Abstract
Background
While many studies have assayed behavioral responses of animals to chemical, temperature and light gradients, fewer studies have assayed how animals respond to humidity gradients. Our novel humidity chamber has allowed us to study the neuromolecular basis of humidity sensation in the nematode Caenorhabditis elegans (Russell et al. 2014).
New Method
We describe an easy-to-construct, low-cost humidity chamber to assay the behavior of small animals, including soft-bodied invertebrates, in controlled humidity gradients.
Results
We show that our humidity-chamber design is amenable to soft-bodied invertebrates and can produce reliable gradients ranging 0.3–8% RH/cm across a 9-cm long x 7.5-cm wide gel-covered arena.
Comparison with Existing Method(s)
Previous humidity chambers relied on circulating dry and moist air to produce a steep humidity gradient in a small arena (e.g. Sayeed & Benzer, 1996). To remove the confound of moving air that may elicit mechanical responses independent of humidity responses, our chamber controlled the humidity gradient using reservoirs of hygroscopic materials. Additionally, to better observe the behavioral mechanisms for humidity responses, our chamber provided a larger arena. Although similar chambers have been described previously, these approaches were not suitable for soft-bodied invertebrates or for easy imaging of behavior because they required that animals move across wire or fabric mesh.
Conclusion
The general applicability of our humidity chamber overcomes limitations of previous designs and opens the door to observe the behavioral responses of soft-bodied invertebrates, including genetically powerful C. elegans and Drosophila larvae.
Keywords: nematode, larva, humidity, hygrotaxis, hygrosensation
1. Introduction
Moisture is essential for life. Therefore many animals display behavioral mechanisms to migrate toward their preferred moisture level (hygrotaxis) (Gunn, 1937; Thomson, 1938; Bursell & Ewer, 1950; Warburg, 1964; Reshetnikov, 1996; Yu et al., 2010; Russell et al. 2014). These behaviors are critical to keep an animal within its niche and regulate essential processes like growth and reproduction. Humidity is one of the most fundamental environmental factors determining the distribution of species (Andrewartha & Birch, 1954). Although the neuro-molecular underpinnings of many sensory modalities have progressed a great deal in the past 30 years, how animals sense and orient to humidity remains enigmatic (Montell, 2007). One proven approach to reveal potentially conserved molecular bases for sensory perception is by studying the invertebrate model organisms Caenorhabditis elegans and Drosophila. Although some progress has been made using adult flies (Sayeed & Benzer, 1996; Lui et al., 2007), it has been difficult to assay how Drosophila larvae respond to moisture gradients without confounding sensory stimuli from food and gravity (Johnson & Crader, 2014). Moreover, humidity responses had not been conducted with C. elegans, perhaps because they quickly desiccate upon removal from a moist substrate.
The need for investigating the humidity responses of soft-bodied invertebrates arose with our observations that C. elegans avoids puddles. Although this behavior suggested their ability to sense humidity levels, it was not reproducible enough gain formal understanding of the genes and neurons essential for the response. Controlled humidity gradients using hygroscopic materials have been described in previous literature, however, none were amenable for use with C. elegans because they required the animals to move across a wire or fabric mesh (Suppl. Fig. 1A, 1B) (Kennedy & Gunn 1937, Barlow & Nicholls 1961). More recent studies determining the genetic basis for humidity sensation of Drosophila were conducted utilizing a binary choice-chamber apparatus (Suppl. Fig.1C) (Sayeed & Benzer 1996, Liu et. al. 2007). Their experimental design relied on circulating dry and moist air to produce very steep humidity gradients. Because C. elegans reacts to air currents, we were wary of this approach due to its potentially confounding stimuli. Additionally, we were interested in observing animals under more naturalistic humidity gradients in order to characterize physiologically meaningful behavior. Here we describe the construction and implementation of our simple, low-cost experimental set-up for characterizing humidity responses in the soil nematode C. elegans (Suppl. Fig.1D). We also illustrate its potential for broader use with other soft-bodied invertebrates as both a research and teaching tool.
2. Materials and methods
2.1 Culturing of C. elegans for hygrotaxis assays
Wild-type and mutant C. elegans are raised on agar plates seeded with an OP50 strain bacterial lawn for food as described (Brenner, 1974). C. elegans displayed the strongest humidity preference when they were starved (Russell et al. 2014). Therefore we learned to qualify the degree of starvation by observing the condition of the worms and the bacterial lawn. Worms may be rinsed off of seeded plates onto unseeded plates to control the starvation conditions – 12 to 18 hours works well. We found, however, that we could achieve similar results by more conveniently allowing the worms to eat all of the bacteria on their original culture plate. We found that a plate was optimal for testing this way when the lawn was completely devoured. This was evident by the absence of trails left by the worms in the bacterial lawn when viewed by oblique illumination. In either case, starved plates were optimum if they still contained plenty of unhatched eggs. If left to starve to the point where all of the eggs hatched, the hatched larvae would display L1-stage larval arrest and the adult worms would appear unhealthy and perform poorly in our assay.
2.2 Humidity chamber
In brief, the assay consists of a polymerized methyl methacrylate (Lucite) chamber with troughs on each end which hold substances to control humidity (Suppl. Fig.2). Steep humidity gradients (10%-90% RH over 9 cm) can be generated using a desiccant such as calcium sulfate (Drierite) and water poured in opposite toughs. Shallow humidity gradients can be produced with the same chamber using different concentration aqueous NaOH solutions (Suppl. Fig 3). The behavioral field that the worms are placed on consists of a semi-desiccated agarose gel placed on a glass plate (Fig.1). This allows the worms to be illuminated from below facilitating observation via steromicroscope. Once the worms are introduced on the agarose field, the chamber is sealed with plastic wrap to establish the humidity gradient. The response of the worms to the humidity gradient is observed through the plastic wrap. We quantified humidity preference by counting the number of worms that reach the dry and humid sides of the chamber at 60 minutes. The humidity gradient forms within 10 minutes and is maintained for over 90 minutes (see section 2.3 below), however, so other time-points could be recorded to accommodate the performance of slower worms. Once the behavioral assay is complete, the chambers should be cleaned with distilled water or a dilute (70%) ethanol solution and reused.
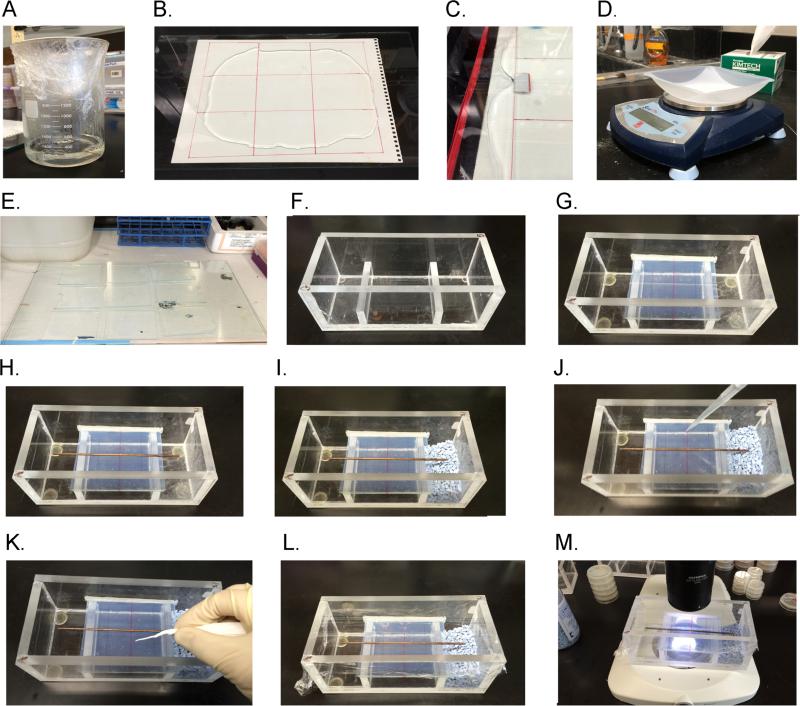
Fig. 1. Preparing a hygrotaxis assay.
(A) Melt a 250-mL batch of 5% agarose in a 2-L Pyrex beaker. (B) Pour molten agarose onto tempered glass. (C) Press a second sheet of tempered glass with 0.06” spacers over top so that molten agarose spreads in all directions, covering the template page below glass. (D) After agarose cools, cut it into 9 rectangle pads as per template and weigh each pad. (E) Place gel pads in laminar flow hood and dry ~45 min rotating 180 degrees after 25 min. (F) Get clean humidity chamber. (G) Place folded rectangular Parafilm strip on one side and then wedge glass plate with gel into chamber making sure that the fit is snug. (H) Heat copper strip and press into gel gently. (I) Add humidity controlling substances or solutions to each trough. (J) Rinse and pipette worms onto gel assay pad in 35 μL of 200-mOsm sorbitol solution. (J) Use rolled Kimwipe or sterile cotton swab to absorb excess sorbitol solution and to spread worms out along midline. (K) Cover humidity chamber with plastic wrap. (M) Observe worms under microscope with dark-field optics.
2.3 Quantifying humidity gradient
To quantify the humidity gradient within the apparatus we utilized the colorimetric moisture indicator cobalt (II) chloride. Each molecule of the indicator absorbs five water molecules. Each water bond shifts the indicator's color from bright blue to magenta. We uniformly infused cobalt (II) chloride powder into blotter paper to generate re-useable humidity measurement strip. The water was driven out of the strip by microwaving until it was bright blue. Dried strips were kept in an air-tight container with desiccant until use.
Because we were interested in the humidity gradient that animals experience across the behavioral field, we placed the humidity strip across the entire length of the field on the glass substrate without agarose. Then we placed desiccant and water respectively in the two troughs and enclosed the apparatus in plastic wrap allowing the humidity gradient to form. We then photographed the indicator strip under full-spectrum lighting with a digital SLR camera at different time intervals beginning at 10 minutes. To quantify the moisture absorption along the length of the strip, we extracted the red and blue values along the length of the photo of the strip via Image J. The ratio of the red and blue values at the respective points along the strip were then plotted. This revealed a mostly linear gradient of moisture absorption across the indicator strip. Photographic analysis of the humidity strip at multiple time points suggested that a linear humidity gradient persisted from 10 minutes through 90 minutes.
3. Results and discussion
3.1 Constructing chamber and preparation of assay components
3.1.1 Assemble chamber and glass substrate (Supplemental video 1)
The humidity chamber was constructed out of inexpensive and widely obtainable polymerized methyl methacrylate (Lucite). A sheet of Lucite was cut into rectangular pieces according to specifications outlined in diagram (Suppl. Fig 2). The individual pieces were then secured together with a clear, thin solvent cement (Weld-On #4, Compton, CA). Once the chambers are assembled we tested them for water-tightness by filling with water. Small leaks were filled through treating with a low viscosity polymer (Rain-X, Houston, TX). The chambers were allowed to air out for 72 hours prior to assay worms.
Tempered glass plates, to be used as a substrate, were cut 0.5 mm more narrow than the inside chamber dimensions (~74.5 mm). This allowed the glass to remain tightly positioned in the chamber through placing strips of Parafilm (West Chester, PA) on the inside of one chamber wall and securing the glass in place through friction just prior to the assay. Tempered glass should be used since the glass plates will be repeatedly exposed to hot molten agar. Lines to demark zones for scoring can be drawn on the glass with a permanent marker.
3.1.2 Preparing solution for transferring animals
When working with populations of very small animals like C. elegans (adults are ~1 mm), it is easy to deposit them in a liquid droplet on the center of the agarose assay pad. For C. elegans, we used a solution of sterile 200 mOsm sorbitol because it lacks salts but its physiological osmolarity does not put the animal in hypotonic stress. Moreover, Ward (1973) previously found that worms are neither attracted to nor repelled by sorbitol. A sterile cotton-swab can then be used to wick away excess liquid from the droplet of worms and to distribute the animals uniformly across the midline.
3.1.3 Preparing NaOH solutions
To test animals in a defined shallow humidity gradients we used different concentrations of NaOH in either trough. Salt solutions are well known for their deliquescence, readily dissolving in the water they absorb due to their charged interaction with the polar water molecule. The strong ionic interaction of sodium hydroxide binds water molecules, reducing the amount of free water and therefore the water vapor pressure. A common example of this is salt shakers becoming clogged under humid conditions if a desiccant such as rice is not added to the shaker. By exploiting the hygroscopic properties of NaOH salt it is possible to generate gradients as shallow as 1.5% across the 9-cm assay surface. Supplemental Figure 3 demonstrates the relationship between the concentration of NaOH solution and the RH% obtained at 20°C. Supplemental Table 1 shows the weight of NaOH to add to 100-ml water in order to produce various relative humidities at 20°C (Madge, 1961).
3.1.4 Copper dividers
By dividing the assay field longitudinally, different groups of animals may be tested in parallel. For example, control animals on one half and experimental animals on the other. We use 2-mm copper tubing cut into 12 cm lengths. Worms may crawl under the copper tube if it is not properly sealed to the agarose assay pad. Pre-heat the copper tube over ethanol lamps to seal the tube to the agarose surface (Suppl. Video 1). Because C. elegans are repelled by copper, the worms are less likely to crawl near the tube, which helps prevent the tube from obscuring the worms from view. Alternatively, Teflon strips may be used in place of copper tubes to avoid potential toxicity of copper; however, in our experience many worms tend to cling to the strips, obscuring individual worms from view.
3.1.5 Gel pad
To obtain agarose assay pads of uniform thickness, we attached spacers cut from architectural 0.06 inch thick modeling plastic to the edges of a 20 cm x 30 cm tempered glass plate (Fig 1B,C).
3.2 Prepare agarose pads
Although nematodes stick to plastic, they move fine on agarose. Since agarose, a hydrogel, can influence the humidity gradient that the animal experiences in the assay chamber, we dehydrated it slightly, inducing a skin to form which prevents water in the substrate from confounding the humidity gradient (Suppl. Video 1).
3.2.1
We then prepared the agarose pads by mixing up 250 mL of a 5% solution in a 2-L beaker. Next, we secured 6 sheets of plastic wrap over the top of the beaker before microwaving at full power for 3 minutes. After microwaving, the molten agarose cooled causing the plastic wrap to implode, facilitating a vacuum which served to degas the agarose (Fig 1A, Suppl. video 1). The degasing of the agarose ensures that there are no bubbles in the gel-pads which interfere with the uniformity of the substrate and obstruct viewing the worms.
3.2.2
Just after pouring the molten agarose over one sheet of tempered glass we then pressed the tempered glass with the spacers on the bottom on top of the molten agarose and shimmied the glass until the spacers on all sides are touching the bottom glass plate. This ensured that all the gels were the correct thickness (Fig 1B, 1C).
3.2.3
Once the agarose had cooled, we removed the top sheet of tempered glass. Then using the gel-template beneath as a guide we used a sterile ruler and razor blade to cut the agarose into the correct size gel pads (S video 1).
3.2.4
We then weighed each pad on a scale before placing them to dry out the in a fume hood for ~50 minutes. Then we reweighed the gel pads and determined the hydration of the gels. Once they dried to between 70-80% of their original weight, they were ready for use. The time to dry given here is for a lab at 60% RH and 21°C. The time to dry will be variable depending on the humidity and temperature. The gel assay pads could be used immediately for experiments, or saved for later use kept sandwiched between two sheets of glass and plastic wrap. This allowed us to use them up to five days later (Fig 1D, 1E, Suppl. Video 1).
3.3.5 Secure gel-pads inside hygrotaxis chamber
We used a 1-inch large, ethanol-sterilized spatula to pick up each gel pad and center it on top of a glass plate prepared with scoring marks. Next, we trimmed the edges of the agarose flush with the glass on all sides. We then folded several rectangular Parafilm strips down their length and placed them fold-side down against one side of the chamber. One side of a glass assay plate was then pressed against the Parafilm pressing flush. The Parafilm served as a seal, keeping the gel assay pad secure even if the assay chamber is turned upside down. Sealing the assay substrate in this way ensured that the humidity in the air trapped under the substrate field did not influence the linearity of the humidity gradient of the assay environment (Fig 1G).
3.3.6 Copper divider to assay multiple animal groups in parallel
In order to be able to run two assays side by side a copper rod was placed down the middle to divide the worms. We first heated the copper tube over an alcohol flame for about 15 seconds before placing in the middle of the agar-pad. Do not overheat, or else the tube will melt completely through the agar pad. Use the flat end of the spatula to gently press down on the rod slightly embedding it in the agarose gel pad (Fig 1H).
3.3.7 Establishment of humidity gradient
When we ran steep humidity gradients, we put 50-mL of Drierite in one trough and 15 mL of dH2O in the other trough. When we tested worms in more shallow gradients, we placed 15 mL of the NaOH solutions in their respective troughs.
3.3.8 Place worms on gel assay pad
We rinsed worms off their cultivation plate with 1.5 mL of 200-mOsm sorbitol with 1:10,000 of Triton X 100 detergent into an Eppendorf tube. The detergent prevents the worms from sticking to the side of the plastic tube and pipettes (Davis et al. 2010). We then rinsed the animals by allowing them to settle by gravity for 2 minutes and then decant all but ~100 μL, repeating this three times. We then deposited the cleaned worms onto the middle of the assay field in 35 μL of sorbitol solution. We immediately wicked the excess liquid with a sterile cotton swab and also used the swab to spread the worms out along the midline (Fig. 1J 1K). Lastly, we sealed the humidity chamber with plastic wrap (Fig. 1L). In some cases, groups of worms form piles in the middle of the chamber and fail to migrate. This can be prevented by spreading them out as diffusely as possible across the midline. If needed, the piles of worms can be illuminated with a blue-light laser pointer for a few seconds through the plastic wrap. This will disperse the pile and does not seem to induce any directional bias.
Although this method outlined above works well for C. elegans, other soft-bodied invertebrates may need to be handled more gently. When assaying Drosophila larvae, we found that it was better to wash and transfer them using standard fly protocols.
3.3.9 Scoring the assay
To observe the worms for scoring the assay, we used a low-power upright microscope that has dark-field optics. We recorded the number of worms in each zone of the substrate at 60 minutes. Animal orientation schemes can be assessed through collecting and analyzing video if desired (Suppl. Video 1). In our previously reported research, worms on each side of the assay were counted after 60 min to compute a performance index for hygrotaxis, Ihtx, representing the number of worms on the dry side minus the number on the humid side, divided by the total number on both sides. With this scheme, perfect hygrotaxis toward the dry side yields an Ihtx of +1, perfect hygrotaxis toward water yields an Ihtx of −1, and no preference yields an Ihtx of 0 (Russell et al., 2014).
3.4 Typical results
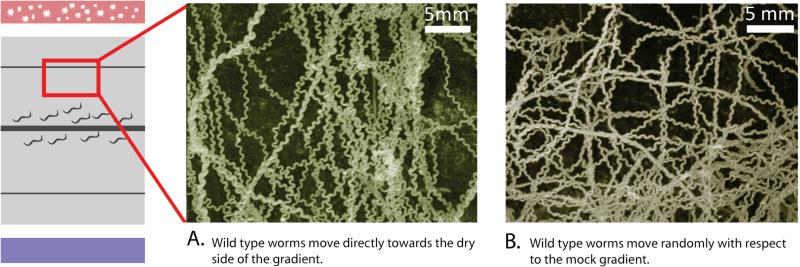
When a humidity gradient is established they showed robust preference (Fig. 2 center). However, under isohumid conditions with a mock gradient, the worms dispersed randomly (Fig. 2 right).
Fig. 2. C. elegans orients within a humidity gradient.
Worms within a humidity gradient orient towards their preferred humidity level. Starved worms show robust preference for dry side of the chamber. Under isohumid conditions (mock gradient) the worms distribute randomly. The images were generated from a montage of 300 superimposed photos of worms taken over 10 min in part of the hygrotaxis chamber. Trajectories appear as contiguous images of individual worms.
3.5 Cleaning humidity chamber
The humidity chamber can be washed after use with dH20 and/or a dilute ethanol solution (70%). Chambers should be handled with gloves so as to prevent any odors from being impregnated in the Lucite. Periodically washing with a dilute gentle detergent such as Alconox cleans contamination due to improper handling.
3.6 Other considerations
3.6.1 Inadvertent gaseous gradients
Although calcium sulfate (Drierite) and sodium hydroxide solutions are effective water absorbing agents, they also can absorb carbon dioxide from the atmosphere. This property is similar to all other alkali salts as well as alternative desiccants such as sulfuric acid and glycerol (Brunauer 1942, Johnson 1940, Solomon 1951). This quality was considered advantageous by mid-century invertebrate ethologists since it counter acted the buildup of CO2 from the animals. However, because C. elegans has been shown to sense carbon dioxide, we were concerned the worms may be responding to the CO2 gradient the assay also produces (Hallem et al. 2008). However when we tested worms in a humidity gradient produced by placing water in one trough while leaving the other trough empty, the behavioral response was indistinguishable to that with the desiccant (Russell et al. 2014).
3.6.2 Concomitant temperature gradients
Researchers assaying humidity responses must be aware of concomitant temperature gradients. Because the humidity gradient results in the hydrogel evaporating more quickly on the side closest to the desiccant a slight thermal gradient is produced (Russell et al. 2014). We characterized the temperature of the substrate under various conditions though IR imaging. We were able to obtain clear IR images of the assay by isolating the camera in a cardboard box in which a hole was cut out for the lens. Also we used a lens which allowed us to put the camera right up on the plastic wrap allowing us to avoid thermal reflections by taking them out of the plane of focus. We detected 0.01°C per cm temperature gradient on the dry side of the chamber for a 10-90% RH gradient. Researchers must also be aware that even if they use a non-moist substrate, water will evaporate from the animal on the dry side of the chamber. For C. elegans, we found that temperature cues appear to be integrated with mechanical cues to determine humidity levels.
3.7 General applicability
The assay detailed here allows for the characterizations of both crawling insects as well as soft-bodied invertebrates such as worms and larvae (Suppl. Fig. 1D). This flexibility will allow the study of animals previously intractable with all past humidity chamber designs. A recent publication suggested that Drosophila larvae in an upright food cylinder shows a moisture preference before pupation (Johnson & Carder 2012). The migration patterns of larvae, however, were also potentially influenced by the presence of food at the bottom of the cylinder and gravity. Our novel humidity chamber described here could provide controlled conditions to directly probe humidity responses of Drosophila larvae as well as other insect larvae. For testing larger animals, the humidity chamber can be scaled up provided that the ratio of the dimensions remains consistent. It is not recommended to increase the height/width ratio since that will lead to an unstable humidity gradient due to humidity convection currents. Although the cost of electrophoresis grade agarose is the most cost-prohibitive component of the assay design, it is still possible to run ~150 assays for each 100 g of agarose used. We have not tried to use less-expensive agar in place of agarose but feel that could work provided the animals you are testing do not respond to components of the agar.
Importantly this assay also provides a simple method for demonstrating sensory neuroscience principles at close to no cost. This gives K-12 educational programs the potential for utilizing this assay as a teaching tool providing an intuitive hands-on way for school kids to augment their sensory neuroscience coursework. Because this method facilitates the testing of a wide variety of small terrestrial animals school kids could collect and the humidity relations of a variety of local worms, arthropods, or insect larvae.
Supplementary Material
HIGHLIGHTS.
New experimental set-up for studying behavioral responses to humidity gradient
Novel assay design is applicable for soft-bodied invertebrate animals
Tunable humidity gradient allows characterization of naturalistic humidity responses
Simple, low-cost design is amenable for research labs or K-12 classrooms
Acknowledgements
We thank Layla Young for expert assistance. Some strains were provided by the Caenorhabditis Genetic Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440) and the National Bioresource Project of Japan. Funding was provided by a NSF Graduate Fellowship to (J.R.) and a NIH NINDS grant NS075541 (J.P.-S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Supplementary figures, table and video related to this article can be found in the online version.
References
- Andrewartha HG, Birch CL. The distribution and abundance of animals. University of Chicago Press; Chicago: 1954. [Google Scholar]
- Barlow CA, Nicholls CF. A humidity gradient apparatus for insects. The Canadian Entomologist. 1961;93.10:860–864. [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunauer S. Adsorption of Gases and Vapors. Vol. 1. Princeton University Press; Princeton, N. J.: 1942. [Google Scholar]
- Bursell E, Ewer DW. On the reactions to humidity of Peripatopsis moseleyi (Wood-Mason). Journal of Experimental Biology. 1950;26.4:335–353. [Google Scholar]
- Gunn DL. The humidity reactions of the wood-louse, Porcellio scaber (Latreille) Journal of Experimental Biology. 1937;14.2:178–186. [Google Scholar]
- Gunn DL, Kennedy JS. Apparatus for investigating the reactions of land arthropods to humidity. Journal of Experimental Biology. 1936:450–459. [Google Scholar]
- Hallem EA, Sternberg PW. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proceedings of the National Academy of Sciences USA. 2008;105.23:8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WA, Carder JW. Drosophila nociceptors mediate larval aversion to Dry surface environments utilizing both the painless TRP channel and the DEG/ENaC subunit, PPK1. PLoS One. 2012;7.3:e32878. doi: 10.1371/journal.pone.0032878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CG. The maintenance of high atmospheric humidities for entomological work with glycerol-water mixtures. Annals of Applied Biology. 1940;27:295–299. [Google Scholar]
- Liu L, et al. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450.7167:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- Madge DS. The control of relative humidity with aqueous solutions of sodium hydroxide. Entomologia Experimentalis et Applicata. 1961;4.2:143–147. [Google Scholar]
- Montell C. TRP channels: it's not the heat, it's the humidity. Current Biology. 2008;18.3:123–126. doi: 10.1016/j.cub.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Reshetnikov AN. Hygrotactic and olfactory orientation in juvenile common toads (Bufo bufo) during the postmetamorphic period. Advances in Amphibian Research in the Former Soviet Union. 1996;1:181–190. [Google Scholar]
- Russell J, et al. Humidity sensation requires both mechanosensory and thermosensory pathways in Caenorhabditis elegans. Proceedings of the National Academy of Sciences USA. 2014;111.22:8269–8274. doi: 10.1073/pnas.1322512111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed O, Benzer S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proceedings of the National Academy of Sciences USA. 1996;93.12:6079–6084. doi: 10.1073/pnas.93.12.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon ME. Control of humidity with potassium hydroxide, sulphuric acid, or other solutions. Bulletin of Entomological. Research. 1951;42:543–554. [Google Scholar]
- Thomson RC. The reactions of mosquitoes to temperature and humidity. Bulletin of Entomological Research. 1938;29.02:125–140. [Google Scholar]
- Warburg MR. The response of isopods towards temperature, humidity and light. Animal Behaviour. 1964;12.1:175–186. [Google Scholar]
- Ward S. Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proceedings of the National Academy of Sciences USA. 1973;70.3:817–821. doi: 10.1073/pnas.70.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HP, et al. Hygropreference behaviour and humidity detection in the yellow spined bamboo locust, Ceracris kiangsu. Physiological Entomology. 2010;35.4:379–384. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.