Abstract
Sleep is a vital segment of life, however, the mechanisms of diet promoting sleep are unclear and are the focus of research. Insomnia is a general sleep disorder and functional foods are known to play a key role in the prevention of insomnia. A number of studies have demonstrated that major insomnia risk factors in human being are less functional foods in dietary. There are higher functional components in functional foods promoting sleep, including tryptophan, GABA, calcium, potassium, melatonin, pyridoxine, L-ornithine and hexadecanoic acid; but wake-promoting neurochemical factors include serotonin, noradrenalin, acetylcholine, histamine, orexin and so on. The factors promoting sleep in human being are the functional foods include barley grass powder, whole grains, maca, panax, Lingzhi, asparagus powder, lettuce, cherry, kiwifruits, walnut, schisandra wine, and milk; Barley grass powder with higher GABA and calcium, as well as potassium is the most ideal functional food promoting sleep, however, the sleep duration for modern humans is associated with food structure of ancient humans. In this review, we put forward possible mechanisms of functional components in foods promoting sleep. Although there is clear relevance between sleep and diet, their molecular mechanisms need to be studied further.
Keywords: Bioactive component, dietary, functional food, human being, insomnia, molecular mechanisms, sleep promoting.
INTRODUCTION
Sleep affects brain function, which is not only a common phenomenon in vertebrates, but is also as a result of environmental, psychophysiological and pharmacological factors and remains one of the mysterious sciences. Sleep disorders (insomnia and so on) constitute a global epidemic that affects 45% of the world's population [1], but less than 1% have been treated by functional foods for insomnia prevention; sleep disorders cost the US $150 billion each year. Insomnia qualifies to be the world's most common sleep disorder affecting 2.2 billion in 2014, which accounts for 30% of the population of the world. The pathogenetic mechanism of insomnia or agrypnia constitutes visceral thalamus degeneration (familial insomnia), autoantibodies blocking the voltage-gated potassium channels and GABAergic synapses down-regulated within the limbic system [2].
Dietary supplementation of tryptophan can stimulate serotonergic activity and promote sleep, whereas serotonin released into diencephalon and cerebrum might play a key inhibitory role in insomnia prevention [3]. Prolonged-release melatonin was approved in Europe for insomnia prevention, which suggest a beneficial role of sleep-wake cycle stabilization in the treatment of insomnia [4]. Benzodiazepines for rapidly modulate GABA signaling in central neurons are widely used in the treatment of a variety of neurological and psychiatric conditions including anxiety, insomnia and epilepsy [5]. FDA-approved medications for treatment of chronic insomnia include γ-aminobutyric acid (GABA), melatonin, benzodiazepine, and histamine 4 receptor agonist [6]. T-type calcium channels have been proposed as therapeutic targets for lots of diseases such as insomnia, epilepsy, pain, cancer and hypertension [7]. The immunoglobulin G targeting dipeptidyl-peptidase-like protein-6 is not only a regulatory subunit of neuronal Kv4.2 potassium channels, but also a biomarker for an immunotherapy-responsive multifocal neurologic disorder (insomnia and so on) of the central and autonomic nervous systems [8]. There are important role for Kv2.2-expressing neurons in the regulation of the sleep-wake cycle [9]. The polyunsaturated fatty acids were associated with sleep efficiency and rapid eye movement sleep (REMS) [10]. L-ornithine has the potential to improve sleep quality for fatigue [11]. The advantage of orexin antagonists is the promotion and maintenance of physiological sleep which should avoid hangover phenomena of approved treatments [12]. The robust REMS-inducing effect of melanin is likely related to the deactivation of monoaminergic, orexinergic, glutamatergic, cholinergic and GABAergic neurons [13]. Diet and nutrient intake contribute to insomnia [14]. The notion that food directly affects sleep is difficult to address, as which diet can boost sleep remains unclear. We aimed to seek constituents of functional foods promoting sleep by complex interaction between diet and sleep, which may help generate molecular mechanisms for future studies in human beings.
MAJOR INSOMNIA RISK FACTORS
Heredity is a Major Factor that Cause Insomnia
Insomnia may be influenced by an epigenetic control process between sleep mechanisms and gene-environment interactions having an impact on brain plasticity [15]. Insomnia is 43% to 55% heritable in humans [16]. High heritability of insomnia persists except for psychiatric disorders [17]. The dysregulation of ROR1, PLCB1, or PLCB4 by PAX6 and CTCF is one neural mechanism associated with pancreatic dysfunction in insomnia [18]. Sleep is controlled by PGC-1α genes, but Apoε4 plays an important role in insomnia [19]. Melatonin receptor genes may be associated with insomnia in schizophrenia patients [20]. The intronic SNPs in the PRIMA1 and near GBP4 gene have been identified as influencing caffeine-induced insomnia [21]. PER3 genotype contributed variance in insomnia severity for alcohol-dependent patients [22]. Opioid receptor mu1 gene is involved in the physiology of heroin and alcohol addiction [23]. Fatal familial insomnia is a hereditary autosomal-dominant mutation of the prion protein gene by motor disturbances in which the thalamus is a crucial switch [24]. Chronic sleep deprivation causes the expression of more than 700 genes, which are related to inflammation and immune and stress response [25]. Sleep duration is genetically regulated by rs2031573 and rs1037079 that may contribute to the regulation of sleep duration via gene expression [26]. The molecular mechanisms for the circadian clock regulate sleep such that a novel signaling molecule is mediated via distinct neuronal pathways [27]. Humans homozygous for the PER3(5/5) allele are more sensitive to non-image-forming light effects, as indexed by specific changes in sleep EEG activity [28].
The Loss of Components from whole Grain to Polished Grain Foods are Key Factors that Cause Insomnia
GABA is an inhibitory neurotransmitter and promotes parasympathetic activity for improved sleep, which is a functional food ingredient of safety use [29]. Some nutrients (calcium, potassium and magnesium) are associated with improved sleep [30]. Cav2.3 E-/R-type voltage-gated calcium channels modulate sleep [31]. The ion channel subunits contribute to the somatodendritic delayed rectifier, A-type and calcium-activated SK potassium currents, IH, L- and T-type calcium currents in the control of sleep, learning and nervous system disorders [32]. Ketamine and magnesium association reduces the post-operative morphine consumption after scoliosis surgery, which seems to provide better sleep quality and improves patient satisfaction [33]. Rice and wheat are staple foods of more than 70% of the worlds’ populations. The loss of GABA from brown rice to white rice in the world in 2013 was estimated to be 70,323 tons; The loss of potassium and magnesium as well as calcium from brown rice to white rice in the world in 2013 were 929,765 tons and 485,875 tons as well as 31,599 tons, respectively (unpublished data). The loss of potassium and magnesium as well as calcium from whole grains to white flour of wheat in the world in 2013 were 4,782,778 tons and 1,414626 tons as well as 162,944 tons, respectively (unpublished data). The loss of GABA and other nutrition-related sleep from whole grains to polished grain foods are key factors that cause insomnia.
Some Foods and Beverages are Key Factors that Cause Insomnia
Foods rich in sugar, caffeine, green tea, etc can contribute to insomnia. The poor sleep quality and short sleep duration are more closely related with prediabetes than long sleep duration [34]. Sleep duration is inversely associated with higher intake of sugar [35]. Protein and carbohydrate intake in diet is associated with insomnia [36]. Short sleep duration is associated with increased energy consumption [37]. The sleep duration is inversely associated with serum leptin and dietary energy intake, while, short sleep contributes to hyperphagia, insulin resistance and obesity [38]. Excessive caffeine consumption (i.e >500 mg per day) can cause negative health consequences such as insomnia, psychomotor agitation, headache and gastrointestinal complaints [39]. Habitual coffee consumption is associated with reduced mortality and risks for arrhythmias, but caffeine at high doses can increase insomnia, anxiety and calcium loss [40]. In addition, smoking is associated with increased insomnia [41].
Disease: A Major Factor Causing Insomnia
Insomnia not only remains a significant risk factor for depression, anxiety, fibromyalgia, rheumatoid arthritis, whiplash, arthrosis, osteoporosis, headache, asthma and myocardial infarction, but is also significantly associated with the incidence of angina, hypertension, obesity and stroke [42]. Some sleep disorders and related problems are reported as risks leading to dementia [43]. It was observed that moderate traumatic brain injury patient (70.73%) had significantly higher occurrence of insomnia than the mild cases (19.67%) [44]. The similarity and difference in global gene expressions among patients with sporadic Creutzfeldt-Jakob disease, fatal familial insomnia and Alzheimer's disease, may help understand the common mechanism of neurodegenerative diseases [45]. Melatonin has a protective effect against lung inflammation associated with insomnia [46]. Sleep disorders are also known to be associated with diabetes and obesity [47]. Sleep apnea and obesity are strongly related, which highlights that sleep apnea is independently associated with early atherosclerotic plaque burden for coronary artery calcium in nonobese patients [48]. Epidemiological studies have supported an association between decreased self-reported sleep duration and an increased incidence of type 2 diabetes, obesity, and cardio-vascular disease, which may help in the development of new preventive and therapeutic approaches against obesity and type 2 diabetes based on increasing the quality and/or quantity of sleep [49].
Psychology: The Most Important Risk Factor Causing Insomnia
Sleep is a very complex physiological process. Insomnia has been suggested to cause depression and other mental disorders [50] such as, a correlation between global cognitive function and sleep disturbances in Parkinson's disease patients has been observed [51]. Chronic insomnia is best managed using cognitive behavior therapy [52]. Insomnia symptoms are positively associated with suicidal ideation, which is accounted for by depressive symptoms as evident in Japanese white-collar workers [53]. Melanin concentrating hormone stimulation can counteract the arousal neurons in the treatment of insomnia [54]. The alterations in the diurnal activity increase the risk of mood disorders among insomniacs [55]. Short or long sleep duration is an important sleep-related factor associated with memory impairment [56].
NATURAL FUNCTIONAL FOODS PROMOTING SLEEP IN HUMAN BEINGS
Sleep has an influence on eating behaviors, with insufficient sleep causing changes in the brain activity that may increase caloric consumption [57]. The consumption of high-protein and carbohydrate intake may have a significant influence on sleep [58]. Diet is a modifiable factor for improving sleep, but the associations of macronutrient intakes with insomnia are inconsistent.
Major Strategies of Functional Foods with High Components Promoting Sleep
Functional Components Promote Sleep
Zolpidem is a widely used hypnotic drug and a positive allosteric modulator of GABA with α 1-subunit containing GABAA receptors (α1-GABAARs) [59], however, α1-GABAARs play a significant role in hypnosis, electro-encephalogram sleep and anticonvulsant effects [60], but currently approved treatments for insomnia primarily target GABA-A receptor signaling [61]. A deregulation of Ca2+ signaling based on the neurons and inflammatory responses in the microglia and astrocytes may influence cognition by interfering with the rhythm rheostat that controls the sleep/wake cycle [62]. Sleep disorders are cardinal manifestations of voltage-gated potassium channel complex autoimmunity in association with a spectrum of neurologic presentations [63]. The hypoglossal motor activity in REMS is a muscarinic receptor mechanism linked to G-protein-coupled rectifying potassium channels [64]. A moderate magnesium deficiency may enhance inflammatory, including disrupted sleep/sleep deprivation [65]. Zinc and copper mediate sleep in the central nervous system, however, Zn/Cu ratio in the serum and hair is associated with sleep duration [66]. Reduced selenium or calcium intake has also been highlighted to be associated with insomnia [30]. Tryptophan and serotonin as well as melatonin in foods may be the most useful in promoting sleep [67]. The sleep-wake cycle is not only associated with many neurotransmitters, which include GABA, serotonin, orexin, melanin, cholinergic, galanin, noradrenaline, and histamine, but is also related with excessive nutrition, which includes carbohydrate, tryptophan, valerian, melatonin and so on [68].
Maca Promotes Sleep
Yunnan is the biggest production base for maca in China [69]. Maca grown in altitudes 2800-4500 m in Yunnan province of China not only enhances fertility chances, but is also reduces the glucose levels and prostate size, lowers blood pressure, improves memory, anxiety and depression, provides relief in osteoporosis as well as show, antioxidant, antiaging, antifatigue, antiviral activity, etc [70-72]. Moreover, a number of functional components are associated with improved sleep, e.g. hexadecanoic acid (22.73%) in fatty acids, calcium (974.63 mg/100g) and potassium (903.65 mg/100g) [73,74]. The main components of 90 compounds of essential oils for maca are [(1,1-dimethylethoxy) methyl-benzene, N-(phenylmethyl)]- acetamide, phthalic acid hexyl octyl ester, 1-isocyano-2-methyl-benzene,benzyl nitrile and methoxy-acetaldehyde [75].
Panax Promotes Sleep
Panax notoginseng flower and leaf showed improvement in sleep function [76]. Effective parts in Oplopanax elatus have a significant role of improving sleep. The best effective dose for 64 g /kg is during 7 days, which is related to central neurotransmitter and nitric oxide adjustment mechanism [77]. Panax ginseng demonstrated anxiolytic effects in a human trial [78]. The red ginseng extract intake can improve the quality of sleep [79]. There is significant alleviation in insomnia, flushing, perspiration and appetite by Korean red ginseng consumption [80]. The improvement of fermented ginseng acts via GABAergic modification [81].
Lingzhi Promotes Sleep
Ganoderma lucidum has been used as a tranquilizing agent for the treatment of insomnia in China, which is associated with the modulation of cytokines such as TNF-α [82]; Its basidiocarp, mycelia and spores contain 400 different bioactive compounds, some of the ingredients can promoting sleep [83]. Cracked Lingzhi Spores Powder could improve the sleep function of mice [84].
Other Foods Promote Sleep
Instant asparagus powder could improve the sleeping quality of mice [85]. Enzyme-treated asparagus extract intake is effective to modulate the sleep state among those with low sleep efficiency or excess sleep time [86]. The Gastrodia elata or its ultrafine powder at 40 mg/kg.BW dosage could improve the sleeping quality of mice [87, 88].
Sleep Duration Associated with Barley Grass
Barley grass powder promotes sleep due to the presence of GABA, and calcium, magnesium as well as B vitamins. Barley grass powder for Yungong brand was made from the leaves and stem of seedling barley, which is rich in a lot of high functional components improving sleep, because its GABA concentrations are 62.5 times than that of polished rice; calcium (845 mg/100g) and potassium (3110 mg/100g) concentrations in barley grass powder are 99.6 times and 31 times higher than those of polished rice [89]. The downregulated GABAB-R2 receptors in GABA-signaling are essential for sleep maintenance via l-LNv neurons in the circadian clock circuit [90]. A decline in GBAB-A receptor signaling triggers hyperactive sleep disorders, which reveals the GABAergic neurotransmission for pentameric ligand-gated ion channels [91]. The natural Mg2+, N-methyl-D-aspartic acid antagonist and GABA agonist play a key role in sleep regulation [92]. The loss of GABA, potassium, magnesium and calcium from brown rice to white rice of China in 2013 was 20,403 tons, 269,763 tons, 140,972 tons, and 9,168 tons, respectively; but the loss of potassium and magnesium as well as calcium from whole grains to white flour of wheat of China in 2013 was 835,128 tons, 247,010 tons, and 28,452 tons, respectively (unpublished data). Rice and wheat are staple foods of more than 70% of the worlds’ population. The dietary consumption of rice with high glycemic index is associated with good sleep [93]. Therefore, ideal foods to improve sleep for modern people are polished rice or wheat flour plus barley grass powder as well as its products.
The dietary flexibility of early hominins for grasses represents a significant distinction between great apes and common ancestors [94]. Adequate sleep is associated with low calorie foods in overweight adults [95]. Excess weight and obesity due to high fat or low fibre foods are factors that are strongly associated with the risk for Obstructive Sleep Apnea [96]. Sleep deficiencies associated with metabolic dysregulation may contribute to obesity, diabetes and cardiometabolic disease [30], potentially by altering food intake, inflammation, impairing glucose tolerance and insulin sensitivity [97].
Major Methods of Vegetable-Fruits with High Components Promote Sleep
Food choices based on vegetable and fruit consumption are significantly associated with sleep duration (>8 h/night) [98]. A low intake of vegetables and fish and high intake of confectionary and noodles are independently associated with poor sleep quality [99]. Traditionally, lettuce has been recommended for its hypnotic property, with the main component found in n-butanol fraction of this plant [100]. Cherry ingestion may contribute to establish a high-quality sleep and be used as a potential nutraceutical tool to prevent sleep disorders with the advancing of age [101]. The consumption of a tart cherry juice concentrate provides an increase in melatonin that is beneficial in managing disturbed sleep [102]. The walnut may contribute to improved sleep based on the highest content in the antioxidants melatonin, serotonin and total polyphenols [103]. Kiwifruit consumption may improve sleep onset, duration and efficiency in adults with self-reported sleep disturbances [104].
Functional Beverages with high Components Promoting Sleep
Schisandra wine could improve the sleep of mice as a healthy food [105]. Moreover, milk with high melatonin and calcium consumed at night improves sleep quality in rats [106]. Tryptophan can increase the production of niacin, serotonin and melatonin. The alcoholic and malolactic fermentation during the making of 5 monovarietal wines plays a key role in the formation of melatonin and other 11 biogenic amines [107].
MOLECULAR MECHANISM OF FUNCTIONAL FOODS PROMOTING SLEEP
Mechanism of Tryptophan Promoting Sleep
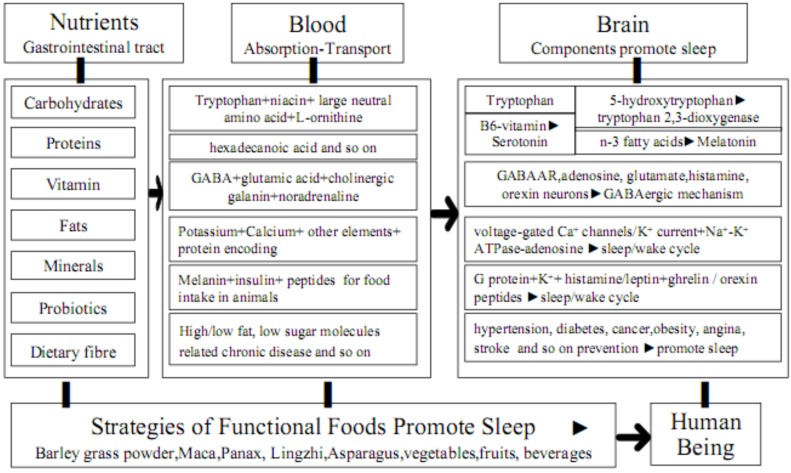
Molecular mechanism of functional foods with high tryptophan content promotes sleep (see Fig. 1), especially barley green of Puritan's Pride (1.20%). It has an impact on the availability of tryptophan as well as the synthesis of serotonin and melatonin, aiding in promoting sleep [57]. Tryptophan is not only a precursor of the neurotransmitter serotonin and the neurosecretory hormone melatonin for sleep/wake [108], but it is also metabolized in the brain along different pathways to serotonin, melatonin and niacin and is partly used as a source of protein synthesis [67]. The dietary tryptophan through the blood-brain barrier is favored by competing large neutral amino acids and carbohydrates; 5-hydroxytryptophan is converted to serotonin by aromatic L-amino acid decarboxylase and pyridoxine, however, serotonin is converted to melatonin by arylalkylamine-N-acetyltransferase and n-3 fatty acids [67]. Acetylcholine neurons modulate both arousal and REMS in combination with disturbed melatonin metabolism that might be involved in the sleep disorders in Cockayne syndrome [109].
Fig. (1).
Possible mechanisms of functional components in foods promote sleep.
Mechanism of GABA Promotes Sleep
Molecular mechanism of functional foods with high GABA content promotes sleep (see Fig. 1), especially barley grass powder of Yungong (0.33%) and red ginseng as well as Ganoderma lucidum. GABA is the main inhibitory neurotransmitter of the central nervous system and activation of GABA(A) receptors favors sleep [67]. GABAergic medullary parafacial zone neurons can potently trigger a slow wave sleep and modulate the cortical electro-encephalogram [110]. The receptor was crystallized bound to a previously unknown agonist, benzamidine, opening a new avenue for the rational design of GABAAR modulators [91]. The level of α/β-subunits of GABAAR was reduced, but glutamic acid decarboxylase were over-expressed in the hypothalamus. Red ginseng extract increased non-REMS via GABAAergic systems [111]. Ganoderma lucidum aqueous extract significantly decreased sleep latency, increased sleeping time, non-REM sleep time and light sleep time in pentobarbital-induced sleep via a GABAergic mechanism [112]. The potentiation effects of kiwifruit peel ethanol extracts on pentobarbital-induced sleep in mice may be modulated by a GABAergic mechanism [113]. The increased glutamate levels in the posterior hypothalamus activate histamine neurons and contribute to caffeine-induced waking and alertness, but GABA levels do not significantly change [114]. The robust REMS-inducing effect of melanin-concentrating hormone is likely related to the deactivation of monoaminergic, orexinergic, glutamatergic, cholinergic and GABAergic neurons involved in the generation of wakefulness and the inhibition of REM sleep [13]. The sleep-promoting action of adenosine can be reversed by orexin A applied to the lateral hypothalamus and exciting glutamatergic input to orexin neurons via the action of orexin receptor 1 [115]. A2AR-mediated activation of median preoptic nucleus and ventrolateral preoptic for two hypothalamic area GABAergic neurons contributes to adenosinergic regulation of sleep [116]. A2AR plays a predominant role in sleep induction, whereas A1R regulates the sleep-wake cycle in a site-dependent manner [117]. Ethanol promotes sleep by increasing adenosine in the orexinergic perifornical hypothalamus, resulting in A1 receptor-mediated inhibition of orexin neurons [118].
Mechanism of Ca-K Promote Sleep
Molecular mechanism of functional foods with high Ca and K content promotes sleep (see Fig. 1), especially barley grass powder of Yungong and Maca. The key role is played by Ca2+ entry through thalamic T channels in the modulation of other voltage-dependent channels that are important for the generation of both slow waves and sleep spindles [119]. Voltage-gated Ca2+ channels are key elements in mediating thalamocortical rhythmicity. Low-voltage activated CaV2.3 R-type Ca2+ channels in the thalamocortical loop and extra-thalamocortical circuitries regulate rodent sleep architecture, which are related to the generation of non-rapid eye movement sleep [120]. The orexin activates mTORC1 via extracellular calcium influx and the lysosome pathway involves v-ATPase and Rag GTPases for chronic sleep disorder [121]. The upregulation of proteins encoding inward rectifier K+ current, delayed rectifier K+ current, acetylcholine activated K+ current, transient outward K+ current and ultra-rapid delayed rectifier potassium current as well as downregulation of protein encoding L-type Ca2+ current were found after chronic obstructive sleep apnea [122]. The Na+-K+ ATPase-dependent adenosine efflux is likely to provide the regulation of sleep by adenosine-mediated activity [123].
Mechanism of Hormones Promoting Sleep
Molecular mechanism of some hormones for food intake in animals promotes sleep (see Fig. 1). Histamine inhibits melanin-concentrating hormone neurons for the regulation of REMS through histamine-3 receptors by activating G protein-dependent inwardly rectifying potassium channels and wake-active histaminergic neurons for the regulation of sleep and arousal [124]. The insulin may be responsible for paradoxical sleep deprivation-induced dysregulation in energy metabolism for food intake, however, reduced leptin levels are compensated by increased expression of leptin receptors in the hypothalamus, whereas no compensations occur in insulin receptors [125]. Sleep restriction is not only associated with a decrease in circulating levels of leptin and an increase in circulating levels of ghrelin, but also a stimulation of brain regions sensitive to food stimuli, which is likely to contribute to the current epidemics of type 2 diabetes and obesity [126]. Some neurons in the thalamus contain acetylcholine, dopamine, cholecystokinin and histamine, however, the forebrain / hypothalamic projections to posterior and lateral posterior may play a role in the migraine attacks triggered by disrupted sleep, skipping meals and emotional reactions [127]. The orexin peptides and their receptors are involved in the regulation of sleep/wakefulness state, energy homeostasis and reward seeking [128].
Mechanism of Disease Prevention Promoting Sleep
Molecular mechanism of functional foods for chronic diseases prevention promotes sleep (see Fig. 1), such as hypertension [129], diabetes [130] and cancer [131]. Gongmi No.3 with the highest retrograded resistant starch in the world is the most ideal rice product to prevent chronic diseases in the World, especially diabetes [132]. Sleep disorder has detrimental effects on metabolic health, which may help in the development of new preventive and therapeutic approaches against obesity and type 2 diabetes based on increasing the quality and/or quantity of sleep [133]. The sleep disorders may be important, modifiable Coronary Artery Disease risk factors in the Indian population [134]. The short self-reported sleep duration is related significantly to an increased risk of reduced glomerular filtration rate in a hypertensive population [135]. Insomnia associated with physiological hyperarousal is associated with a significant risk of hypertension [136].
CONCLUSION AND FUTURE PROSPECTS
This review reveals that sleep disorders are related to functional components of some diets. Experimental studies have demonstrated that major insomnia risk factors are as follows: heredity, chronic disease, psychology, the loss of components from whole grain to polished grain foods, some foods and beverages. Tryptophan is an ingredient producing serotonin for inducing calmness and drowsiness; GABAergic parafacial zone is a sleep promoting center; Voltage-gated Ca2+ channels are key elements in mediating thalamocortical rhythmicity; Orexin peptides and their receptors are involved in the regulation of sleep/wake; and Functional foods for chronic diseases prevention promote sleep. There are higher functional components in functional foods that promote sleep, including tryptophan, GABA, calcium, potassium, melatonin, pyridoxine, L-ornithine and hexadecanoic acid; but wake-promoting neurochemical factors including serotonin, noradrenalin, acetylcholine, histamine, orexin and so on. Although diet promoting sleep is limited for clinical evidence, they may be useful in a number of cases. According to the reviewed studies, eating functional foods may promote sleep, including barley grass powder, whole grains, maca, panax, Lingzhi, asparagus powder, lettuce, cherry, kiwifruits, walnut, schisandra wine, and milk; Barley grass powder for Yungong brand rich in a lot of high functional components improves sleep, because its GABA concentrations are 62.5 times than that of polished rice; calcium and potassium concentrations in barley grass powder are 99.6 times and 31 times than that of polished rice, respectively. Barley grass powder with higher GABA and calcium, potassium as well as tryptophan is the most ideal functional foods to improve sleep. These results suggest that the sleep duration for modern humans is associated with food structure of ancient humans. Overall, there are higher functional components in functional foods most helpful in promoting sleep. These results warrant that future research may highlight the importance of diet promoting sleep quality.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This research was supported by China Agriculture Research System (CARS-05), the National Natural Science Foundation of China (No.31260326), the Science and Technology to Benefit the People (2014RA060) and the Exploit of Emphases New Production (2014BD001) from Yunnan Provincial Scientific and Technology Department. Thank you for copy editing and figures improvement for professional editing team at Eureka Science.
REFERENCES
- 1.Noor ZM, Smith AJ, Smith SS , et al. A study protocol: a community pharmacy-based intervention for improving the management of sleep disorders in the community settings. BMC Health Serv Res. 2014;14:74. doi: 10.1186/1472-6963-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Provini F. Agrypnia excitata. Curr Neurol Neurosci Rep. 2013;13(4):341–0. doi: 10.1007/s11910-013-0341-8. [DOI] [PubMed] [Google Scholar]
- 3.Melancon MO, Lorrain D, Dionne IJ. Exercise and sleep in aging: emphasis on serotonin. Pathol Biol. 2014;62(5):276–83. doi: 10.1016/j.patbio.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Hajak G, Lemme K, Zisapel N. Lasting treatment effects in a postmarketing surveillance study of prolonged-release melatonin. Int Clin Psychopharmacol. 2015;30(1):36–42. doi: 10.1097/YIC.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lévi S, Le Roux N, Eugène E , et al. Benzodiazepine ligands rapidly influence GABAA receptor diffusion and clustering at hippocampal inhibitory synapses. Neuropharmacology. 2015;88:199–208. doi: 10.1016/j.neuropharm.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer DN. Chronic insomnia. Continuum. 2013;19(1):50–66. doi: 10.1212/01.CON.0000427213.00092.c1. [DOI] [PubMed] [Google Scholar]
- 7.Powell KL, Cain SM, Snutch TP , et al. Low threshold T-type calcium channels as targets for novel epilepsy treatments. Br J Clin Pharmacol. 2014;77(5):729–39. doi: 10.1111/bcp.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobin WO, Lennon VA, Komorowski L , et al. DPPX potassium channel antibody: frequency,clinical accompaniments,and outcomes in 20 patients. Neurology. 2014;83(20):1797–803. doi: 10.1212/WNL.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermanstyne TO, Subedi K, Le WW , et al. Kv2 : a novel molecular target to study the role of basal forebrain GABAergic neurons in the sleep-wake cycle. Sleep. 2013;36(12):1839–48. doi: 10.5665/sleep.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papandreou C. Independent associations between fatty acids and sleep quality among obese patients with obstructive sleep apnoea syndrome. J Sleep Res. 2013;22(5):569–72. doi: 10.1111/jsr.12043. [DOI] [PubMed] [Google Scholar]
- 11.Miyake M, Kirisako T, Kokubo T , et al. Randomised controlled trial of the effects of L-ornithine on stress markers and sleep quality in healthy workers. Nutr J. 2014;13(1):53–0. doi: 10.1186/1475-2891-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boss C. Orexin receptor antagonists-a patent review (2010 to August 2014) Expert Opin Ther Pat. 2014;24(12):1367–81. doi: 10.1517/13543776.2014.978859. [DOI] [PubMed] [Google Scholar]
- 13.Monti JM, Torterolo P, Lagos P. Melanin-concentrating hormone control of sleep-wake behavior. Sleep Med Rev. 2013;17(4):293–8. doi: 10.1016/j.smrv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Zadeh SS, Begum K. Comparison of nutrient intake by sleep status in selected adults in Mysore, India. Nutr Res Pract. 2011;5(3):230–5. doi: 10.4162/nrp.2011.5.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palagini L, Biber K, Riemann D. The genetics of insomnia-evidence for epigenetic mechanisms? Sleep Med Rev. 2014;18(3):225–35. doi: 10.1016/j.smrv.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Drake CL, Friedman NP, Wright KPJ , et al. Sleep reactivity and insomnia genetic and environmental influences. Sleep. 2011;34(9):1179–88. doi: 10.5665/SLEEP.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wing YK, Zhang J, Lam SP , et al. Familial aggregation and heritability of insomnia in a community -based study. Sleep Med. 2012;13(8):985–90. doi: 10.1016/j.sleep.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Ban HJ, Kim SC, Seo J , et al. Genetic and metabolic characterization of insomnia. PLoS ONE. 2011;6(4):e18455–0. doi: 10.1371/journal.pone.0018455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CC, Lung FW. The role of PGC-1 and Apoe4 in insomnia. Psychiatr Genet. 2012;22(2):82–7. doi: 10.1097/YPG.0b013e32834dc438. [DOI] [PubMed] [Google Scholar]
- 20.Park HJ, Park JK, Kim SK , et al. Association of polymorphism in the promoter of the melatonin receptor 1A gene with schizophrenia and with insomnia symptoms in schizophrenia patients. J Mol Neurosci. 2011;45(2):304–8. doi: 10.1007/s12031-011-9522-6. [DOI] [PubMed] [Google Scholar]
- 21.Byrne EM, Johnson J, McRae AF , et al. A genome-wide association study of caffeine-related sleep disturbance confirmation of a role for a common variant in the adenosine receptor. Sleep. 2012;35(7):967–75. doi: 10.5665/sleep.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brower KJ, Wojnar M, Sliwerska E , et al. PER3 polymorphism and insomnia severity in alcohol dependence. Sleep. 2012;35(4):571–7. doi: 10.5665/sleep.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang SC, Tsou HH, Chen CH , et al. Genetic polymorphisms in the opioid receptor mu1 gene are associated with changes in libido and insomnia in methadone maintenance patients. Eur Neuropsychopharmacol. 2012;22(10):695–703. doi: 10.1016/j.euroneuro.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Cortelli P, Fabbri M, Calandra-Buonaura G , et al. Gait disorders in fatal familial insomnia. Mov Disord. 2014;29(3): 420–4. doi: 10.1002/mds.25786. [DOI] [PubMed] [Google Scholar]
- 25.Möller-Levet CS, Archer SN, Bucca G , et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. PNAS. 2013;110(12):E1132–E1141. doi: 10.1073/pnas.1217154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ollila HM, Kettunen J, Pietiläinen O , et al. Genome-wide association study of sleep duration in the Finnish population. J Sleep Res. 2014;23(6):609–18. doi: 10.1111/jsr.12175. [DOI] [PubMed] [Google Scholar]
- 27.Kunst M, Hughes ME, Raccuglia D , et al. Calcitonin gene-related peptide neurons mediate sleep-specific circadian output in Drosophila. Curr Biol. 2014;24(22):2652–64. doi: 10.1016/j.cub.2014.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chellappa SL, Viola AU, Schmidt C , et al. Light modulation of human sleep depends on a polymorphism in the clock gene Period3. Behav Brain Res. 2014;271:23–9. doi: 10.1016/j.bbr.2014.05.050. [DOI] [PubMed] [Google Scholar]
- 29.Takeshima K, Yamatsu A, Yamashita Y , et al. Subchronic toxicity evaluation of ?-aminobutyric acid (GABA) in rats. Food Chem Toxicol. 2014;68C:128–34. doi: 10.1016/j.fct.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Grandner MA, Jackson N, Gerstner Jr , et al. Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res. 2014;23(1):22–34. doi: 10.1111/jsr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider T, Dibué-Adjei M. Cav23 E-/R-type voltage-gated calcium channels modulate sleep in mice. Sleep. 2014 doi: 10.5665/sleep.4518. PMID 2551511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dufour MA, Woodhouse A, Goaillard JM. Somatodendritic ion channel expression in substantia nigra pars compacta dopaminergic neurons across postnatal development. J Neurosci Res. 2014;92(8):981–99. doi: 10.1002/jnr.23382. [DOI] [PubMed] [Google Scholar]
- 33.Jabbour HJ, Naccache NM, Jawish RJ , et al. Ketamine and magnesium association reduces morphine consumption after scoliosis surgery prospective randomised double-blind study. Acta Anaesthesiol Scand. 2014;58(5):572–9. doi: 10.1111/aas.12304. [DOI] [PubMed] [Google Scholar]
- 34.Engeda J, Mezuk B, Ratliff S , et al. Association between duration and quality of sleep and the risk of pre-diabetes evidence from NHANES. Diabet Med. 2013;30(6):676–80. doi: 10.1111/dme.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hjorth MF, Quist JS, Andersen R , et al. Change in sleep duration and proposed dietary risk factors for obesity in Danish school children. Pediatr Obes. 2014;9(6):e156–9. doi: 10.1111/ijpo.264. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka E, Yatsuya H, Uemura M , et al. Associations of protein, fat, and carbohydrate intakes with insomnia symptoms among middle-aged Japanese workers. J Epidemiol. 2013;23(2):132–8. doi: 10.2188/jea.JE20120101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dweck JS, Jenkins SM, Nolan LJ. The role of emotional eating and stress in the influence of short sleep on food consumption. Appetite. 2014;72:106–13. doi: 10.1016/j.appet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Stern JH, Grant AS, Thomson CA , et al. Short sleep duration is associated with decreased serum leptin, increased energy intake and decreased diet quality in postmenopausal women. Obesity. 2014;22(5):E55–61. doi: 10.1002/oby.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wierzejska R. Caffeine-common ingredient in a diet and its influence on human health. Rocz Panstw Zakl Hig. 2012;63(2):141–7. [PubMed] [Google Scholar]
- 40.Bhatti SK, O'Keefe JH, Lavie CJ. Coffee and tea perks for health and longevity? Curr Opin Clin Nutr Metab Care. 2013;16(6):688–97. doi: 10.1097/MCO.0b013e328365b9a0. [DOI] [PubMed] [Google Scholar]
- 41.Mehari A, Weir NA, Gillum RF. Gender and the association of smoking with sleep quantity and quality in American adults. Women Health. 2014;54(1):1–14. doi: 10.1080/03630242.2013.858097. [DOI] [PubMed] [Google Scholar]
- 42.Sivertsen B, Lallukka T, Salo P , et al. Insomnia as a risk factor for ill health results from the large population-based prospective HUNT Study in Norway. J Sleep Res. 2014;23(2):124–32. doi: 10.1111/jsr.12102. [DOI] [PubMed] [Google Scholar]
- 43.Tagaya H, Murayama N, Hakamata Y. Sleep disorders. Nihon Rinsho. 2014;72(4):739–43. [PubMed] [Google Scholar]
- 44.Jain A, Mittal RS, Sharma A , et al. Study of insomnia and associated factors in traumatic brain injury. Asian J Psychiatr. 2014;8(1):99–103. doi: 10.1016/j.ajp.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Tian C, Liu D, Xiang W , et al. Analyses of the similarity and difference of global gene expression profiles in cortex regions of three neurodegenerative diseases sporadic Creutzfeldt-Jakob disease (sCJD), fatal familial Insomnia (FFI), and Alzheimer's disease (AD) Mol Neurobiol. 2014;50(2):473–81. doi: 10.1007/s12035-014-8758-x. [DOI] [PubMed] [Google Scholar]
- 46.Kim JY, Lee YD, Kim BJ , et al. Melatonin improves inflammatory cytokine profiles in lung inflammation associated with sleep deprivation. Mol Med Rep. 2012;5(5):1281–4. doi: 10.3892/mmr.2012.814. [DOI] [PubMed] [Google Scholar]
- 47.Gandolphi LR, Okazaki KM, Nozoe KT , et al. Influence of sleep disorders on television viewing time, diabetes and obesity. Diabet Med. 2015;32(1):141–2. doi: 10.1111/dme.12603. [DOI] [PubMed] [Google Scholar]
- 48.Luyster FS, Kip KE, Aiyer AN , et al. Relation of obstructive sleep apnea to coronary artery calcium in non-obese versus obese men and women aged 45-75 years. Am J Cardiol. 2014;114(11):1690–4. doi: 10.1016/j.amjcard.2014.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nedeltcheva AV, Scheer FA. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2014;21(4):293–8. doi: 10.1097/MED.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashizume Y. The importance of sleep in the mental health. Nihon Rinsho. 2014;72(2):341–6. [PubMed] [Google Scholar]
- 51.Kim EJ, Baek JH, Shin DJ , et al. Correlation of sleep disturbance and cognitive impairment in patients with Parkinson's disease. J Mov Disord. 2014;7(1):13–8. doi: 10.14802/jmd.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cunnington D, Junge MF, Fernando AT. Insomnia prevalence, consequences and effective treatment. Med J Aust. 2013;199(8):S36–40. doi: 10.5694/mja13.10718. [DOI] [PubMed] [Google Scholar]
- 53.Kato T. Insomnia symptoms, depressive symptoms, and suicide ideation in Japanese white-collar employees. Int J Behav Med. 2014;21(3):506–10. doi: 10.1007/s12529-013-9364-4. [DOI] [PubMed] [Google Scholar]
- 54.Konadhode RR, Pelluru D, Blanco-Centurion C , et al. Optogenetic stimulation of MCH neurons increases sleep. J Neurosci. 2013;33(25):10257–63. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasler BP, Germain A, Nofzinger EA , et al. Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. J Sleep Res. 2012;21(5):515–26. doi: 10.1111/j.1365-2869.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu L, Jiang CQ, Lam TH , et al. Short or long sleep duration is associated with memory impairment in older Chinese the Guangzhou Biobank Cohort Study. Sleep. 2011;34(5):575–80. doi: 10.1093/sleep/34.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaput JP. Sleep patterns, diet quality and energy balance. Physiol behav. 2014;134(1):86–91. doi: 10.1016/j.physbeh.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Lindseth G, Lindseth P, Thompson M. Nutritional effects on sleep. Western J Nurs Res. 2013;35(4):497–513. doi: 10.1177/0193945911416379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vlainic J, Jembrek MJ, Štrac D , Pericic D. The effects of zolpidem treatment and withdrawal on the in vitro expression of recombinant a1ß2?2s GABAA receptors expressed in HEK 293 cells. Naunyn- Schmiedeberg's Arch Pharmacol. 2010;382(3):201–12. doi: 10.1007/s00210-010-0539-0. [DOI] [PubMed] [Google Scholar]
- 60.Fitzgerald AC, Wright BT, Heldt SA. The behavioral pharmacology of zolpidem evidence for the functional significance of a1-containing GABA(A) receptors. Psychopharmacology (Berl) 2014;231(9):1865–96. doi: 10.1007/s00213-014-3457-x. [DOI] [PubMed] [Google Scholar]
- 61.Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2014;171(2):283–93. doi: 10.1111/bph.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berridge MJ. Calcium regulation of neural rhythms, memory and Alzheimer's disease. J Physiol. 2014;592(2):281–93. doi: 10.1113/jphysiol.2013.257527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cornelius Jr, Pittock SJ, McKeon A , et al. Sleep manifestations of voltage-gated potassium channel complex autoimmunity. Arch Neurol. 2011;68(6):733–8. doi: 10.1001/archneurol.2011.106. [DOI] [PubMed] [Google Scholar]
- 64.Grace KP, Hughes SW, Shahabi S , et al. K+ channel modulation causes genioglossus inhibition in REM sleep and is a strategy for reactivation. Respir Physiol Neurobiol. 2013;188(3):277–88. doi: 10.1016/j.resp.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 65.Nielsen FH, Johnson LK, Zeng H. Magnesium supplementation improves indicators of low magnesium status and inflammatory stress in adults older than 51 years with poor quality sleep. Magnes Res. 2010;23(4):158–68. doi: 10.1684/mrh.2010.0220. [DOI] [PubMed] [Google Scholar]
- 66.Song CH, Kim YH, Jung KI. Associations of zinc and copper levels in serum and hair with sleep duration in adult women. Biol Trace Elem Res. 2012;149(1):16–21. doi: 10.1007/s12011-012-9398-5. [DOI] [PubMed] [Google Scholar]
- 67.Peuhkuri K, Sihvola N, Korpela R. Diet promotes sleep duration and quality. Nutr Res. 2012;32(5):309–19. doi: 10.1016/j.nutres.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Halson SL. Sleep in elite athletes and nutritional interventions to enhance sleep. Sports Med. 2014;44(S1):S13–23. doi: 10.1007/s40279-014-0147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang SH, Li GZ, Xue RG , et al. Present situation and promoting strategies of maca industry in Yunnan Province. World Sci Technol. 2012;14(4):1921,–5. [Google Scholar]
- 70.Del Valle Mendoza J, Pumarola T, Gonzales LA , et al. Antiviral activity of maca (Lepidium meyenii) against human influenza virus. Asian Pac J Trop Med. 2014;7(S1):S415–20. doi: 10.1016/S1995-7645(14)60268-6. [DOI] [PubMed] [Google Scholar]
- 71.Gonzales GF, Villaorduña L, Gasco M , et al. Maca (Lepidium meyenii Walp), a review of its biological properties. Rev. Peru Med Exp Salud Publica. 2014;31(1):100–10. [PubMed] [Google Scholar]
- 72.Uchiyama F, Jikyo T, Takeda R , et al. Lepidium meyenii (Maca) enhances the serum levels of luteinising hormone in female rats. J Ethnopharmacol. 2014;151(2):897–902. doi: 10.1016/j.jep.2013.11.058. [DOI] [PubMed] [Google Scholar]
- 73.Ai Z. Study on the antidepressant and sleep-improving effect and mechanism of Maca (Lepidum meyenii) extract. MsD dissertation. Wuhan Huazhong University of Science and Technology. 2013 [Google Scholar]
- 74.Sun XD, Tang H, Du P , et al. Nutritional components and antioxidative activity of polysaccharide in vitro from Maca cultivated in Lijiang. Chinese J Spectro Lab. 2013;30(5):2365–71. [Google Scholar]
- 75.Meng QQ, Zeng XY, Yang YK , et al. Differences of the main compounds of essential oils extracted from Lepidium meyenii Walpers cultivated in Yunnan with different solvents. Fine Chem. 2013;30(4):442–6. [Google Scholar]
- 76.Zhao A, Ma N, Cui XM , et al. Experimental study on the improvement sleep function of mouse by Panax notoginseng flower glycosides capsules. Chin Med J Res Prac. 2013;27(3):25–7. [Google Scholar]
- 77.Li TL, Yu S, Xin J. The study of the sleep improvement function and mechanism of the effective parts of Cirenshen. Pharmacol Clin Chin Materia Med. 2012;28(5):105–8. [Google Scholar]
- 78.Provino R. he role of adaptogens in stress management. Australian J Med Herbalism. 2010;22(2):41–50. [Google Scholar]
- 79.Han HJ, Kim HY, Choi JJ , et al. Effects of red ginseng extract on sleeping behaviors in human volunteers. J Ethnopharmacol. 2013;149(2):597–9. doi: 10.1016/j.jep.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 80.Yang M, Lee HS, Hwang MW , et al. Effects of Korean red ginseng (Panax Ginseng Meyer) on bisphenol A exposure and gynecologic complaints single blind, randomized clinical trial of efficacy and safety. BMC Complement Altern Med. 2014;14:265. doi: 10.1186/1472-6882-14-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kitaoka K, Uchida K, Okamoto N , et al. Fermented ginseng improves the first-night effect in humans. leep. 2009;32(3):413–21. doi: 10.1093/sleep/32.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cui XY, Cui SY, Zhang J , et al. Extract of Ganoderma lucidum prolongs sleep time in rats. J Ethnopharmacol. 2012;139(3):796–800. doi: 10.1016/j.jep.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 83.Sanodiya BS, Thakur GS, Baghel RK , et al. Ganoderma lucidum a potent pharmacological macrofungus. Curr Pharm Biotechnol. 2009;10(8):717–42. doi: 10.2174/138920109789978757. [DOI] [PubMed] [Google Scholar]
- 84.Zhang ML, Bao HY, Fu WW , et al. Studies of cracked Lingzhi spores powder on hypnotic effects in mice. Ginseng Res. 2013;(4):17–9. [Google Scholar]
- 85.Ma SF, Wang F, Li HC , et al. Study on the sleep improvement effect of the instant asparagus powder. Sci Technol Food Industry. 2012;33(18):359–61. [Google Scholar]
- 86.Ito T, Goto K, Takanari J , et al. Effects of enzyme-treated asparagus extract on heat shock protein 70, stress indices, and sleep in healthy adult men. J Nutr Sci Vitaminol (Tokyo) 2014;60(4):283–90. doi: 10.3177/jnsv.60.283. [DOI] [PubMed] [Google Scholar]
- 87.Qi LJ, Gao S, Ma L , et al. An experimental study of the effect of Gastrodia elata on sleeping. apital J Public Health. 2012;6(2):66–8. [Google Scholar]
- 88.Li W, Fu LH, Kuang YS , et al. Study on the absorptive ability and sleep improvement effect of ultrafine powder of Gastrodia elata Bl. Food Res Develop. 2014;35(20):27–31. [Google Scholar]
- 89.Zeng YW, Pu XY, Zhang J , et al. Synthetic research and utilization on industrial development of barley in Southwestern China. J Agric Sci Technol. 2013;15(3):48–56. [Google Scholar]
- 90.Gmeiner F, Kolodziejczyk A, Yoshii T , et al. GABA(B) receptors play an essential role in maintaining sleep during the second half of the night in Drosophila melanogaster. J Exp Biol. 2013;216(20):3837–43. doi: 10.1242/jeb.085563. [DOI] [PubMed] [Google Scholar]
- 91.Miller PS, Aricescu AR. Crystal structure of a human GABAA receptor. Nature. 2014;512(7514):270–5. doi: 10.1038/nature13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abbasi B, Kimiagar M, Sadeghniiat K , et al. The effect of magnesium supplementation on primary insomnia in elderly A double-blind placebo-controlled clinical trial. J Res Med Sci. 2012;17(12):1161–9. [PMC free article] [PubMed] [Google Scholar]
- 93.Yoneyama S, Sakurai M, Nakamura K , et al. Associations between rice, noodle, and bread intake and sleep quality in Japanese men and women. PLoS One. 2014;9(8):e105198–0. doi: 10.1371/journal.pone.0105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wynn JG, Sponheimer M, Kimbel WH , et al. Diet of Australopithecus afarensis from the Pliocene Hadar Formation, Ethiopia. PNAS. 2013;110(26):10495–501. doi: 10.1073/pnas.1222559110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tasali E, Chapotot F, Wroblewski K , et al. The effects of extended bedtimes on sleep duration and food desire in overweight young adults A home-based intervention. Appetite. 2014;80:220–4. doi: 10.1016/j.appet.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith SS, Waight C, Doyle G , et al. Liking for high fat foods in patients with Obstructive Sleep Apnoea. Appetite. 2014;78:185–92. doi: 10.1016/j.appet.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 97.Depner CM, Stothard ER, Wright KPJ. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14(7):507–0. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kruger AK, Reither EN, Peppard PE , et al. Do sleep-deprived adolescents make less-healthy food choices? Br J Nutr. 2014;111(10):1898–904. doi: 10.1017/S0007114514000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Katagiri R, Asakura K, Kobayashi S , et al. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleepquality among middle-aged female Japanese workers. J Occup Health. 2014;56(5):359–68. doi: 10.1539/joh.14-0051-oa. [DOI] [PubMed] [Google Scholar]
- 100.Ghorbani A, Rakhshandeh H, Sadeghnia HR. Potentiating effects of Lactuca sativa on pentobarbital-induced sleep. Iran J Pharm Res. 2013;12(2):401–6. [PMC free article] [PubMed] [Google Scholar]
- 101.Garrido M, González-Gómez D, Lozano M , et al. A Jerte valley cherry product provides beneficial effects on sleep quality Influence on aging. J Nutr Health Aging. 2013;17(6):553–60. doi: 10.1007/s12603-013-0029-4. [DOI] [PubMed] [Google Scholar]
- 102.Howatson G, Bell PG, Tallent J , et al. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur J Nutr. 2012;51(8):909–16. doi: 10.1007/s00394-011-0263-7. [DOI] [PubMed] [Google Scholar]
- 103.Tapia MI, Morgado JS, García-Parra J , et al. Comparative study of the nutritional and bioactive compounds content of four walnut (Juglans regia L. cultivars. J Food Compos Anal. 2013;31:232–7. [Google Scholar]
- 104.Lin HH, Tsai PS, Fang SC , et al. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. sia Pac J Clin Nutr. 2011;20(2 ): 169–74. [PubMed] [Google Scholar]
- 105.Yin LP, Li ZX, Zhang CX. Study on the effect of schisandra wine on sleep improvement of mice. Sci Technol Food Industry. 2013;34(14):346–9. [Google Scholar]
- 106.Milagres MP, Minim VP, Minim LA , et al. Night milking adds value to cow's milk. J Sci Food Agric. 2014;94(8):1688–92. doi: 10.1002/jsfa.6480. [DOI] [PubMed] [Google Scholar]
- 107.Rodriguez-Naranjo MI, Ordóñez JL , et al. elatonin is formed during wine making at safe levels of biogenic amines. Food Chem Toxicol. 2013;57:140–6. doi: 10.1016/j.fct.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 108.Jones BE. Neurobiology of waking and sleeping. Handb Clin Neurol. 2011;98:131–49. doi: 10.1016/B978-0-444-52006-7.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Okoshi Y, Tanuma N, Miyata R , et al. Melatonin alterations and brain acetylcholine lesions in sleep disorders in Cockayne syndrome. Brain Dev. 2014;36(10):907–13. doi: 10.1016/j.braindev.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 110.Anaclet C, Ferrari L, Arrigoni E , et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci. 2014;17(9):1217–24. doi: 10.1038/nn.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee CI, Kim CS, Han JY , et al. Repeated administration of Korea red ginseng extract increases non -rapid eye movement sleep via GABAAergic systems. J Ginseng Res. 2012;36(4):403–410. doi: 10.5142/jgr.2012.36.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chu QP, Wang LE, Cui XY , et al. Extract of Ganoderma lucidum potentiates pentobarbital-induced sleep via a GABAergic mechanism. Pharmacol Biochem Behav. 2007;86(4):693–8. doi: 10.1016/j.pbb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 113.Yang H, Lee YC, Han KS , et al. Green and gold kiwifruit peel ethanol extracts potentiate pentobarbital-induced sleep in mice via a GABAergic mechanism. Food Chem. 2013;136(1):160–3. doi: 10.1016/j.foodchem.2012.07.111. [DOI] [PubMed] [Google Scholar]
- 114.John J, Kodama T, Siegel JM. Caffeine promotes glutamate and histamine release in the posterior hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2014;307(6):R704–10. doi: 10.1152/ajpregu.00114.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cun Y, Tang L, Yan J , et al. Orexin A attenuates the sleep-promoting effect of adenosine in the lateral hypothalamus of rats. Neurosci Bull. 2014;30(5): 877–86. doi: 10.1007/s12264-013-1442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kumar S, Rai S, Hsieh KC , et al. Adenosine A(2A) receptors regulate the activity of sleep regulatory GABAergic neurons in the preoptic hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2013;305(1):R31–41. doi: 10.1152/ajpregu.00402.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang ZL, Zhang Z, Qu WM. Roles of adenosine and its receptors in sleep-wake regulation. Int Rev Neurobiol. 2014;119:349–71. doi: 10.1016/B978-0-12-801022-8.00014-3. [DOI] [PubMed] [Google Scholar]
- 118.Sharma R, Sahota P, Thakkar MM. Role of adenosine and the orexinergic perifornical hypothalamus in sleep-promoting effects of ethanol. leep. 2014;37(3):525–33. doi: 10.5665/sleep.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crunelli V, David F, Leresche N , et al. Role for T-type Ca2+ channels in sleep waves. Eur J Physiol. 2014;466(4):735–45. doi: 10.1007/s00424-014-1477-3. [DOI] [PubMed] [Google Scholar]
- 120.Siwek ME, Müller R, Henseler C , et al. The CaV2 R-type voltage-gated Ca2+ channel in mouse sleep architecture. Sleep. 2014;37(5):881–92. doi: 10.5665/sleep.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Z, Liu S, Kakizaki M , et al. Orexin/hypocretin activates mTOR complex 1 (mTORC1):via an Erk/Akt-independent and calcium-stimulated lysosome v-ATPase pathway. J Biol Chem. 2014;289(46):31950–9. doi: 10.1074/jbc.M114.600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao J, Xu W, Yun F , et al. Chronic obstructive sleep apnea causes atrial remodeling in canines mechanisms and implications. Basic Res Cardiol. 2014;109(5):427–0. doi: 10.1007/s00395-014-0427-8. [DOI] [PubMed] [Google Scholar]
- 123.Sims RE, Dale N. Activity-dependent adenosine release may be linked to activation of Na+-K+ ATPase an in vitro rat study. PLoS One. 2014;9(1):e87481–0. doi: 10.1371/journal.pone.0087481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Parks GS, Olivas ND, Ikrar T , et al. Histamine inhibits the melanin-concentrating hormone system implications for sleep and arousal. J Physiol. 2014;592(Pt 10):2183–96. doi: 10.1113/jphysiol.2013.268771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moraes DA, Venancio DP, Suchecki D. Sleep deprivation alters energy homeostasis through non-compensatory alterations in hypothalamic insulin receptors in Wistar rats. Horm Behav. 2014;66(5):705–12. doi: 10.1016/j.yhbeh.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 126.Copinschi G, Leproult R, Spiegel K. The important role of sleep in metabolism. Front Horm Res. 2014;42:59–72. doi: 10.1159/000358858. [DOI] [PubMed] [Google Scholar]
- 127.Kagan R, Kainz V, Burstein R , et al. Hypothalamic and basal ganglia projections to the posterior thalamus possible role in modulation of migraine headache and photophobia. Neuroscience. 2013;248:359–68. doi: 10.1016/j.neuroscience.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu TR, Yang Y, Ward R , et al. Orexin receptors multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Cell Signal. 2013;25(12):2413–23. doi: 10.1016/j.cellsig.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 129.Zeng YW, Du J, Pu XY , et al. Strategies of functional food for hypertension prevention in China. J Med Plants Res. 2011;5(24):5671–6. [Google Scholar]
- 130.Zeng YW, Pu XY, Du J , et al. Use of functional foods for diabetes prevention in China. fr J Pharm Pharmacol. 2012;6(35):2570–9. [Google Scholar]
- 131.Zeng YW, Yang JZ, Pu XY , et al. Strategies of functional food for cancer prevention in human beings. Asian Pac J Cancer Prevt. 2013;14(3):1585–92. doi: 10.7314/apjcp.2013.14.3.1585. [DOI] [PubMed] [Google Scholar]
- 132.Zeng YW, Zeng Y, Pu ZG , et al. DNA fingerprint and determination of functional components for rice with diabetes prevention. Adv Mater Res. 2013;635:1566–9. [Google Scholar]
- 133.Ruble K, George A, Gallicchio L , et al. Sleep disordered breathing risk in childhood cancer survivors An exploratory study. Pediatr Blood Cancer. 2015 doi: 10.1002/pbc.25394. doi 10.1002/pbc.25394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sharma M, Sawhney JP, Panda S. Sleep quality and duration - Potentially modifiable risk factors for Coronary Artery Disease? Indian Heart J. 2014;66(6):565–8. doi: 10.1016/j.ihj.2014.10.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guo X, Yu S, Li Z , et al. Self-reported sleep duration is associated with reduced glomerular filtration rate among adults with hypertension a population-based study from rural northeast China. J Sleep Res. 2015 doi: 10.1111/jsr.12274. doi 10.1111/jsr.12274. [DOI] [PubMed] [Google Scholar]
- 136.Li Y, Vgontzas AN, Fernandez-Mendoza J , et al. Insomnia with physiological hyperarousal is associated with hypertension. Hypertension. 2015 doi: 10.1161/HYPERTENSIONAHA.114.04604. pii HYPERTENSIONAHA.114.04604. [DOI] [PubMed] [Google Scholar]