Abstract
Nicotine is a psychomotor stimulant with ‘reinforcement enhancing’ effects – the actions of nicotine in the brain increase responding for non-nicotine rewards. We hypothesized that this latter effect of nicotine depends on increased incentive properties of anticipatory cues; consistent with this hypothesis, multiple laboratories have reported that nicotine increases sign tracking, i.e. approach to a conditioned stimulus (CS), in Pavlovian conditioned-approach tasks. Incentive motivation and sign tracking are mediated by mesolimbic dopamine (DA) transmission and nicotine facilitates mesolimbic DA release. Therefore, we hypothesized that the incentive-promoting effects of nicotine would be impaired by DA antagonists. To test this hypothesis, separate groups of rats were injected with nicotine (0.4 mg/kg base) or saline prior to Pavlovian conditioning sessions in which a CS (30 s illumination of a light or presentation of a lever) was immediately followed by a sweet reward delivered in an adjacent location. Both saline and nicotine pretreated rats exhibited similar levels of conditioned approach to the reward location (goal tracking), but nicotine pretreatment significantly increased approach to the CS (sign tracking), regardless of type (lever or light). The DAD1 antagonist SCH-23390 and the DAD2/3 antagonist eticlopride reduced conditioned approach in all rats, but specifically reduced goal tracking in the saline pretreated rats and sign tracking in the nicotine pretreated rats. The non-selective DA antagonist flupenthixol reduced sign-tracking in nicotine rats at all doses tested; however, only the highest dose of flupenthixol reduced goal tracking in both nicotine and saline groups. The reductions in conditioned approach behavior, especially those by SCH-23390, were dissociated from simple motor suppressant effects of the antagonists. These experiments are the first to investigate the effects of dopaminergic drugs on the facilitation of sign-tracking engendered by nicotine and they implicate dopaminergic systems both in conditioned approach as well as the incentive-promoting effects of nicotine.
Keywords: Nicotine, conditioning, sign tracking, goal tracking, dopamine receptor antagonist
1. Introduction1
Nicotine, one of the most widely used addictive substances in the world (Ague, 1972, Lerman and Audrain-McGovern, 2010), is considered to be the reinforcing agent in tobacco products (USDHHS, 1988). Several lines of evidence suggest that nicotine plays a critical role in smoking and other forms of tobacco use (Chaudhri et al., 2006, Rose, 2006, Caggiula et al., 2009, Le Foll and Goldberg, 2009). Nicotine supports operant behavior in humans (Perkins et al., 2001) as well as several non-human species (Henningfield and Goldberg, 1983). Nicotine replacement therapies are one of the most widely used treatments for smoking cessation and they improve cessation rates by 50–70% (Stead et al., 2012). Also, cessation products that do not include nicotine or include nicotine reduction (e.g., Quest® cigarettes) have been commercial failures, whereas smoke-free cigarettes that provide a nicotine vapor, while not accepted as cessation therapies, are gaining market share as alternatives to tobacco delivery products (Odum et al., 2012).
The central role of nicotine in tobacco dependence is undeniable; however, the role of nicotine in dependence may not be as straightforward as with other abused drugs. Nicotine is a moderate stimulant with weak and unreliable reinforcing properties (Caggiula et al., 2009). While nicotine infusions by themselves support operant behavior in non-human animals (Donny et al., 2003); the effects of nicotine are weak, and under progressive-ratio reinforcement schedules the motivation to obtain nicotine infusions is relatively low (Chaudhri et al., 2007). In choice situations, nicotine is less preferred than other abused drugs, such as cocaine (Manzardo et al., 2002). Despite its relatively low reinforcing efficacy, responding for nicotine is robustly enhanced by inclusion of other environmental stimuli (Palmatier et al., 2006). For example, visual reinforcers included with nicotine infusions increase operant responding (Donny et al., 2003) and motivation to obtain the reinforcer(s) (Chaudhri et al., 2007). This interaction between nicotine and non-nicotine stimuli has recently been replicated in human smokers (Perkins and Karelitz, 2013b, a). Also, human research suggests that the ‘euphoric’ effects of nicotine self-administered in cigarettes are weak and their subjective pleasure may be heavily influenced by environmental factors (Dar et al., 2007) and that individuals with high reactivity to food-associated cues also have high reactivity to nicotine-associated cues (Mahler and de Wit, 2010).
Evidence from our laboratory and others has stressed that incentive motivation may be critical to the interaction between nicotine and non-nicotine stimuli (Olausson et al., 2004a 2004, Palmatier et al., 2012a, Palmatier et al., 2012b, Peartree et al., 2012). For example, we recently found that nicotine-induced increases in motivation in an operant task did not depend on the strength of the reinforcer used, but was exquisitely sensitive to the strength of the ‘cues’ associated with that reinforcer (Palmatier et al., 2012b). We also found that nicotine increased approach to a conditioned stimulus (CS) that was spatially separated from an unconditioned stimulus (US) in a Pavlovian conditioned approach task (Palmatier et al., 2012a). This increase in ‘sign tracking’ (i.e., approach to CS) was not accompanied by a change in approach to the US (‘goal tracking’), and the nicotine-induced increase in sign tracking was systematically related to in the intensity of the US. Specifically, greater nicotine-induced increases in sign tracking were observed when the CS was paired with 20% sucrose, relative to rats that had the CS paired with 5% sucrose. Collectively, these findings suggest that nicotine increases incentive-based motivation, which may help to explain why the reinforcing (Donny et al., 2003, Chen et al., 2011) and rewarding (Peartree et al., 2012) effects of nicotine are more robust when non-nicotine reinforcers and rewards are included in the test paradigm.
Incentive motivation is widely considered to depend on the mesotelencephalic dopamine (DA) system (Berridge and Robinson, 1998, Wightman and Robinson, 2002, Uslaner et al., 2008, Flagel et al., 2011, Saunders and Robinson, 2012, Anselme et al., 2013). For example, in selectively bred rats that display more sign tracking behavior, phasic DA responses to a CS are more pronounced in the core of the nucleus accumbens (NAc). Systemic administration of the non-selective DA antagonist flupenthixol reduces acquisition of sign tracking in these rats (Flagel et al., 2011). Reduced expression of sign tracking was observed after local administration of flupenthixol to the NAc (Saunders and Robinson, 2012). Also, genetic reconstruction of D1-receptor function in the NAc core in DA D1 receptor knock-out mice preferentially increased acquisition of sign-tracking responses, whereas reconstruction of D1 function in the NAc shell did not restore sign- or goal-directed conditioned responses (Gore and Zweifel, 2013). Thus, the NAc, and in particular DA D1 receptors in the core, appear to play a critical role in the acquisition and expression of sign tracking.
We hypothesized that nicotine-facilitated sign tracking is dependent on mesolimbic DA transmission. To address this hypothesis, we replicated our previous findings in which injections of nicotine prior to testing sessions increase approach to a CS paired with a sweet reward. In Experiment 1, the CS (30 s presentation of a light) was presented inside of a receptacle that could monitor approach (head entries). The US (0.1 ml presentation of 5% chocolate solution) was delivered in a separate, identical receptacle in which head entries could also be monitored. Once we established that nicotine increased approach to the CS, we investigated the role of DA receptors by pre-treating rats with the D1 receptor antagonist SCH-23390, the D2/3 receptor antagonist eticlopride, and the non-selective DA receptor antagonist flupenthixol. A video-recording system installed in the testing chambers and automated behavioral monitoring software were used to monitor approach and non-specific effects of the antagonists. In Experiment 2 the findings from Experiment 1 were confirmed with a CS that is more comparable to previous studies of sign tracking – a lever was inserted into the chamber for 30 s and a light above the lever was illuminated. Sucrose solution (20% w/v) served as the US in Experiment 2.
2. Method
2.1. Subjects
2.1.1. Experiment 1
Ten male Sprague Dawley rats weighing 274–300 were purchased from Charles River Laboratories (Raleigh, NC) and were housed in a temperature- and humidity-controlled environment. The rats were non-naïve, as they initially participated as control subjects in a previous operant-conditioning experiment with a sucrose reinforcer (20% w/v). In that study, rats were randomly assigned to NIC (0.4 mg/kg nicotine) or SAL (vehicle) exposure conditions (n=5/group). Rats in both groups received 24 sessions (approximately 1 h per day) in the operant test chambers and the NIC group received 26 exposures to nicotine, but these injections were temporally separated from chamber exposures (at least 1 h after sessions). To reduce generalization between studies, the rats were shifted to new chambers, the levers were removed, and Nesquick® chocolate was used as the US. In the original study, sucrose was delivered in a liquid dipper for pressing a lever located on one wall of the chamber. For the present study, the CS and US locations were on the opposite wall and Nesquick was delivered via syringe pump to a receptacle well (see Apparatus). All rats had water ad libitum and were fed 20 g food per day, after the daily conditioning session.
2.1.2. Experiment 2
Sixteen male Sprague Dawley rats weighing 274–300 were purchased from Charles River Laboratories (Raleigh, NC) and were housed in the same manner as Experiment 1. All rats in Experiment 2 were naïve before acquisition.
2.2. Drugs and Solutions
Nicotine hydrogen tartrate salt was purchased from Sigma-Aldrich (St. Louis, MO) and mixed in sterile saline, the pH was adjusted to 7.0 (±0.2) with a dilute NaOH solution. Nicotine dose (0.4 mg/kg) was calculated from the freebase form and the solution was injected subcutaneously 15 min before testing sessions unless otherwise noted. SCH-23390, (−)-eticlopride hydrochloride, and flupenthixol dihydrochloride were purchased from R&D Systems (Minneapolis, MN) and mixed in sterile saline. All DA antagonists were injected into the intraperitoneal cavity (ip) 30 min before test sessions. Powdered Nesquick® (chocolate) was purchased from a local market and dissolved in tap water at a concentration of 5% (w/v).
2.3. Apparatus
Ten standard modular operant chambers housed in sound attenuating cubicles were used in this experiment. The chambers, cubicles, interfacing and software were purchased from Med Associates (St Albans, VT). Each chamber had two walls fitted with three modular panels for intelligence devices. One of the walls was fitted with two receptacles equipped with LED panel lamps and infrared head-entry detectors, a liquid well and an 18 g pipe for fluid delivery. In Experiment 1, fluid was delivered to the US receptacle via syringe pump (Razel Scientific, St. Albans, VT) with a 10 RPM motor, and the syringe was fitted with a blunted 18 g needle and connected to the US receptacle with Tygon chemical resistant microbore tubing (10.16 mm, ID). The syringe pump was programmed to deliver 0.1 ml of the Nesquick® solution for each US presentation. The receptacles were located on the left and center panels of the wall. Because of the size of the head-entry detector units, the height of each receptacle had to be offset – the left receptacle was mounted slightly lower, with the bottom edge approximately 1.5 cm above the floor of the chamber, the right receptacle was higher, with the bottom edge located 6 cm above the floor. The light stimuli were Dialight LED panel lamps (white, 20 mA, 100 foot-lambert luminous intensity) purchased from Newark-Element 14 (Newark, NJ). The opposite wall was fitted with a liquid dipper and head entry receptacle (center panel), two levers and stimulus lights located above each lever (left and right panels). The lever in the right panel was fitted with a wire that was attached to a contact lickometer and could record lever contacts. For rats in Experiment 1, the preceding study included lever-press operants and sucrose delivered via the liquid dipper on this wall. For rats in Experiment 2, the lever mounted in the right panel and and the stimulus light directly above it served as the CS.
Each operant test chamber was fitted with a network surveillance camera (TV-IP572PI, TrendNet, Torrance, CA) that was mounted to the ceiling of the sound attenuating cubicle and attached to a local router for data collection. The cameras were equipped with near-infrared emitters for low-light recordings. Video recordings from CS and pre-CS periods were triggered by a background procedure written in MED-PC software (Med Associates) and a local application written by the first author. The background procedure and software initiated a recording from each network camera by creating an instance of VLC Media Player (VideoLAN, Paris, France) which was terminated after 30 s. The recording from each chamber was appended to one of two video files (pre-CS or CS) compiled throughout the session for each rat. Each video file contained a 10 min compilation of the pre-CS or CS periods from an entire session. To conserve disk space, video was only recorded during DA antagonist test sessions and preceding/intervening placebo ‘washout’ tests. Video files were stored on a local server for later analysis with AnyMaze video tracking software (Harvard Apparatus, Holliston, MA). Background procedures and locally created software are available upon request from the first author. VLC Media Player is free, open source software (www.videolan.org).
2.4. Procedure
2.4.1 Experiment 1
To reduce the possibility that prior NIC exposure might alter goal or sign tracking in our control group, the rats with prior exposure to nicotine were assigned to the NIC group and received NIC pretreatments 15 min before test sessions. Rats in the SAL group received placebo injections 15 min before test sessions. Our previous experiments (Palmatier et al., 2012a) found that prior NIC exposure is not needed to obtain the increases in sign tracking observed in the present studies. Sessions were conducted on consecutive days (7 days/week). Each session was 60 min long with 20 trials randomly distributed throughout each session. For each trial, the CS was illuminated for 30 s and the US was delivered immediately after CS presentation. Conditioning proceeded as previously described (Palmatier et al., 2012a); initially both the CS and US were delivered in the left (lower) receptacle. After robust conditioned approach was established (10 sessions), the US was shifted to the right (upper) receptacle, however, CS presentations remained in the left receptacle. Antagonist tests were carried out after stable approach was established with CS and US presentations in separate receptacles (less than 30% variance in average approach to US receptacle in both groups). Rats were tested with SCH-23390, then eticlopride, then flupenthixol in the order listed. Dose order was randomized and one ‘washout’ session was given between each antagonist test. Before all non-drug sessions, placebo (saline) was administered ip 30 min before session onset.
2.4.2 Experiment 2
To confirm the findings of Experiment 1, naïve rats received a more traditional sign tracking/goal tracking procedure in which insertion of a lever served as the CS. Rats were randomly assigned to one of two groups (PRE or POST, n=8 per group). For these rats, nicotine pretreatment began the day before acquisition started (Day 0) and continued throughout testing. During testing, all rats received two injections – one injection 15 min before testing sessions and one injection in the home cage at least one hour after testing sessions were completed. For the PRE group, 0.4 mg/kg nicotine injections were administered 15 min before testing and placebo was administered in the home cage. This order was reversed in the POST group. This procedure was used to investigate whether exposure to nicotine outside of the conditioning context (home cage, POST group) promoted sign-tracking in a manner that was comparable to acute nicotine pretreatment (conditioning apparatus, PRE group). Before conditioning began, all rats were trained to access 20% sucrose from a liquid dipper within 5 s in two consecutive sessions, nicotine (PRE) or saline (POST) injections were administered before these sessions; 1 h after these sessions rats received the reverse injection. Conditioning was comparable to Experiment 1 in that each session was 60 min, CS presentations were 30 s in duration, and there were 20 pairings of the CS and US randomly distributed during the session. However, in Experiment 2 insertion of the left lever and illumination of the stimulus light above the lever served as the CS and the US (20% sucrose, w/v) was presented in the liquid dipper for 5 s. After the 21 acquisition sessions, post-session injections were discontinued and all rats received only their assigned pre-session injection – 0.4 mg/kg NIC for the PRE group and 0.9% saline for the POST group. Conditioning continued for 5 more sessions to habituate rats to the new procedures and to reduce the likelihood that post-session nicotine injections would influence DA antagonist tests. Rats were then tested with SCH-23390 (0.05 mg/kg) or eticlopride (0.1 mg/kg) in randomized order, followed by a combination of both antagonists (0.025 mg/kg SCH-23390 + 0.05 mg/kg eticlopride). Dose selection was based on the findings of Experiment 1; two consecutive saline ‘washout’ tests were included between each antagonist test.
2.5. Data Analyses
2.5.1. Elevation and Duration Scores – Experiment 1
Conditioned approach behavior was quantified using the elevation score, which has been described previously (Brooks, Besheer et al., 2004, Palmatier et al., 2004). The elevation score is a difference score calculated by subtracting receptacle entries that occur during the pre-CS period (30 s before the CS) from receptacle entries that occur during the CS; it quantifies to what degree receptacle entries during CS presentation are elevated from baseline levels. This measure has the principal advantage of being continuous, so changes in receptacle entries during the CS are easily detected compared to ratio or probabilistic transformations in which scaling can obscure changes in behavior. Inclusion of pre-CS baseline in this measure also accounts for differences in overall activity, making it a specific measure of conditioned approach. To complement the elevation score, the duration of receptacle entries during each CS and pre-CS was also measured. This temporal measure was converted to a ‘duration score’, which was calculated in the same way as the elevation score and quantifies how much time spent in a receptacle during CS presentation is elevated from baseline levels.
2.5.2. Lever contacts and Receptacle Entries – Experiment 2
Because the lever is not presented during the pre-CS period, approach to the CS in Experiment 2 was quantified as lever contacts recorded by the contact lickometer during CS presentations and served as the principal measure of sign tracking. In order to make the measures of conditioned approach comparable in Experiment 2, US entries (during CS presentation) was the principal measure of goal tracking.
2.5.2. Video Tracking
Video was recorded during the pre-CS and CS intervals. Tracking of each rat was conducted by dividing the chamber image into 5-cm2 grids. A ‘CS zone’ was operationally defined as a 15 cm × 10 cm grid in front of the left receptacle. A ‘US zone’ the same size was defined in front of the right receptacle. Because of slight differences in the height of the CS and US, these zones included the visible openings of each receptacle and wall space above/below each receptacle; therefore they included floor space which extended approximately 12.2 cm away from the wall containing both receptacles. The rest of the chamber (not included in the CS and US zones) was considered the ‘neutral zone’ and was used to quantify general activity. Measures of activity included distance traveled (m) and frequency of grid-line crosses; these measures were based on the center mass of the subject. Measures of conditioned approach included time spent in each zone and frequency of zone entries; these were based on the location of the subject’s head in the test chamber.
2.5.3. Statistical Tests
Multivariate analyses of variance (MANOVAs) were used to determine statistical reliability of the results because our principal dependent measures (sign tracking and goal tracking) are related. Each approach measure (goal tracking, sign tracking) was treated as an outcome variable in the analyses and both were included in the Omnibus model. Univariate tests included in the model were used to parse which outcome(s) contributed to significant main effects and interactions. Where appropriate, Session or Dose (DA antagonist dose) served as within-subjects factors/repeated measures, and Drug (e.g., NIC vs. SAL) was a between-subjects factor. For antagonist tests, a profile analysis of the within-subjects factor (Dose) was performed with second-order, simple-effects tests for NIC and SAL rats comparing the 0 dose (baseline) to sign and goal tracking at each of the antagonist test doses. For the MANOVAs, Pillai’s trace was used for all significance tests because it provides the most protection against Type I error with small sample sizes. SPSS statistical software (IBM, New York) was used to perform significance tests, and significance was set at an alpha of p≤0.05. For follow-up pairwise contrasts on antagonist tests, Bonferroni’s method was used to correct for alpha inflation on each measure (sign or goal tracking), and the critical alpha was set at p≤0.017 (Experiment 1) or p≤0.05 (Experiment 2).
3. Results
3.1 Experiment 1: Receptacle Entry
3.1.1. Acquisition
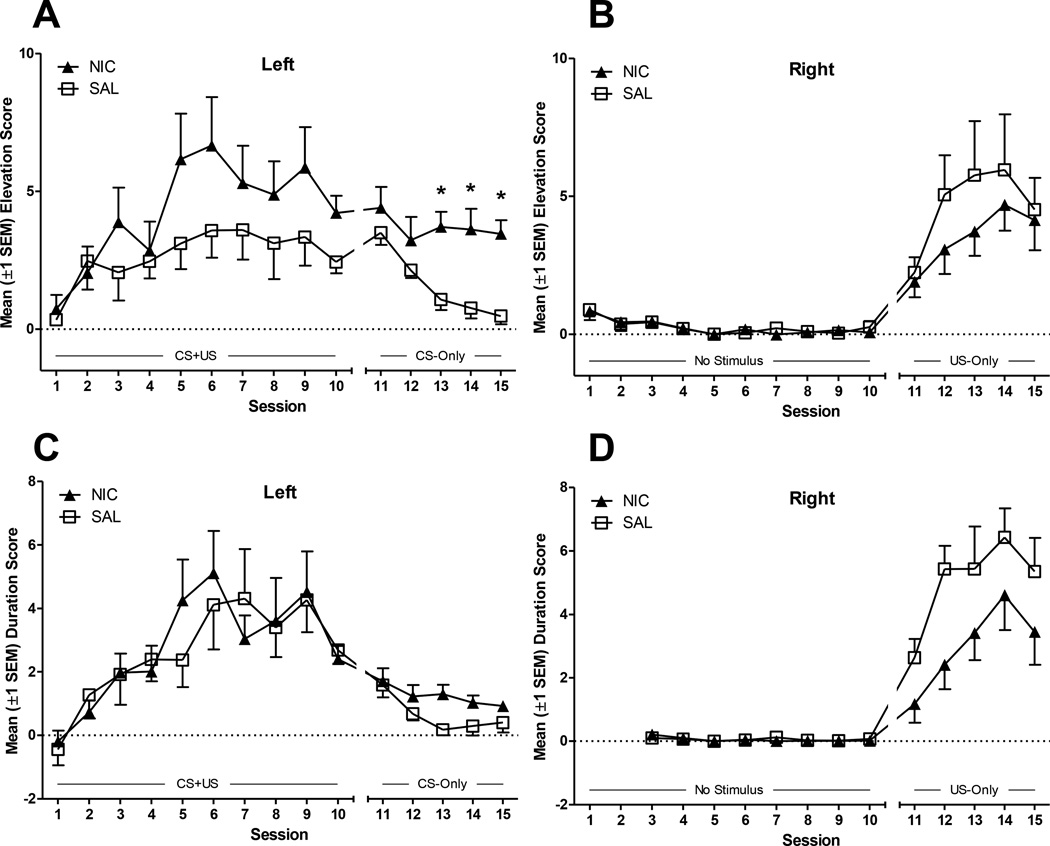
During the first 10 sessions, all rats learned to approach the left receptacle during the CS (Fig. 1). The MANOVA on elevation scores (Fig. 1A) confirmed that there was a significant main effect of Session [F(18,144)=4.4, p<0.001]. However, the Drug × Session interaction was not significant (F<1). An identical pattern emerged for duration scores (Fig. 1C), with significant main effect of Session [F(14,112)=2.6, p<0.01] but no interaction (F<1). Due to a programming error, duration scores were not calculated for the right receptacle during the first two acquisition sessions, so these sessions were eliminated from analyses.
Figure 1.
Panels A and B illustrate elevation scores (the frequency of receptacle entries during the CS minus the frequency of receptacle entries during the Pre-CS period) over acquisition tests in the receptacles on left (A) and right (B) side of the chamber. Panels C and D illustrate duration scores (duration of entries during the CS minus duration of entries during the PreCS) for the same receptacles and sessions. During the first 10 sessions, both the CS and US were presented in the receptacle on the left side of the chamber and all rats learned to approach the CS, as illustrated by elevation (A) and duration (C) scores above 0. For sessions 11–15, the US was presented in the receptacle on the right side; while all rats learned to approach this receptacle during CS presentations (B, D), rats pretreated with nicotine (NIC group) made more approach responses in the CS receptacle (Left, A). *Indicates a significantly higher elevation score for NIC group relative to SAL group, p<0.01.
Shifting the US to the right receptacle resulted in a shift in conditioned approach by both groups (Fig 1); however, the NIC group maintained high levels of approach to the left (CS) receptacle, whereas the SAL group reduced approach to the CS during this phase. This was confirmed by MANOVAs on elevation scores including Session (11–15) as a within-subjects factor and Drug as a between-subjects factor and side (left-CS, right-US) as outcome variables. For elevation scores (Fig 1A-B), there was a significant main effect of Session and a Side × Session interaction [Fs(8,64)≥2.42, p≤0.02]. Second-order, simple effects contrasting Drug on each dependent variable across trials were used for follow-up analyses. For goal tracking (elevation scores from the right-US receptacle), these contrasts were not significant (ps≥0.27). However, for sign tracking (elevation scores from the left-CS receptacle) there were significant differences between NIC and SAL rats on Sessions 13–15 (ps<0.01). For duration scores, this pattern was not replicated, probably due to the reduction in time spent in the left receptacle and increase in time spent in the right receptacle that occurred after the goal switch (Fig. 1C-1D). MANOVA revealed a significant main effect of Session [F(8,64)=3.21, p=0.004], but the Session × Drug interaction was not significant (p=0.23). On each measure, there was a significant main effect of Drug [Fs(1,8)≥5.4, ps<0.05], suggesting that NIC tended to increase time spent in the left (sign) receptacle (Fig. 1C) and reduce time spent in the right (goal) receptacle (Fig. 1D) during these sessions.
3.1.2. SCH-23390
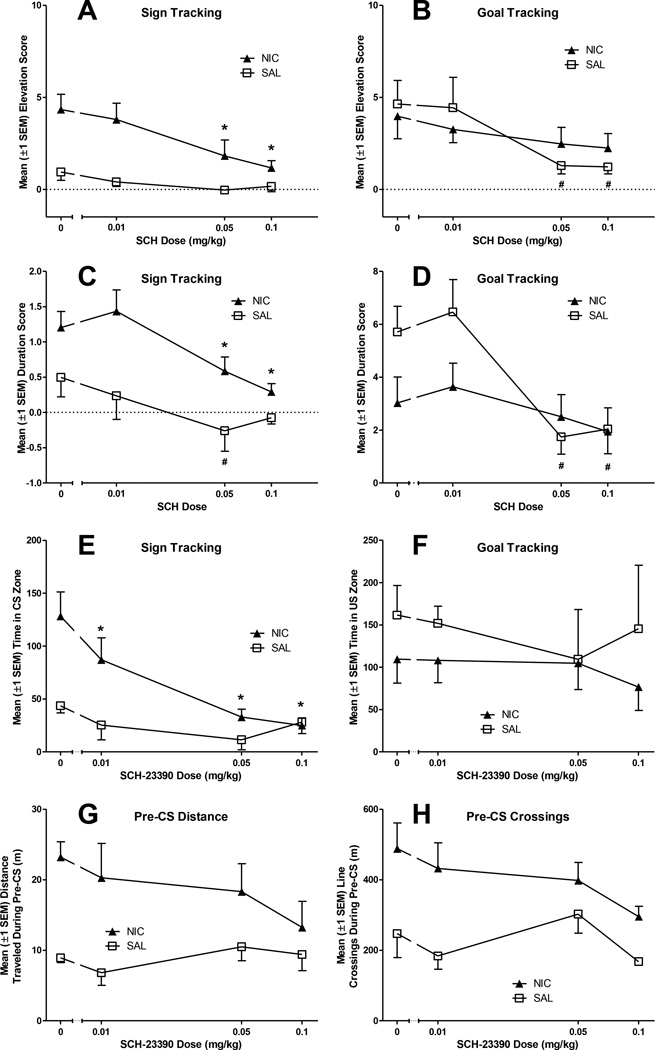
The DAD1 antagonist SCH-23390 reduced conditioned behaviors in all rats, but specifically goal tracking in the SAL group and sign tracking in the NIC group (Fig 2A-B). The MANOVA confirmed a significant main effect of Dose (SCH dose) as well as a significant Dose × Drug interaction [Fs(6,48)≥3.4, ps≤0.02] on elevation scores for receptacle entries. Univariate tests within the model confirmed that SCH-23390 reduced both goal- and sign-tracking responses (ps<0.01). For NIC rats, the 0.05 and 0.1 mg/kg SCH-23390 doses significantly reduced sign-tracking entries (ps<0.001), but SCH-23390 did not alter goal tracking entries at any dose for these rats (ps≥0.17). In contrast, for the SAL rats the 0.05 and 0.1 mg/kg SCH-23390 doses reduced goal tracking entries (ps<0.017) but did not alter sign tracking entries (ps≥0.1).
Figure 2.
SCH-23390 reduced goal-tracking in rats pretreated with saline (SAL group) and reduced sign-tracking in rats pretreated with nicotine (NIC group). Panels A and B illustrate elevation scores (the frequency of receptacle entries during the CS minus the frequency of receptacle entries during the Pre-CS period) as a function of SCH-23390 dose. Panels C and D illustrate duration scores (duration of entries during CS-PreCS) across SCH-23390 doses. The abscissa displays dose on a log scale. SCH-23390 reduced time spent in CS zone (G) only in rats pretreated with nicotine, without altering other measures of conditioned approach or locomotion. Panels E and F illustrate the effects of SCH-23390 on locomotion; distance traveled (E) and line crossings (F) during Pre-CS intervals served as general measures of activity. Panels G and H illustrate the effects of SCH-23390 on additional measures of conditioned approach behavior: time spent in CS (G) and US (H) zones during the CS intervals. The abscissa displays dose on a log scale. *Indicates that SCH-23390 significantly reduced behavior in NIC group, relative to placebo (0mg/kg), p<0.017. #Indicates a significant reduction in conditioned approach for SAL rats, relative to placebo (0mg/kg SCH-23390), p<0.017.
For duration scores, there was a similar pattern (Fig 2C-D), with a significant main effect of Dose and a significant Dose × Drug interaction [Fs(6,48)≥3.6, ps<0.01]. Univariate tests included in the model confirmed that SCH-23390 reduced the duration of both goal and sign tracking responses (ps<0.01). Second-order simple contrasts confirmed that SCH-23390 pretreatment (0.05 and 0.1 mg/kg) caused a significant reduction in duration scores for sign tracking in NIC rats at both doses (ps<0.017), but did not alter goal tracking (ps≥0.17). SCH-23390 (0.05 and 0.1 mg/kg) significantly reduced duration scores in the goal receptacle (ps<0.017), relative to the 0 mg/kg baseline for SAL rats. There was also a significant decrease in duration scores in the sign receptacle for these rats, but only after pretreatment with 0.05 mg/kg SCH-23390 (p<0.01).
3.1.3. Video Tracking and Reliability
Three dependent measures (distance traveled during the Pre-CS, time spent in CS zone, and time spent in US zone) were automatically calculated by Any-Maze software. Because this measure was novel, reliability analyses were performed on the CS and US zone time measures from 15 randomly selected video samples. Manual video scoring was performed by a research assistant who was blind to the experimental conditions and data sampling. The analyses confirmed that Any-Maze video analysis was comparable to blind human observation for CS and US zone time (r=0.90) and CS and US zone entries (r=0.87). One of the video cameras malfunctioned during testing, so one rat from the SAL group was eliminated from these video tracking analyses (NIC: n=5, SAL: n=4).
Video - Approach during the CS
SCH-23390 did not reduce zone time in the goal location for any group at any dose. However, time spent in the CS zone was reduced at all SCH-23390 doses (0.01–0.1 mg/kg) for NIC rats, but not for SAL rats (Fig. 2E-F). There was a significant main effect of Dose, as well as a significant Drug × Dose interaction [Fs(6,42)≥2.5, ps≤0.04]. Univariate follow-ups confirmed that the main effect of Dose and the Drug × Dose interaction were both based on changes in the sign-tracking response [Fs(3,21)≥5.71, ps<0.01]. Second-order, simple contrasts confirmed that 0.01–0.1 mg/kg SCH-23390 reduced zone time in the CS location for NIC rats (ps≤0.01). SCH-23390 did not reduce zone time in the goal location for either group (ps≥0.28).
Video - Activity during the Pre-CS
To assess drug effects on general locomotor activity, we analyzed line crossings and distance traveled during the Pre-CS period. NIC pretreatment increased both measures of activity during the Pre-CS, but SCH-23390 did not reduce basal activity (SAL groups) or NIC-induced activity in this experiment (Fig. 2G-H). The MANOVA included both outcome variables (distance traveled and line crossings), as they are related measures of activity, and revealed a significant main effect of Drug [Fs(1,7)≥6.26, ps≤0.04], but no main effect of Dose nor a Dose × Drug interaction (ps≥0.14). For line crossings, MANOVA revealed significant main effects of Drug [F(1,7)=10.88, p=0.01] and Dose [F(3,21)=3.29, p=0.04]. Line crossings did not significantly differ from the 0 mg/kg dose after any other SCH-23390 dose (ps≥0.42).
3.1.4. Eticlopride
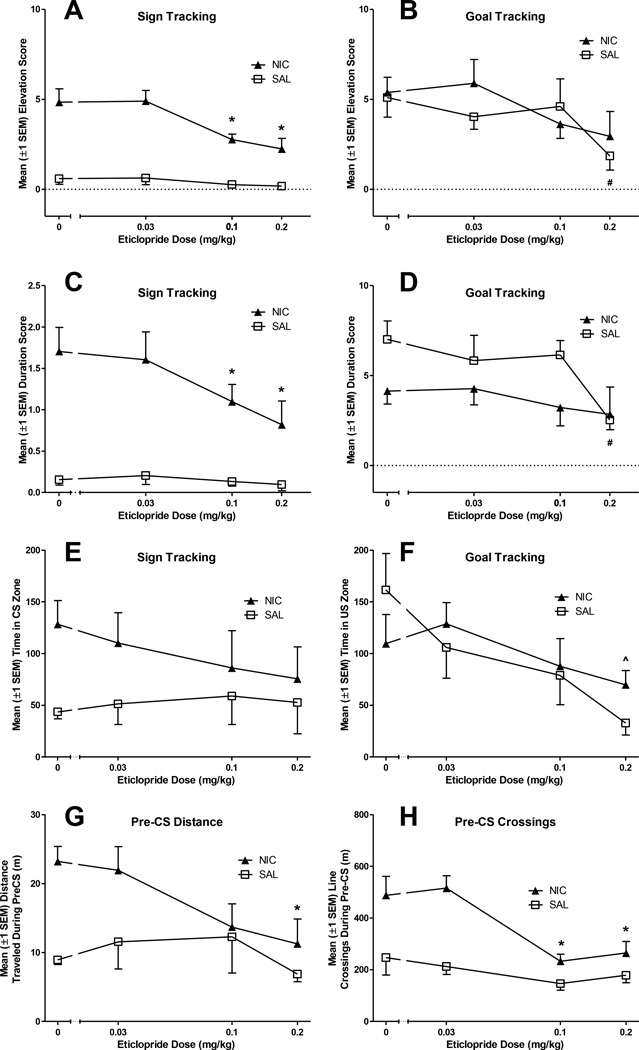
Similar to SCH-23390, the DAD2/3 antagonist eticlopride reduced goal tracking in the SAL group and sign tracking in the NIC group, but its effect was more potent in the NIC rats (Fig 3A-B). The MANOVA revealed a significant main effect of Dose and a significant Dose × Drug interaction [Fs(6,48)≥2.91, ps≤0.02]. Univariate tests conducted as part of the model revealed that eticlopride reduced approach to both the sign and goal (ps≤0.02). Second-order, simple contrasts confirmed that eticlopride (0.1–0.2 mg/kg) reduced sign-tracking (ps≤0.005) but not goal-tracking entries (ps≥0.02) in NIC rats. In SAL rats, the 0.2 mg/kg dose reduced goal-tracking entries (p=0.005), but none of the doses altered sign-tracking entries (ps≥0.49).
Figure 3.
Eticlopride reduced goal-tracking in rats pretreated with saline (SAL group) and reduced sign-tracking in rats pretreated with nicotine (NIC group). Panels A and B illustrate elevation scores (the frequency of receptacle entries during the CS minus the frequency of receptacle entries during the Pre-CS period) as a function of eticlopride dose. Panels C and D illustrate duration scores (duration of entries during CS-PreCS) across eticlopride doses. The abscissa displays dose on a log scale. Eticlopride dose-dependently reduced activity in rats pretreated with nicotine (NIC group), but did not reduce activity in rats pretreated with saline (SAL group). Eticlopride did not alter time spent in the CS zone but the highest eticlopride dose reduced time spent in the US zone in in both groups. Panels E and F illustrate the effects of eticlopride on additional measures of conditioned approach behavior; time spent in CS and US zones during the CS intervals (E and F, respectively). Panels G and H illustrate the effects of eticlopride on locomotion; distance traveled (G) and line crossings (H) during Pre-CS intervals served as general measures of activity. The abscissa displays dose on a log scale. ^Indicates eticlopride significantly reduced behavior in NIC and SAL groups, relative to placebo (0mg/kg), p<0.017. *Indicates that eticlopride significantly reduced behavior in NIC group, relative to placebo (0mg/kg), p<0.017. #Indicates a significant reduction in conditioned approach for SAL rats, relative to placebo (0mg/kg eticlopride), p<0.017.
A comparable effect was observed for duration scores, as the highest dose of eticlopride (0.2 mg/kg) reduced goal tracking in the SAL group and the two highest doses (0.1–0.2 mg/kg) reduced sign tracking in the NIC group (Fig 3C-D). MANOVA revealed a significant main effect of Dose and a significant Dose × Drug interaction [Fs(6,48)≥3.99, ps≤0.003]. Second-order simple contrasts confirmed that the 0.2 mg/kg dose of eticlopride significantly reduced goal tracking in SAL rats (p=0.005) without affecting sign-tracking duration, and that the 0.1 and 0.2 mg/kg eticlopride doses reduced sign tracking in the NIC rats (ps≤0.001) without affecting goal-tracking duration.
Video – Approach during the CS
Eticlopride (0.2 mg/kg) reduced time spent in the goal zone for both NIC and SAL rats, but did not alter time spent in the CS zone for either group (Fig. 3E-F). The omnibus MANOVA revealed only a significant main effect of Dose [F(6,42)=2.4, p=0.044]. Follow-ups confirmed that this was based on a significant reduction in goal zone time for rats pretreated with the highest eticlopride dose (p<0.01).
Video - Activity during the Pre-CS
Eticlopride did not reduce activity in SAL rats and only reduced NIC-induced activity at the highest doses (Fig 3G-H). MANOVA on locomotion revealed a significant main effect of Dose [F(6,42)=5.56, p<0.001] as well as a significant Dose × Drug interaction [F(6,42)=2.98, p=0.015]. Second-order contrasts revealed that no dose of eticlopride altered activity in SAL rats (ps≥0.14). However, for NIC rats, the 0.1 and 0.2 mg/kg eticlopride doses significantly reduced line crossings (ps<0.017), but only the 0.2 mg/kg dose significantly reduced distance traveled in NIC rats (p=0.01).
3.1.5. Flupenthixol
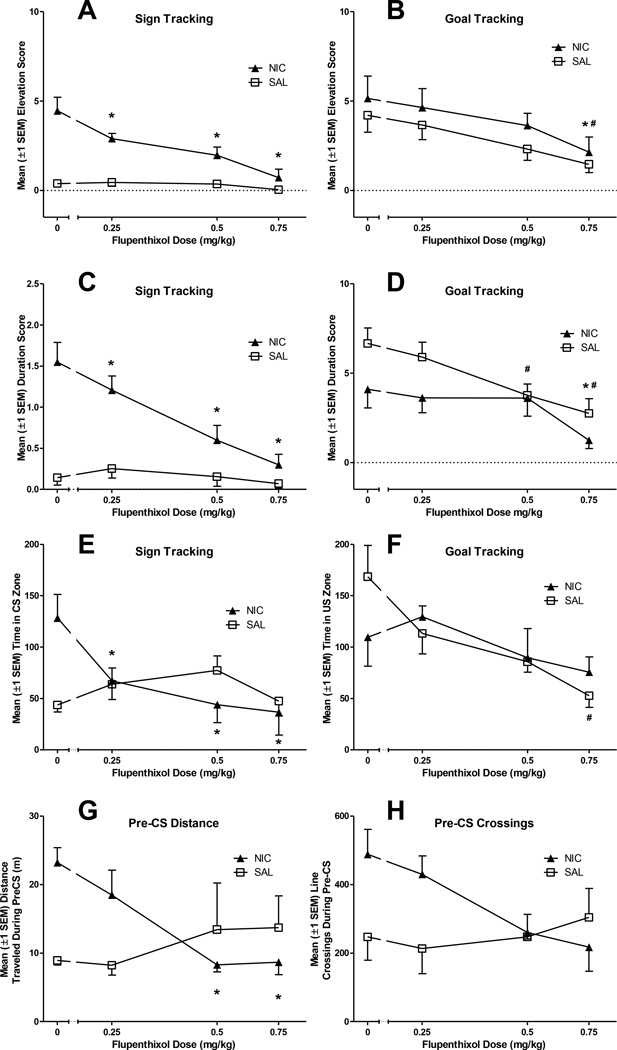
The highest dose of the non-selective DA antagonist flupenthixol reduced goal tracking in both NIC and SAL groups. However, all three doses of flupenthixol reduced sign-tracking in only NIC rats (Fig 4A-B). The MANOVA on elevation scores confirmed a significant main effect of Dose and a significant Dose × Drug interaction [Fs(6,48)≥4.02, ps≤0.002], and univariate tests included in the model confirmed that flupenthixol reduced both sign- and goal-tracking entries (ps<0.001). Second-order, simple contrasts confirmed that the 0.75 mg/kg dose of flupenthixol significantly reduced goal-tracking entries in both NIC and SAL rats (ps≤0.01). However, all three flupenthixol doses (0.03–0.75 mg/kg) significantly reduced sign-tracking entries in the NIC rats (ps≤0.002) with no effect on SAL rats.
Figure 4.
Flupenthixol (FLU) reduced goal-tracking in all rats, but only reduced sign-tracking in rats pretreated with nicotine (NIC group). Panels A and B illustrate elevation scores (the frequency of receptacle entries during the CS minus the frequency of receptacle entries during the Pre-CS period) as a function of FLU dose. Panels C and D illustrate duration scores (duration of entries during CS-PreCS) across FLU doses. The abscissa displays dose on a log scale. Flupenthixol (FLU) significantly reduced distance traveled in rats pretreated with nicotine (NIC group), but did not significantly reduce line crossings in either group. All doses of FLU reduced time spent in the CS zone in NIC rats, while the highest dose reduces time spent in the US zone in SAL rats. Panels E and F illustrate the effects of FLU on additional measures of conditioned approach behavior: time spent in CS and US zones during the CS intervals (E and F, respectively). Panels G and H illustrate the effects of FLU on locomotion; distance traveled (G) and line crossings (H) during Pre-CS intervals served as general measures of activity. The abscissa displays dose on a log scale. *Indicates that FLU significantly reduced behavior in NIC group, relative to placebo (0mg/kg), p<0.017. #Indicates that FLU significantly reduced behavior in SAL group, relative to placebo (0mg/kg), p<0.017.
A similar pattern was observed for duration scores; however, this measure was more sensitive to changes in goal tracking induced by flupenthixol (Fig 4C-D). The MANOVA on duration of receptacle entries revealed a significant main effect of Dose and a significant Dose × Drug interaction [Fs(6,48)≥5.3, ps<0.001], and univariate tests included in the model confirmed that flupenthixol decreased the duration of both the sign- and goal-receptacle entries during the CS (ps<0.001). Follow-up comparisons confirmed that for SAL rats, 0.5 and 0.75 mg/kg flupenthixol significantly reduced duration scores in the goal location (ps≤0.002), while the decrease in NIC rats did not reach significance (ps≥0.02, critical alpha = 0.017). In contrast, all three doses (0.03–0.75 mg/kg) of flupenthixol significantly reduced duration scores in the sign location in NIC rats (ps≤0.01), but not SAL rats (ps≥0.03).
Video – Approach during the CS
Flupenthixol reduced approach to the sign at all doses for the NIC group, but did not alter approach to the sign in the SAL group. In contrast, flupenthixol only reduced goal tracking in SAL rats, and only at the highest dose tested (0.75 mg/kg; Fig. 4E-F). The omnibus MANOVA revealed a significant main effect of Dose, as well as a significant Drug × Dose interaction [Fs(6,42)≥2.74, ps≤0.024]. Second-order contrasts confirmed that all flupenthixol doses (0.25–0.75 mg/kg) reduced CS zone time for NIC rats (ps≤0.01); however, flupenthixol did not alter US zone time for this group (ps ≥0.36). For SAL rats, flupenthixol significantly reduced US zone time (0.75 mg/kg, p<0.017) but not CS zone time (ps≥0.08).
Video – Activity during the Pre-CS
Flupenthixol did not reduce activity in SAL rats and only reduced NIC-induced activity at the highest doses (Fig 4G-H). MANOVA revealed a significant Dose × Drug interaction [F(6,42)=2.6, p=0.03]. Second-order contrasts confirmed that flupenthixol did not reduce activity in SAL rats at any dose (ps≥0.25). For NIC rats, the two highest doses of flupenthixol (0.5–0.75 mg/kg) reduced distance traveled (ps<0.017), but none of the flupenthixol doses significantly reduced line-crossings in this group (p≥0.033, critical alpha = 0.017).
3.3. Experiment 2: Lever Contacts and Receptacle Entries
3.3.1. Acquisition
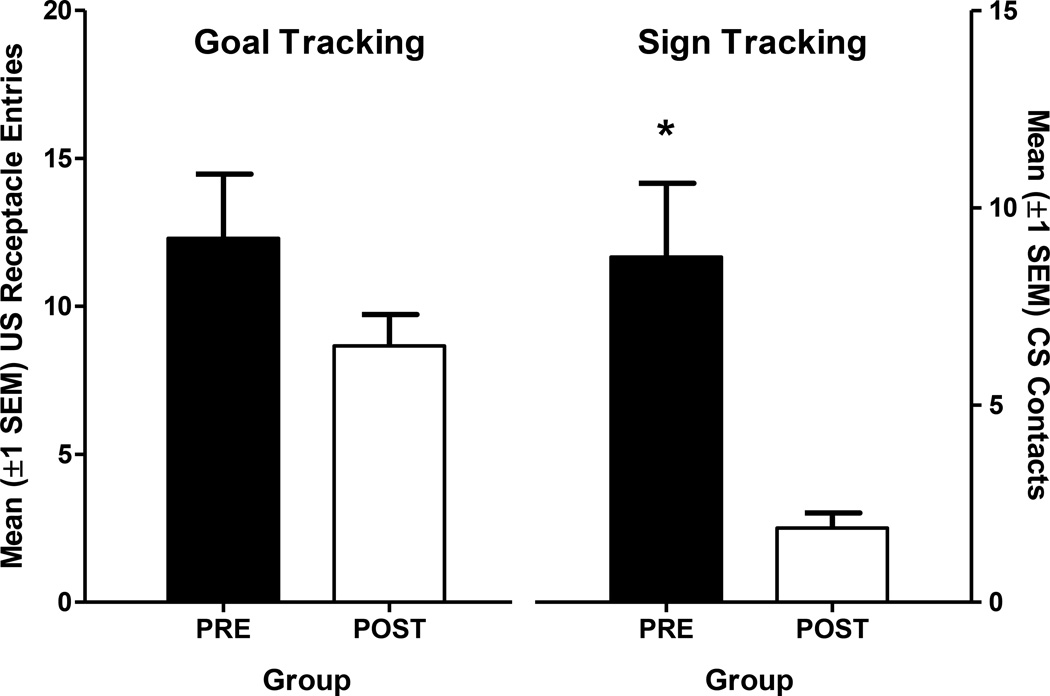
At the end of acquisition testing there were no significant differences in goal tracking but the PRE group approached the lever CS significantly more than the POST group (Fig. 5). This was confirmed by MANOVA including both lever contacts (sign tracking) and receptacle entries (goal tracking) as response variables. There was a significant effect of Group [F(2,13)=21.2, p<0.001] and univariate tests included in the model confirmed that this effect was based on a significant difference in lever contacts [F(1,14)=12.9, p<0.01] but not receptacle entries (p=0.16).
Figure 5.
In Experiment 2 pretreatment with nicotine increased approach to the lever-CS, but did not alter approach to the US receptacle. Receptacle entries during CS presentation are plotted on the left ordinate, lever contacts during CS presentation are plotted on the right ordinate. The bars represent the average number of approach responses to each location for each rat on the final three acquisition sessions (19–21). *indicates significantly more lever contacts for the PRE group, relative to the POST group, p<0.05.
3.3.2. SCH-23390
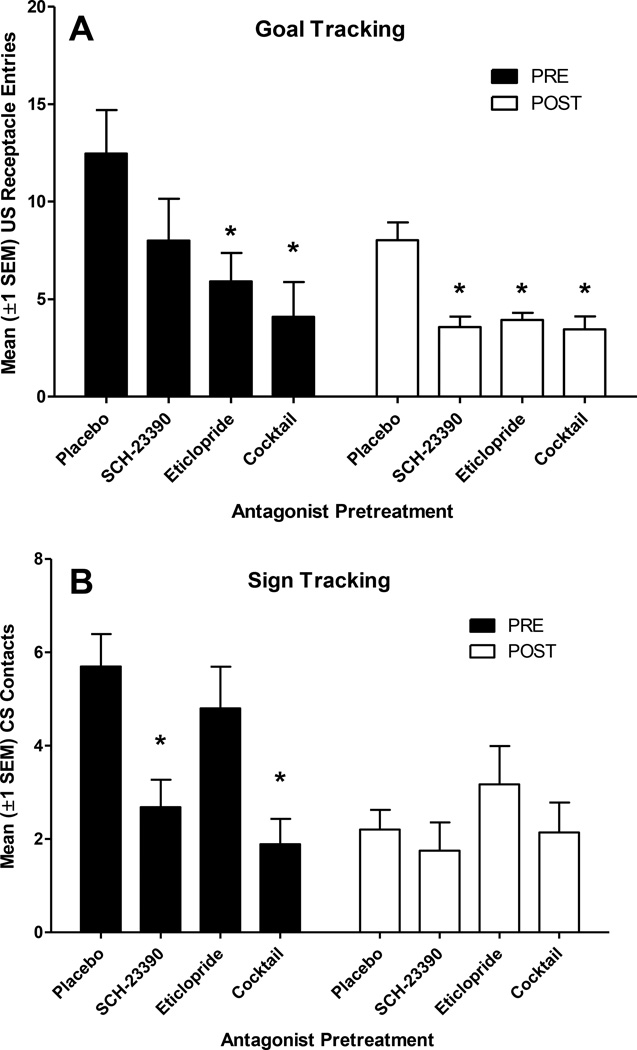
SCH-23390 (0.05 mg/kg) reduced goal tracking (Fig. 6a) and sign tracking (Figure 6b) in rats pretreated with nicotine (PRE group) and significantly reduced goal tracking (Fig. 6a) in rats pretreated with saline (POST group). MANOVA revealed significant main effects of Group and Dose, as well as a significant Group × Dose interaction [Fs(2,13)≥4.8, ps≤0.027]. Univariate follow-ups confirmed that the main effect of SCH-23390 pretreatment was based on reductions of both lever contacts and receptacle entries [Fs(1,14)≥9.3, ps<0.01]. The Drug × Dose interaction was based primarily on reductions in lever-contacts in the PRE group [F(1,14)=8.6, p=0.011]. Second-order, simple contrasts confirmed that 0.05 mg/kg SCH-23390 reduced receptacle entries for POST rats (p<0.05) but not PRE rats (p=0.14) and reduced lever contacts for PRE rats (p<0.001), but not POST rats (p=0.47).
Figure 6.
In Experiment 2, the selective DA D1 antagonist SCH-23390 (0.05 mg/kg) reduced goal tracking in the POST group (Panel A) and sign-tracking in the PRE group (Panel B). The DA D2/3 antagonist eticlopride (0.1 mg/kg) reduced goal tracking in both the PRE and POST groups (Panel A), but had no effect on sign tracking. The combination of both SCH-23390 (0.025 mg/kg) and eticlopride (0.05 mg/kg) had summative effects on approach – goal tracking was reduced in both groups and sign tracking was only reduced in the PRE group. *indicates significantly fewer approach responses after antagonist pretreatment, relative to placebo control pretreatment, p<0.05.
3.3.3. Eticlopride
Eticlopride (0.1 mg/kg) reduced goal tracking in both groups (Fig. 6a) but did not significantly alter sign-tracking (Fig. 6b). MANOVA revealed significant main effects of Group and Dose [Fs(2,13)≥11.9, ps≤0.01], but the Group × Dose interaction did not reach statistical significance (p=0.63). Univariate follow-ups confirmed that the main effect of eticlopride pretreatment (Dose) was based on reductions of receptacle entries [F(1,14)=24.8, p<0.001] but not changes in lever contacts (F<1). Second-order, simple contrasts confirmed that 0.1 mg/kg eticlopride reduced receptacle entries for both PRE and POST groups (ps≤0.002).
3.3.4. SCH-23390 and Eticlopride Cocktail
The mixture of SCH-23390 (0.025 mg/kg) and eticlopride (0.05 mg/kg) reduced goal tracking in both groups (Fig. 6a) but only reduced sign-tracking in rats that were pretreated with nicotine (PRE group, Fig. 6b). MANOVA revealed significant main effects of Group and Dose as well as a significant Group × Dose interaction [Fs(2,13)≥5.5, ps≤0.02]. Univariate follow-ups confirmed that the Group × Dose interaction was based on reductions in lever contacts [F(1,14)=16.4, p<0.001] but not changes in receptacle entries (p=0.23). Second-order, simple contrasts confirmed that the SCH-23390 and eticlopride cocktail reduced receptacle entries for both PRE and POST groups (ps≤0.014), whereas lever contacts were only reduced in the PRE group (p<0.001).
4. Discussion
The present studies confirm our previous findings (Palmatier et al., 2012a) that nicotine promotes approach to a CS (sign tracking) which is spatially separated from the US, but has only marginal effects on approach to the US (goal tracking). These studies also implicate dopaminergic systems in the incentive-promoting effects of nicotine; systemic pretreatments with both selective and non-selective DA receptor antagonists reduced the facilitation of sign-tracking engendered by nicotine. Surprisingly, the antagonists selectively reduced sign-tracking behaviors in nicotine pretreated rats but reduced goal-tracking behaviors in saline pretreated rats. The findings of Experiment 2 add a second confirmation (see Palmatier et al., 2012a) that the facilitation of sign-tracking by nicotine does not depend on acquisition procedures (e.g., shifting the location of the US). In addition, Experiment 2 demonstrates that nicotine-induced increases in sign-tracking are observed using the more traditional lever-CS paradigm. An important additional component of these studies was the measurement of locomotion within the chamber by using video recordings, and the distance and line-crossing data confirmed that the DA antagonists had marginal effects on basal activity in the operant test chambers (SAL groups). Although eticlopride and flupenthixol both reduced measures of nicotine-induced hyperactivity at the highest doses tested, these reductions were not always associated with reductions in conditioned approach. Thus, the reductions in incentive motivation observed in the present study cannot be explained by simple motor suppressant effects of the antagonists. These experiments are the first to investigate the effects of dopaminergic drugs on the facilitation of sign-tracking engendered by nicotine.
The present studies confirm that the dopamine system is critically involved in the increased sign tracking observed in rats pretreated with nicotine. Several previous studies have implicated mesotelencephalic DA systems in behavioral responses to nicotine including primary reinforcement (Corrigall et al., 1992, Corrigall et al., 1994), locomotor sensitization (Palmatier and Bevins, 2002, Sheppard et al., 2009), and conditioned place-preference (Acquas et al., 1989). DA antagonists also reduce effects of nicotine that may be related to its incentive-promoting effects. For example, the ability of nicotine to reduce current threshold on intracranial self-stimulation is blocked by the non-selective DA antagonist haloperidol (Ivanova and Greenshaw, 1997). Surprisingly, very little attention has been paid to the role of dopamine systems in the ‘reinforcement-enhancing’ or incentive-amplifying effects of nicotine (Olausson et al., 2004a, b, Liu et al., 2007a, Liu et al., 2007b, Palmatier et al., 2008). Among the many reasons that researchers have avoided investigating dopaminergic drugs in operant tasks is the fact that DA systems are critically involved in primary reinforcement by multiple rewards including food (Sharf et al., 2005), brain stimulation (Phillips and Fibiger, 1979) and access to a receptive mate (Pfaus and Phillips, 1991). Moreover, DA antagonists can impair motor function when they are administered systemically (Hoffman and Beninger, 1985).
In the paradigm employed in Experiments 1–2, we were better equipped to describe the topography of conditioned approach responses – including both the frequency and duration of receptacle entries and using measures that take baseline levels of receptacle entry into account (the elevation and duration scores). In addition, the video-tracking system allowed us to examine gross changes in activity as well as approach to the receptacles that may have been below the threshold of the receptacle-entries (time spent in proximity to the CS and US receptacles). By including these measures, we could dissociate the effects of the DA antagonists on approach behavior from their effects on gross activity – confirming that D1, D2/3, and non-selective DA antagonists reduced the facilitation of sign tracking engendered by nicotine. In addition, an important dissociation between the receptacle entry and video-tracking findings emerged. Both D1 and D2/3 antagonists reduced entries into the sign receptacle for NIC pretreated rats and reduced entries into the goal receptacle for SAL pretreated rats. However, video-tracking data suggest that SCH-23390 preferentially reduced sign tracking in NIC rats (Figure 5A), whereas eticlopride only reduced goal tracking in SAL rats (Figure 6B). These observations suggest the possibility of a dissociation between DA receptor subtypes in some aspects of the topography of conditioned approach behaviors; however, additional data are needed for confirmation. One potential reason that the DA antagonists did not reduce sign tracking in saline-pretreated rats is a ‘floor effect’ – basal approach to the CS was so low it would be difficult to measure reductions. However, this conclusion may follow from presentation of the data, in which approach is averaged across all trials and not summed across the session. For example, basal approach to the CS in saline-pretreated rats included approximately 40 lever contacts per session in Experiment 2. Thus, a floor effect seems to be an unlikely account for the selective effects of the DA antagonists.
Sign-tracking is receiving increasing attention in pre-clinical models of substance use disorders because of its apparent critical association with incentive motivation and mesotelencephalic DA systems (Saunders and Robinson, 2013). Studies in awake, behaving rats suggest that phasic DA release is associated with the delivery of unexpected rewards (Mirenowicz and Schultz, 1996), reward-predictive CSs (Schultz, 1998b, a, Phillips et al., 2003, Roitman et al., 2004), and salient sensory stimuli (Robinson et al., 2002, Robinson and Wightman, 2004). However, the DA release that is time-locked to presentation of incentives (Day et al., 2007, Brown et al., 2011) and is robust in sign-tracking rats (Flagel et al., 2011) has been almost exclusively observed in the NAc core, which has been extensively linked to Pavlovian approach behavior (Parkinson et al., 1999, Di Ciano et al., 2001, Parkinson et al., 2002, Dalley et al., 2005). Systemic administration of flupenthixol blocked the acquisition of sign-tracking in rats that were selectively-bred for high responsiveness to novel environments (bHR rats) (Flagel et al., 2011), and local infusion of flupenthixol into the NAc core reduced the expression of sign tracking (Saunders and Robinson, 2012). Similarly, the present data found that systemic flupenthixol dose-dependently dampened the expression of sign-tracking in rats that showed NIC-enhanced sign-tracking behavior. Extending these results, SCH-23390 and eticlopride also dose-dependently reduced sign-tracking behavior in these rats, indicating that both D1- and D2-type receptor activation contribute to conditioned approach to the CS.
Although learning of cue-reward associations that lead to goal-tracking behavior is dopamine-independent, the expression of the goal-tracking response seems to be dopamine dependent. In the present study, flupenthixol, SCH-23390 and eticlopride reduced goal-tracking behavior in SAL rats, albeit with generally less potency than their effects on sign-tracking, indicating that both conditioned responses are sensitive to DA receptor blockade at doses that do not produce non-specific motor effects. These findings are consistent with Flagel and colleagues (2011), who investigated the effects of flupenthixol on the acquisition vs. expression of Pavlovian conditioned approach in bHR (sign-tracking) or bLR (goal-tracking) rats. Goal tracking was reduced by pretreatment with flupenthixol during acquisition in bLR rats. However, in a subsequent ‘drug-free’ expression test goal tracking recovered to control levels. This finding demonstrates that global antagonism of DA receptors disrupts expression of the conditioned response, without altering acquisition of the associations that lead to the conditioned response. The site of DA action in goal tracking may be specific; when flupenthixol was infused directly into the NAc core, only minimal reductions in approach to the goal were observed in bLR rats (Saunders and Robinson, 2012). Finally, the necessity of DA signaling may differ in the acquisition of sign- versus goal-tracking behavior. Based on the above and current studies, we can conclude that while DA signaling contributes to the expression of both sign-tracking and goal-tracking approach, different neural learning circuits support their acquisition and different cortico-striatal pathways support their expression.
One of the difficulties in distinguishing the neural substrates of sign- and goal-tracking is that both behaviors involve approach to stimuli with incentive properties. The goal is a spatial stimulus with an ambiguous meaning – reward (US) is delivered only at that receptacle but infrequently; most of the time no reward is available in the goal location. The CS is a spatio-temporal stimulus with a discrete meaning – at the end of the CS, a reward is always delivered. The temporal properties of the CS may be critical to the development of sign tracking, as previous studies have shown that inserting a trace between the CS and US delivery reduces sign-tracking (Christian et al., 1983). Mesotelencephalic DA systems have frequently been implicated in timing behavior, as mice with deletions of the gene that encodes the DA transporter display poor temporal control of behavior in operant timing tasks (Meck et al., 2012) and the firing of DA neurons in the VTA shifts from ‘surprising’ rewards to CSs that temporally disambiguate reward delivery (Schultz et al., 1997, Schultz, 1998b, a). Thus, an important feature of the sign-tracking approach response may be the discrete temporal nature of the CS and the responsiveness of incentive systems to temporal stimuli. For example, drugs that facilitate phasic DA release or mimic the effects of DA at post-synaptic receptors may increase attention to temporally discrete incentives. Although more research is needed to investigate this hypothesis, the temporal features of sign-stimuli have been basically ignored in the recent resurgence of sign-tracking studies (cf. Christian et al., 1983). Nevertheless, the goal-tracking response is also time-locked to the presentation of the CS, with the critical difference being the target of the conditioned approach. This may be why DA receptor antagonists diminished the expression of conditioned sign-tracking or goal tracking responses in the present experiment, depending on what the animal exhibited at baseline.
The procedures used in Experiment 1 diverge somewhat from the more traditional procedures currently used in sign tracking experiments that investigate ‘incentive salience’ (Saunders and Robinson, 2012, Anselme et al., 2013). The two principal differences are (1) use of spatially distinct receptacles as the sign and goal and (2) shifting of the US from the sign receptacle to the goal receptacle. The rationale for using two receptacles is to equate response form – a lever stimulus evokes grasping, manipulation, and biting behaviors (Tomie et al., 2008), whereas a receptacle evokes exploratory behavior (Holland, 1979) which includes sniffing and licking at the fluid well (Bueno and Moreira, 1998). Thus, comparisons of approach behaviors are more direct. Although some investigators have suggested that the difference in response forms may make the behaviors qualitatively distinct (Berridge et al., 2009), this assertion has never been empirically confirmed. In fact, evidence from sexual conditioning studies in male Japanese Quail suggests that when signs and goals are similar (e.g., species typical cues used as signs) the sign tracking CR is more robust and more resistant to extinction (Krause et al., 2003). The rationale for shifting the location of the goal was prompted by an insight from one of our previous studies (Palmatier et al., 2012a) in which the sign was originally presented in the same location as the goal. We realized that we had very little control over what is actually learned about the spatial cues (e.g., receptacle, light location, etc.). Lights presented in different spatial locations may be easily discriminated (e.g., Floresco et al., 2008); therefore we felt that the best way to ensure that the sign maintained its full status as an excitatory CS was by moving the goal location while leaving the sign stimuli unaltered – thus any configuration between the temporal features of the light illumination and spatial cues remained intact. Although it is tempting to consider that nicotine may have specifically altered extinction of goal tracking in Experiment 1, such an assertion is problematic for two reasons. First, ‘extinction’ refers to attenuation of a conditioned response that is observed when the CS is presented but the US is withheld. In Experiment 1, US presentation is not withheld and the CS is still a temporal predictor of the US, but is no longer an accurate spatial cue. Thus, one would have to assert that nicotine impaired extinction of spatially-specific conditioned approach responses. This seems an overly complex hypothesis, especially in light of the evidence from Experiment 2 (present studies) and our previous research (Palmatier et al., 2012a) which show that nicotine increases sign tracking when signs and goals are spatially separate from the start of acquisition. The extinction hypothesis is less parsimonious than the hypothesis that nicotine simply increased sign tracking.
In summary, the present studies confirm the role of DA in NIC-induced enhancement of sign tracking to a CS. Moreover, they establish that both D1- and D2-type receptor activation contributes to conditioned approach to the CS in sign-tracking rats and to the US location in goal-tracking rats, although these receptor effects may occur in different striatal subregions. These findings further our understanding of NIC augmentation of non-drug reinforcement that contributes to the neurobiological mechanism of nicotine addiction.
Highlights.
Nicotine increased approach to sucrose-paired conditioned stimuli (sign tracking).
Systemic administration of dopaminergic antagonists was investigated.
A dopamine D1 antagonist reduced facilitation of sign tracking by nicotine.
A dopamine D2/3 receptor selective antagonist reduced approach to the reward location.
The facilitation of sign tracking by nicotine depends on dopamine D1 receptor signaling.
Acknowledgements
The authors thank Dr. Margaret Broadwater for critical reading of the manuscript and Michael Moore for performing reliability checks on the ANY-maze video tracking data. This work was funded by East Tennessee State University – Office of Research and Sponsored Programs. DLR’s effort was supported by NIAAA (P60 AA011605) and the UNC Bowles Center for Alcohol Studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: bHR, bred for high responsiveness to novel environments; bLR, bred for low responsiveness to novel environments; CS, conditioned stimulus; DA, dopamine; NIC, nicotine; SAL, saline; US, unconditioned stimulus
References Cited
- Acquas E, Carboni E, Leone P, Di Chiara G. SCH 23390 blocks drug-conditioned place-preference and place-aversion: anhedonia (lack of reward) or apathy (lack of motivation) after dopamine-receptor blockade? Psychopharmacology. 1989;99:151–155. doi: 10.1007/BF00442800. [DOI] [PubMed] [Google Scholar]
- Ague C. Nicotine content of cigarettes and the smoking habit: their relevance to subjective ratings of preferences in smokers. Psychopharmacologia. 1972;24:326–330. doi: 10.1007/BF00403651. [DOI] [PubMed] [Google Scholar]
- Anselme P, Robinson MJ, Berridge KC. Reward uncertainty enhances incentive salience attribution as sign-tracking. Behavioural brain research. 2013;238:53–61. doi: 10.1016/j.bbr.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain research Brain research reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward:‘liking’,‘wanting’, and learning. Current opinion in pharmacology. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology. 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Brooks D. Recent and remote extinction cues reduce spontaneous recovery. The Quarterly journal of experimental psychology B, Comparative and physiological psychology. 2000;53:25–58. doi: 10.1080/027249900392986. [DOI] [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. European journal of neuroscience. 2011;34:1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno J, Moreira R. Conditional discrimination: the role of CS-alone trials. Behavioural processes. 1998;42:33–45. doi: 10.1016/s0376-6357(97)00060-0. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology. 2007;190:353–362. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chen H, Sharp BM, Matta SG, Wu Q. Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:2629–2638. doi: 10.1038/npp.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian BJ, Hurst P, Allred T, Palya WL. Timing information provided by trial-stimulus onset and offset in a sign-tracking procedure. Perceptual and Motor Skills. 1983;57:1119–1123. [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Lääne K, Theobald DE, Armstrong HC, Corlett PR, Chudasama Y, Robbins TW. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proceedings of the National Academy of Sciences. 2005;102:6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar R, Kaplan R, Shaham L, Frenk H. Euphoriant effects of nicotine in smokers: fact or artifact? Psychopharmacology. 2007;191:203–210. doi: 10.1007/s00213-006-0662-2. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nature neuroscience. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. The Journal of neuroscience. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural brain research. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Gore BB, Zweifel LS. Genetic reconstruction of dopamine D1 receptor signaling in the nucleus accumbens facilitates natural and drug reward responses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:8640–8649. doi: 10.1523/JNEUROSCI.5532-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacol Biochem Behav. 1983;19:989–992. doi: 10.1016/0091-3057(83)90405-7. [DOI] [PubMed] [Google Scholar]
- Hoffman DC, Beninger RJ. The D1 dopamine receptor antagonist, SCH 23390 reduces locomotor activity and rearing in rats. Pharmacology Biochemistry and Behavior. 1985;22:341–342. doi: 10.1016/0091-3057(85)90401-0. [DOI] [PubMed] [Google Scholar]
- Holland PC. Differential effects of omission contingencies on various components of Pavlovian appetitive conditioned responding in rats. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:178–193. doi: 10.1037//0097-7403.5.2.178. [DOI] [PubMed] [Google Scholar]
- Ivanova S, Greenshaw A. Nicotine-induced decreases in VTA electrical self-stimulation thresholds: blockade by haloperidol and mecamylamine but not scopolamine or ondansetron. Psychopharmacology. 1997;134:187–192. doi: 10.1007/s002130050441. [DOI] [PubMed] [Google Scholar]
- Krause MA, Cusato B, Domjan M. Extinction of conditioned sexual responses in male Japanese quail (Coturnix japonica): role of species-typical cues. Journal of Comparative Psychology. 2003;117:76. doi: 10.1037/0735-7036.117.1.76. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb Exp Pharmacol. 2009:335–367. doi: 10.1007/978-3-540-69248-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Audrain-McGovern J. Reinforcing effects of smoking: more than a feeling. Biol Psychiatry. 2010;67:699–701. doi: 10.1016/j.biopsych.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Sved AF. Cholinergic substrates of the reinforcement enhancing effects of nicotine. Psychopharmacology Submitted. 2007a doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Donny EC, Sved AF. Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology. 2007b;194:463–473. doi: 10.1007/s00213-007-0863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Cue-reactors: individual differences in cue-induced craving after food or smoking abstinence. PloS one. 2010;5:e15475. doi: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzardo A, Stein L, Belluzzi J. Rats prefer cocaine over nicotine in a two-lever self-administration choice test. Brain Research. 2002;924:10–19. doi: 10.1016/s0006-8993(01)03215-2. [DOI] [PubMed] [Google Scholar]
- Meck WH, Cheng R-K, MacDonald CJ, Gainetdinov RR, Caron MG, Çevik MÖ. Gene-dose dependent effects of methamphetamine on interval timing in dopamine-transporter knockout mice. Neuropharmacology. 2012;62:1221–1229. doi: 10.1016/j.neuropharm.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Odum LE, O'Dell KA, Schepers JS. Electronic cigarettes: do they have a role in smoking cessation? Journal of pharmacy practice. 2012;25:611–614. doi: 10.1177/0897190012451909. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology. 2004a;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology. 2004b;173:98–104. doi: 10.1007/s00213-003-1702-9. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA. Examination of GABAergic and dopaminergic compounds in the acquisition of nicotine-conditioned hyperactivity in rats. Neuropsychobiology. 2002;45:87–94. doi: 10.1159/000048682. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF. Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2139–2147. doi: 10.1038/sj.npp.1301623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Marks KR, Jones SA, Freeman KS, Wissman KM, Sheppard AB. The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology (Berl) 2012a doi: 10.1007/s00213-012-2892-9. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, O'Brien LC, Hall MJ. The role of conditioning history and reinforcer strength in the reinforcement enhancing effects of nicotine in rats. Psychopharmacology (Berl) 2012b;219:1119–1131. doi: 10.1007/s00213-011-2439-5. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Peterson JL, Wilkinson JL, Bevins RA. Nicotine serves as a feature-positive modulator of Pavlovian appetitive conditioning in rats. Behav Pharmacol. 2004;15:183–194. [PubMed] [Google Scholar]
- Parkinson J, Dalley J, Cardinal R, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston K, Robbins T, Everitt B. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behavioural brain research. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in Effects of Lesions of the Nucleus Accumbens Core and Shell on Appetitive Pavlovian Approach Behavior and the Potentiation of Conditioned Reinforcement and Locomotor Activity byd-Amphetamine. The Journal of neuroscience. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peartree NA, Hood LE, Thiel KJ, Sanabria F, Pentkowski NS, Chandler KN, Neisewander JL. Limited physical contact through a mesh barrier is sufficient for social reward-conditioned place preference in adolescent male rats. Physiology & behavior. 2012;105:749–756. doi: 10.1016/j.physbeh.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Fonte C, Wilson A. Reinforcing effects of nicotine as a function of smoking status. Exp Clin Psychopharmacol. 2001;9:243–250. doi: 10.1037//1064-1297.9.3.243. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Influence of reinforcer magnitude and nicotine amount on smoking's acute reinforcement enhancing effects. Drug Alcohol Depend. 2013a;133:167–171. doi: 10.1016/j.drugalcdep.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology. 2013b;228:479–486. doi: 10.1007/s00213-013-3054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behavioral neuroscience. 1991;105:727. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Fibiger HC. Decreased resistance to extinction after haloperidol: Implications for the role of dopamine in reinforcement. Pharmacology Biochemistry and Behavior. 1979;10:751–760. doi: 10.1016/0091-3057(79)90328-9. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Heien ML, Wightman RM. Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:10477–10486. doi: 10.1523/JNEUROSCI.22-23-10477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Wightman RM. Nomifensine amplifies subsecond dopamine signals in the ventral striatum of freely-moving rats. Journal of neurochemistry. 2004;90:894–903. doi: 10.1111/j.1471-4159.2004.02559.x. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology. 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. The European journal of neuroscience. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in resisting temptation: Implications for addiction. Neuroscience and biobehavioral reviews. 2013;37:1955–1975. doi: 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. The phasic reward signal of primate dopamine neurons. Adv Pharmacol. 1998a;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of neurophysiology. 1998b;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sharf R, Lee DY, Ranaldi R. Microinjections of SCH 23390 in the ventral tegmental area reduce operant responding under a progressive ratio schedule of food reinforcement in rats. Brain Research. 2005;1033:179–185. doi: 10.1016/j.brainres.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Sheppard B, Lehmann J, Cope ZA, Brown RW. Sex differences in nicotine sensitization and conditioned hyperactivity in adolescent rats neonatally treated with quinpirole: role of D2 and D3 receptor subtypes. Behavioral neuroscience. 2009;123:1296–1308. doi: 10.1037/a0017536. [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. The Cochrane database of systematic reviews. 2012;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain research reviews. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS. (US Department of Health and Human Services, O. o. t. A. S. f. H., Office of Smoking and Health, ed) 1988. Nicotine addiction: a report of the surgeon general. [Google Scholar]
- Uslaner J, Dell'Orco J, Pevzner A, Robinson T. The influence of subthalamic nucleus lesions on sign-tracking to stimuli paired with food and drug rewards: facilitation of incentive salience attribution? Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2352–2361. doi: 10.1038/sj.npp.1301653. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Robinson DL. Transient changes in mesolimbic dopamine and their association with 'reward'. Journal of neurochemistry. 2002;82:721–735. doi: 10.1046/j.1471-4159.2002.01005.x. [DOI] [PubMed] [Google Scholar]