each of the three nights, suggesting that, overall, nighttime sleep quality was impaired during each night of hospitalization. On five occasions involving three children, sleep efficiency was less than 80% (range 69.58% – 79.25%), indicating very poor sleep quality. Two children, one of whom had relapsed disease, had less than 75% sleep efficiency on the second and third nights. The third child experienced poor sleep efficiency on the third night; however, this child’s sleep efficiency had been declining each night. In contrast, sleep efficiency exceeded 95% on four occasions. One child with relapsed disease experienced greater than 95% sleep efficiency on the first and second nights. Two additional children experienced greater than 95% sleep efficiency on the second night.
Discussion
Study findings contribute to the growing knowledge of the scope of sleep-wake disturbances experienced by hospitalized children with cancer. As reflected in previous research addressing symptoms in children and adolescents with cancer, children’s developmental stages influence their perception and appraisal of symptoms (Collins et al., 2000, 2002; Hinds et al., 1999; Linder, 2008). Maintaining a developmental perspective contributes to a greater understanding of the unique symptom experience of school-age children with cancer.
Similar to findings in prior studies of hospitalized children with cancer and critically ill children, both sleep quantity and quality in this sample of hospitalized school-age children with cancer were impaired (Corser, 1996; Cureton-Lane & Fontaine, 1997; Hinds, Hockenberry, Rai, Zhang, Razzouk, Cremer et al., 2007; Hockenberry et al., 2010). Children’s sleep was fragmented with frequent awakenings and average sleep episodes less than 90 minutes. Such fragmentation limits children’s opportunities to experience complete sleep cycles with adequate SWS and REM sleep (Mindell & Owens, 2010). As such, children with cancer may not experience the restorative benefits of sleep on their health and well-being, and the risk of adverse physiologic and psychologic consequences of disrupted nighttime sleep would be increased.
Consistent with prior studies, this study identifed a strong significant positive association between the number of awakenings and sleep duration, indicating that longer sleep duration does not necessarily indicate better sleep quality (Hinds, Hockenberry, Rai, Zhang, Razzouk, McCarthy et al., 2007). Sleep duration and the number of awakenings both were negatively associated with sleep efficiency. These findings further emphasize the adverse consequences of frequent nighttime disruptions on children’s sleep quality. Sources of disruptions include the child’s care environment as well as the child’s number of medications and illness-related symptoms such as nausea and pain (Linder & Christian, 2011; in press).
Although children’s total sleep time did not differ significantly across each of the three nights, the mean total sleep time on the second and third nights represented a clinically significant decrease from the first night. These quantitative sleep losses may prevent children from experiencing the restorative physiologic and psychologic benefits of nighttime sleep.
In general, sleep onset was delayed past children’s reported usual bedtimes during hospitalization. For the majority of children, nighttime sleep was not well established until after 10:00 pm, although a few children fell asleep prior to 9:00 pm. Sources of delays in bedtime included ongoing medication administration and related supportive nursing care related to the child’s cancer treatment protocol. Other children anecdotally reported that they decided to remain awake later and engage in activities because of the novelty of the hospital environment or because of a holiday such as New Year’s Eve. Children’s waking times in the hospital also were typically delayed past usual waking times at home. These findings indicated an added burden of the child’s illness and hospitalization on children’s and families’ typical nighttime as well as daytime activities. Previous studies involving hospitalized children have not identified time of sleep onset or waking times (Corser, 1996, Cureton-Lane & Fontaine, 1997; Hinds, Hockenberry, Rai, Zhang, Razzouk, McCarthy et al., 2007; Hockenberry et al., 2010), limiting the ability to compare children’s bedtimes and factors influencing bedtimes across studies.
Although most children were able to fall asleep without difficulty after going to bed, six children (60%) experienced prolonged sleep latency on at least one of the three nights. These findings suggest that some children may benefit from additional, individualized interventions to promote nighttime sleep in the hospital setting.
With the individual child as the focus, the case study design facilitated examination of individual differences that may not be appreciated with only measures of central tendency and traditional statistical analyses to characterize the study sample (Yin, 2003). Graphical analysis of children’s sleep characteristics during the three nights enhanced the identification of individual variability in sleep-wake patterns during children’s hospitalizations. While some children experienced declines in sleep quantity and quality over the course of the three nights, others experienced increases in total sleep time and less fragmented sleep each night. Although children with relapsed disease experienced fewer minutes of sleep each night, other sleep parameters, such as sleep efficiency, were more varied among this subgroup. These findings suggest that multiple factors, including the child’s own intraindividual characteristics may influence nighttime sleep characteristics. The patterns of sleep characteristics identified in this study may represent distinct subgroups of children who may benefit from individualized, tailored interventions to promote sleep quantity and quality.
Implications for Future Research
Validation of the subgroups of sleep-wake patterns (i.e., decreasing total sleep time, increasing total sleep time, and variable sleep time) and their persistence across the treatment continuum using a larger study sample is warranted. Although studies involving adults with cancer have identified subgroups based on symptom intensity (Miaskowski et al., 2006), similar studies have not been undertaken with children with cancer. Investigation of the role of biologic influences, such as polymorphisms in inflammatory cytokines, which are associated with fatigue and sleep disturbances in adults with cancer and other chronic illnesses (Aouizerat et al., 2009; Vallance et al., 2011) may lead to a greater understanding of intra-individual characteristics contributing to healthier and poorer sleep outcomes.
Studies addressing sleep-wake patterns in other hospitalized children as well as subgroups of children with cancer, such as those admitted with fever and neutropenia or undergoing hemato-poietic stem cell transplantation, are needed. These studies will provide opportunities to compare and contrast sleep-wake pattern characteristics between hospitalized children with cancer and other acutely ill children. Study findings could be used to direct institution-based interventions and interventions targeted more specifically at individual children or diagnostic groups.
Longitudinal studies are needed to investigate changes in both nighttime and daytime sleep-wake patterns as well as other cancer-related symptoms which may contribute to altered sleep-wake patterns across the child’s cancer treatment continuum. Such studies would support a greater understanding of trajectories of symptoms and symptom burden and allow evaluation of recovery of sleep-wake patterns following discharge from the hospital. Longitudinal studies could also investigate physiological and functional outcomes associated with altered sleep-wake patterns. Mixed methods approaches could be used in these studies to provide complementary approaches for improving understanding and enhancing knowledge development of sleep-wake patterns in children with cancer (Polit & Beck, 2012; Tashakkori & Teddlie, 2003).
Intervention studies are needed to address sources of sleep disturbances for children with cancer in the hospital setting. Interventions targeted at reducing sources of nighttime disruptions contributing to frequent awakenings are warranted. Potential unit-based interventions could include efforts to reduce nighttime noise levels, structuring medication schedules to minimize the number of doses given during typical bedtime hours, and redesigning unit-based practices, such as frequency of vital signs and evaluating urinary output for children receiving chemotherapy. Potential inividual-based interventions could focus on the development of a sleep hygiene protocol that could be individualized to the child’s personal preferences and home routines.
Research with other groups of hospitalized children and children with chronic illness states is warranted to compare sleep outcomes. Such research could provide insight into whether findings are a function of the underlying diagnosis and treatment or of the hospital environment. Findings then could be used to develop interventions to improve sleep for children with cancer.
Limitations
Limitations of this study include a small sample size and cross-sectional design evaluating nighttime sleep during a single hospitalization. Participants were hospitalized for an admission to receive scheduled chemotherapy and had met protocol-based criteria for admission. Findings can not be generalized to all groups of hospitalized children with cancer, including those with compli-cations such as fever and neutropenia or those undergoing hematopoietic stem cell transplant.
How Might This Information Affect Nursing Practice?
This study contributes to the developing effort to understand nighttime sleep character-istics among hospitalized children with cancer. Hospitalized children with cancer are vulnerable to fragmented nighttime sleep, compromising restorative nighttime sleep quantity and quality. Children’s sleep patterns, however, indicate variability among sleep parameters, including total nighttime sleep, number of nighttime awakenings, and time to fall asleep. Study findings support the development and testing of both system-based and individualized nurse-initiated interventions to improve nighttime sleep for children with cancer. Such efforts can promote children’s health and quality of life both during treatment for cancer and into survivorship (Clanton et al., 2011).
Strengths of this study included the use of direct, noninvasive measures for data collection. The multiple-case study design supported the use of graphical analyses to identify individual variation among study participants that can be missed when only group characteristics are summarized (Brown et al., 2007). The developmental focus of this study contributed to a greater understanding of disrupted sleep-wake patterns in school-age children with cancer.
Acknowledgments
This research was funded by the following sources:
Individual National Research Service Award; National Institute for Nursing Research, (F31NR010175-01)
Doctoral Scholarship in Cancer Nursing; American Cancer Society (DSCN-06-204-1)
Dissertation Grant Scholarship; Western Institute of Nursing and the Council for the Advancement of Nursing Science.
Footnotes
Disclosure: The authors report no actual or potential conflicts of interests.
Contributor Information
Lauri A. Linder, Assistant Professor, University of Utah, College of Nursing, and Clinical Nurse Specialist, Primary Children’s Medical Center, Hematology/Oncology/BMT Service Line, Salt Lake City, UT.
Becky J. Christian, Professor, University of Alabama at Birmingham, Birmingham, AL, USA.
References
- Ambulatory Monitoring Inc. Products: software. 2006 http://www.ambulatorymonitoring.com/software.html. Accessed February 1, 2006.
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Aouizerat BE, Dodd M, Lee K, West C, Paul SM, Cooper BA, Miaskowski C. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biological Research for Nursing. 2009;11:27–41. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- Baggott C, Dodd M, Kennedy C, Marina N, Matthay KK, Cooper BA, Miaskowski C. Changes in children’s reports of symptom occurrence and severity during a course of myelosuppressive chemotherapy. Journal of Pediatric Oncology Nursing. 2010;27:307–315. doi: 10.1177/1043454210377619. [DOI] [PubMed] [Google Scholar]
- Berger AM, Parker KP, Young-McCaughan S, Mallory GA, Barsevick AM, Beck SL, Hall M. Sleep/wake disturbances in people with cancer and their caregivers: State of the science. Oncology Nursing Forum. 2005;32:e98–e126. doi: 10.1188/04.ONF.E98-E126. [DOI] [PubMed] [Google Scholar]
- Brown CG, McGuire DB, Beck SL, Peterson DE, Mooney KH. Visual graphical analysis: A technique to investigate symptom trajectories over time. Nursing Research. 2007;58:195–201. doi: 10.1097/01.NNR.0000270029.82736.5a. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107–113. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- Clanton NR, Klosky JL, Li C, Jain N, Srivastava DK, Mulrooney D, Krull KR. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2011 doi: 10.1002/cncr.25797. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JJ, Byrnes ME, Dunkel IJ, Lapin J, Nadel T, Thaler HT, Portenoy RK. The measurement of symptoms in children with cancer. Journal of Pain and Symptom Management. 2000;19:363–377. doi: 10.1016/s0885-3924(00)00127-5. S0885-3924(00)00127-5 [pii] [DOI] [PubMed] [Google Scholar]
- Collins JJ, Devine TD, Dick GS, Johnson EA, Kilham HA, Pinkerton CR, Portenoy RK. The measurement of symptoms in young children with cancer: The validation of the Memorial Symptom Assessment Scale in children aged 7–12. Journal of Pain and Symptom Management. 2002;23:10–16. doi: 10.1016/s0885-3924(01)00375-x. S088539240100375X [pii] [DOI] [PubMed] [Google Scholar]
- Corser NC. Sleep of 1- and 2-year-old children in intensive care. Issues in Comprehensive Pediatric Nursing. 1996;19:17–31. doi: 10.3109/01460869609026852. [DOI] [PubMed] [Google Scholar]
- Cureton-Lane RA, Fontaine DK. Sleep in the pediatric ICU: An empirical investigation. American Journal of Critical Care. 1997;6:56–63. [PubMed] [Google Scholar]
- Gedaly-Duff V, Lee KA, Nail LM, Nicholson HS, Johnson KP. Pain, sleep disturbance, and fatigue in children with leukemia and their parents: A pilot study. Oncology Nursing Forum. 2006;33:641–646. doi: 10.1188/06.ONF.641-646. [DOI] [PubMed] [Google Scholar]
- Hinds PS, Hockenberry-Eaton M, Gilger E, Kline N, Burelson C, Bottomley S, Quargnenti A. Comparing patient, parent, and staff descriptions of fatigue in pediatric oncology patients. Cancer Nursing. 1999;22:277–287. doi: 10.1097/00002820-199908000-00004. [DOI] [PubMed] [Google Scholar]
- Hinds PS, Gattuso JS, Fletcher A, Baker E, Coleman B, Jackson T, Pui CH. Quality of life as conveyed by pediatric patients with cancer. Quality of Life Research. 2004;13:761–772. doi: 10.1023/B:QURE.0000021697.43165.87. [DOI] [PubMed] [Google Scholar]
- Hinds PS, Hockenberry MJ, Gattuso JS, Srivastava DK, Tong X, Jones H, Pui CH. Dexamethasone alters sleep and fatigue in pediatric patients with acute lymphoblastic leukemia. Cancer. 2007;110:2321–2330. doi: 10.1002/cncr.23039. [DOI] [PubMed] [Google Scholar]
- Hinds PS, Hockenberry MJ, Rai SN, Zhang L, Razzouk BI, McCarthy K, Rodriguez-Galindo C. Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncology Nursing Forum. 2007;34:393–402. doi: 10.1188/07.ONF.393-402. [DOI] [PubMed] [Google Scholar]
- Hinds PS, Hockenberry MJ, Rai SN, Zhang L, Razzouk BI, Cremer L, Rodriguez-Galindo C. Clinical field testing of an enhanced-activity intervention in hospitalized children with cancer. Journal of Pain and Symptom Management. 2007;33:686–697. doi: 10.1016/j.jpainsymman.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Hockenberry MJ, Hooke MC, Gregurich M, McCarthy K, Sambuco G, Krull K. Symptom clusters in children and adolescents receiving cisplatin, doxorubicin, or ifosfamide. Oncology Nursing Forum. 2010;37:E16–E27. doi: 10.1188/10.ONF.E16-E27. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. Retrieved from http://seer.cancer.gov/csr/1975_2008/, based on November 2010, SEER data submission, posted to the SEER web site. [Google Scholar]
- Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain, Behavior, and Immunity. 2002;16:503–512. doi: 10.1016/s0889-1591(02)00003-x. [DOI] [PubMed] [Google Scholar]
- Irwin M, Wang M, Campomayor CO, Collado-Hildago A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Archives of Internal Medicine. 2006;166(16):1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- Kotagal S. Sleep disorders in childhood. Neurologic Clinics of North America. 2003;21:961–981. doi: 10.1016/s0733-8619(03)00034-3. [DOI] [PubMed] [Google Scholar]
- Linder LA. Developmental diversity in symptom management for children with cancer. Journal of Pediatric Nursing. 2008;23:296–309. doi: 10.1016/j.pedn.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Linder LA, Christian BJ. Characteristics of the nighttime hospital bedside care environment (sound, light, and temperature) for children with cancer. Cancer Nursing. 2011;34:176–184. doi: 10.1097/NCC.0b013e3181fc52d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder LA, Christian BJ. Nighttime sleep disruptions, the hospital care environment, and symptoms in school-age children with cancer. Oncology Nursing Forum. doi: 10.1188/12.ONF.553-561. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Cooper BA, Paul SM, Dodd M, Lee K, Aouizerat BE, Bank A. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: A cluster analysis. Oncology Nursing Forum. 2006;33:E79–E89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- Mindell JA, Owens JA. A clinical guide to pediatric sleep: Diagnosis and management of sleep problems. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Swick TJ. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- Polit DF, Beck CT. Nursing research: Generating and assessing evidence for nursing practice. 9. Philadelphia, PA: Wolters Kluwer Health Lippincott Williams & Wilkins; 2012. [Google Scholar]
- Sadeh A. The role and validity of actigraphy in sleep medicine: An update. Sleep Medicine Reviews. 2011;15:259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- SPSS. SPSS, Data mining, statistical analysis software, predictive analysis, predictive analytics, decision support systems. 2006 Available at http://www.spss.com. Accessed November 5, 2006.
- Tashakkori A, Teddlie C, editors. Handbook of mixed methods in social & behavioral research. Thousand Oaks, CA: Sage; 2003. [Google Scholar]
- Vallance K, Yang J, Li J, Crabtree VM, Hinds PS, Mandrell BN. Disturbed sleep in pediatric patients with leukemia: The potential role of interleukin-6 (−174GC) and tumor necrosis factor (−308GA) polymorphism. Oncology Nursing Forum. 2011;38:E365–E372. doi: 10.1188/11.ONF.E365-E372. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Spiegel K. Circadian and sleep control of hormonal secretions. In: Turek FW, Zee PC, editors. Regulation of sleep and circadian rhythms. New York: Marcel Dekker, Inc; 1999. pp. 397–425. [Google Scholar]
- Walker AJ, Gedaly-Duff V, Miaskowski C, Nail L. Differences in symptom occurrence, frequency, intensity, and distress in adolescents prior to and one week after the administration of chemotherapy. Journal of Pediatric Oncology Nursing. 2010;27:259–265. doi: 10.1177/1043454210365150. [DOI] [PubMed] [Google Scholar]
- Yin RK. Case study research: Design and methods. 3. Thousand Oaks, CA: Sage Publications, Inc; 2003. (Applied Social Research Methods Series (5)). [Google Scholar]
- Zupanec S, Jones J, Stremler R. Sleep habits and fatigue of children receiving maintenance therapy for ALL and their parents. Journal of Pediatric Oncology Nursing. 2010;27:217–228. doi: 10.1177/1043454209358890. [DOI] [PubMed] [Google Scholar]


