Abstract
Primary objective
To use breath-hold functional magnetic resonance imaging (fMRI) to localize the brain regions with impaired cerebrovascular reactivity (CVR) in a female patient diagnosed with mild traumatic brain injury (mTBI). The extent of impaired CVR was evaluated two months after concussion. Follow-up scan was performed one year post mTBI using the same breath-hold fMRI technique.
Research design
Case report.
Methods and procedures
fMRI blood oxygenation dependent level (BOLD) signals were measured under breath-hold challenge in a female mTBI patient two months after concussion followed by a second fMRI with breath-hold challenge one year later. CVR was expressed as the percent change of BOLD signals per unit time of breath-hold.
Main outcomes
In comparison with CVR measurement of normal control subjects, statistical maps of CVR revealed substantial neurovascular deficits and hemispheric asymmetry within gray and white matter in the initial breath-hold fMRI scan. Follow-up breath-hold fMRI performed one year post mTBI demonstrated normalization of CVR accompanied with symptomatic recovery.
Conclusions
CVR may serve as an imaging biomarker to detect subtle deficits in both gray and white matter for individual diagnosis of mTBI. The findings encourage further investigation of hypercapnic fMRI as a diagnostic tool for mTBI.
INTRODUCTION
Mild traumatic brain injury (mTBI) or concussion is a major public health concern [1]. Common symptoms associated with mTBI include headache, dizziness, nausea and cognitive impairment. There is significant concern for the potential additive effects of repeated concussions, particularly in situations when brain injury sustained from an initial concussion did not sufficiently recover [2–4]. As the uncomplicated mTBI are always unaccompanied by the presence of skull fracture and a trauma-related intracranial abnormality, the brain can often appear normal on clinical computed tomography and magnetic resonance imaging (MRI) [5]. Depending on the mechanism of impact, brain injury can be heterogeneous among individuals, making it difficult to identify common imaging biomarkers for different patients with mTBI. Current treatment guidelines and assessment of recovery status for mTBI rely upon the outcomes from neuropsychological evaluation. A major focus of mTBI research is to identify biomarkers that can map subtle injuries as well as monitor the long term recovery status for an individual patient with mTBI. Since gross neurovascular deficits in mTBI have been reported via transcranial Doppler ultrasound (TCD) under the breath-hold challenge [6], this study sought to apply breath-hold functional magnetic resonance imaging (fMRI) to obtain regional responses of the brain for an individual patient with mTBI. Breath-hold fMRI was used to map cerebrovascular reactivity (CVR), a measure of cerebrovascular reserve which serves as a regional indicator of healthy brain tissues vs. tissues with neurovascular compromise. Insufficient cerebrovascular reserve as a possible biomarker for mTBI is best measured by blood flow sensitive imaging technique under hypercapnic challenge which is not measurable by other MRI techniques. This study evaluated the usefulness of CVR as a biomarker for mTBI.
Two fMRI sessions separated by one year, were performed on a patient with mTBI under breath-hold challenge. fMRI data were also acquired on five healthy subjects under the same breath-hold protocol for comparison. The abnormal CVR in the mTBI patient demonstrates the sensitivity of breath-hold fMRI technique in localizing subtle brain injuries. The reduction of abnormal CVR in the follow-up scan of the same patient demonstrates the capability of breath-hold fMRI to monitor the recovery of an individual patient with mTBI.
CASE REPORT
A 47-year-old female was diagnosed to have mTBI subsequent to blunt trauma to the right temporal/periorbital region. She did not have loss of consciousness at the time of impact. However, she had headaches and executive deficits (poor focus/attention) for approximately five weeks post mTBI. Her initial fMRI scan with breath-hold challenge was performed two months after the concussion when her symptoms had significantly but not completely subsided. A follow-up fMRI scan with breath-hold challenge was performed one year later with her mTBI symptoms resolved.
In the breath-hold challenge, the patient was instructed via visual cues to do 6 epochs of 30-second breath-hold interleaved with 60–90 seconds of normal breathing. The total duration of the breath-hold protocol lasted 10 minutes. Vital signs including heart rhythm, respiration, blood pressure, end-tidal carbon dioxide level (PETCO2) and oxygen saturation were measured simultaneously with MRI acquisition to ensure that the patient performed the breath-holding task properly. For comparison, the same breath-hold protocol was applied onto five healthy male control subjects (age range, 27–35 years) without previous history of mTBI (HC1–5).
This study measured the changes of blood oxygenation dependent level (BOLD) fMRI signals and the imaging parameters were: TR = 2000 ms, TE = 30 ms, flip angle = 90°, FOV = 220 mm, matrix = 64×64, thickness = 5 mm, gap = 1 mm. The CVR map was generated by subjecting BOLD data acquired during breath-hold challenge to regression analysis. The BOLD data were imported into the software Analysis of Functional NeuroImage (AFNI) [7] (National Institute of Mental Health, http://afni.nimh.nih.gov) for time-shift correction, motion correction, normalization and detrending. Simple regression with a regressor of the onset of breath-hold epochs was used. The map of percent BOLD signal changes per unit time of breath-hold was derived as the CVR responses. CVR map of the mTBI patient and individual healthy subject was registered onto their own anatomical scan and transformed to the standardized space of Talairach and Tournoux [8]. In order to protect against type I error, we used Monte Carlo simulation [9] to correct individual voxel probability threshold of p < 0.005 to the overall significance level to α<0.05 for multiple comparisons. Regions of breath-hold correlated change in cerebrovascular responses were clusters of at least 603 mm3 in which each voxel was consistently active along the time series: t(421) > 2.82, p < 0.005 uncorrected. Analysis of resultant statistical parameter maps for CVR were therefore at the overall corrected threshold of p < 0.05
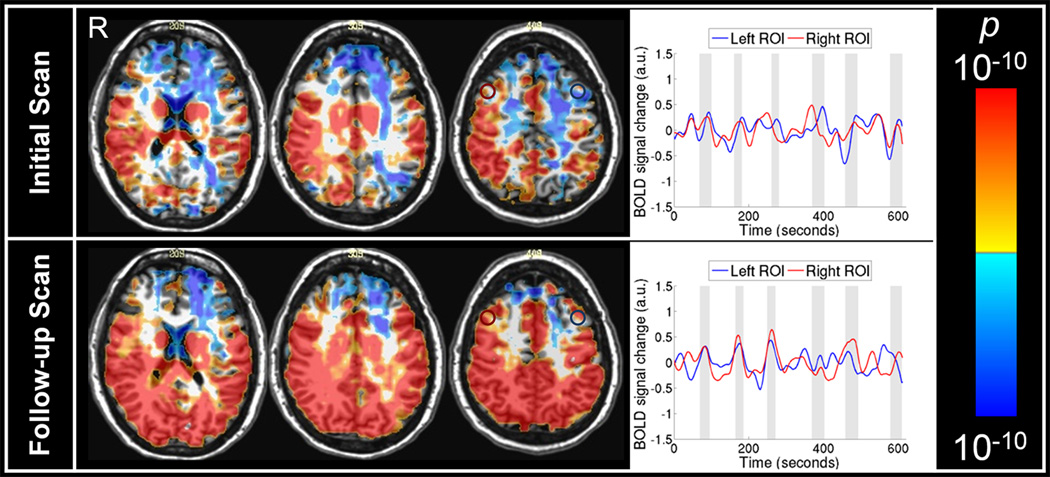
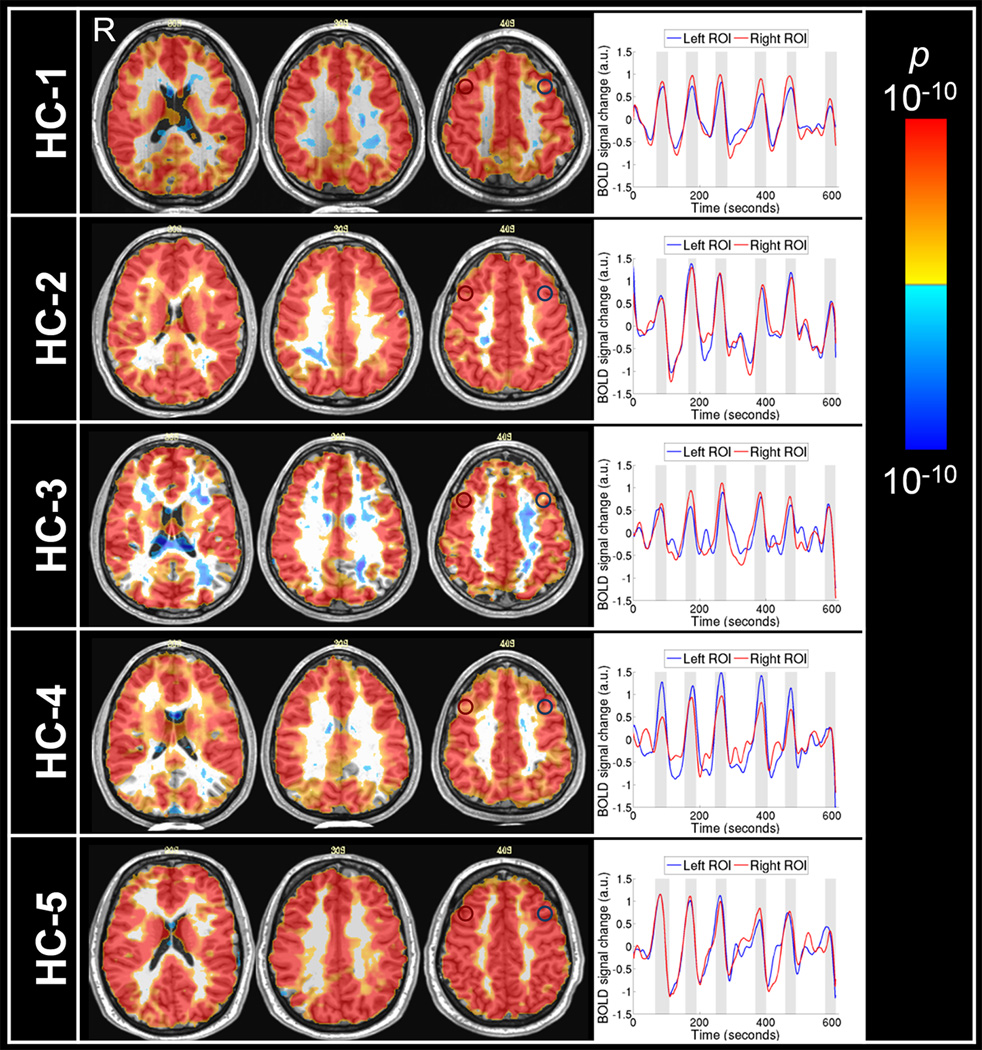
The initial breath-hold fMRI scan at two months post concussion demonstrated hemispheric asymmetry of CVR extending from the frontal gray matter to parietal white matter (Figure 1). A larger extent of CVR abnormality found in left cerebral hemisphere is consistent with the right head impact and subsequent intracranial impact of the brain onto the inner wall of the skull on the left. Clear CVR asymmetry was observed in both gray and white matter in the frontal area. Besides the hemispheric asymmetry, MRI signal response time series in both gray and white matter was asynchronous with the referenced hypercapnic stimulus epochs in breath-hold challenge. In contrast, the MRI signal response time series of healthy control subjects in both gray and white matter followed closely the referenced hypercapnic stimulus time epochs in breath-hold challenges (Figure 2). The source of the difference in CVR between mTBI patient and healthy controls lies in the observation that gray and white matter MRI signal response time series of mTBI patient was asynchronous with the referenced breath-hold time epochs. Both the hemispheric asymmetry and the abnormal pattern of the MRI signal time series are powerful diagnostic markers for mTBI, making it possible to make a diagnosis for an individual patient with mTBI.
Figure 1.
Statistical parametric map of cerebrovascular reactivity (CVR) for a patient with mTBI at the initial scan two months post mTBI (upper panel) and the follow scan one year later (lower panel). Upper panel: Negative CVR indicated by cool colors was observed in both gray and white matter in the frontal and parietal areas, more on the left hemisphere, in the initial scan of the mTBI patient. MRI signal time series were extracted from the selected regions of interest in the left dorsolateral frontal area (blue circle) and in the right dorsolateral frontal area (red circle). The shaded columns in the time series plots indicate breath-hold epochs. The time series from the left medial parietal area was not in synchronous with the breath-hold epochs. Lower panel: Positive CVR indicated by warm colors was observed in the left parietal areas in the follow-up scan of the mTBI patient. The MRI signal time series from the tissues in left (blue circle) and right (red circle) dorsolateral frontal areas were in synchronous with the breath-hold epochs. a.u., arbitrary units.
Figure 2.
Statistical parametric maps of cerebrovascular reactivity (CVR) for five healthy subjects (HC1-HC5). Positive CVR indicated by warm colors was observed in most of the brain regions. The MRI signal time series from the tissues in left (blue circle) and right (red circle) dorsolateral frontal areas of the healthy controls follow closely the breath-hold epochs. The shaded columns in the time series plots indicate breath-hold epochs. a.u., arbitrary units.
In the follow-up breath-hold fMRI scan one year later, hemispheric asymmetry of CVR was significantly reduced together with the normalization of MRI signal response time series that was becoming synchronous with the referenced breath-hold epochs, leading to the normalization of CVR responses. We believe that the follow-up hypercapnic fMRI findings demonstrate objective evidence for mTBI recovery, consistent with the resolution of the patients’ post-concussion symptoms. As is common in patients with mTBI, no brain lesion or vascular lesion was identified by the high resolution T1-weighted MPRAGE, the clinical T2-weighted FLAIR and the MR angiography scans in both initial and follow-up scan sessions.
DISCUSSION
The present findings provide preliminary evidence to support the use of CVR derived in breath-hold fMRI as a biomarker for mTBI. In a TCD study [6], mTBI under breath-hold challenge has been shown to manifest itself in the shape and delay of blood flow velocity time series but not necessarily in the mean amplitude of CVR over breath-hold epochs. Breath-hold fMRI is a significant step over TCD in developing regional CVR as an imaging marker for mTBI. Notably, breath-hold fMRI is unique among MR techniques to be able to demonstrate abnormal neurovascular responses in gray matter as well as white matter [10]. Breath-hold fMRI could supplement diffusion tensor imaging (DTI) of white matter deficits in future research. Similar to previous successful CVR studies of vasculopathies [10–14] and low to intermediate grade gliomas [15, 16], we selected Blood Oxygenation Level Dependent (BOLD) MRI signals instead of MR perfusion parameters derived from dynamic susceptibility contrast (DSC) Gadolinium perfusion and arterial spin labeling (ASL) data. Intravenous injection of gadolinium is not a usual practice for the diagnosis and follow-ups of patients with mTBI in clinical settings although a recent DSC study showed meningeal leakage in acute stage of mTBI [17]. The sensitivity of ASL fMRI to white matter perfusion is controversial [18]. The long repetition time of ASL acquisitions is also less optimal for the short 30-second hypercapnic stimulus epochs which were designed to maximize safety by minimizing the risk of triggering of mTBI symptoms (e.g. migraine attacks). The robust CVR responses we obtained from our breath-hold epochs is noteworthy as most ASL CVR studies used CO2 inhalation and the long hypercapnic epochs reported in the literature typically range from 45 seconds to 3 minutes [10, 19, 20].
Given the fundamental feature of heterogeneity of concussion injuries across the mTBI population [21], individual diagnosis is an important goal to optimize the treatment and management of patients with mTBI. Hypercapnic fMRI with its high sensitivity and high spatial resolution appears to be a promising technology for individual-based assessments whereas other popular fMRI approaches such as cognitive fMRI [22] and resting state [23] normally require group averaging to resolve subtle functional deficits and some of them require a priori hypothesis-driven fMRI analysis approach.
Interpretations of the present study should be considered in the context of acknowledged limitations. Although the sample sizes were very small, we contend that the compelling findings observed in the case study presented here may serve as proof of concept for future work on the breath-hold BOLD-fMRI technique in larger samples of patients with mTBI. Although the CVR map from breath-hold challenge replicates the primary features of abnormal CVR reported under CO2 challenge, changes in respiration (i.e., apnea during breath-hold and ventilatory increases during CO2-inhalation) are known to serve as sources of variance in the BOLD signal [24]. Future research of breath-hold effects would be needed to establish a balance between high SNR provided by CO2 inhalation and the convenience of the breath-hold protocol which is widely practiced in clinics [14, 15].
Taken together the findings in the presented case support the use of breath-hold fMRI to map CVR as a biomarker for mTBI. As a complement to the CVR maps, our detailed analyses of the shape of the MRI signal time series strengthens our confidence in the aberrant findings observed in each of the presented cases. Compared to the referenced breath-hold epochs, the change in MRI signal was asynchronous in the patient and synchronous in the healthy controls (Figure 2). Moreover, the hemispheric asymmetry and asynchronous MRI signal time series observed initially in the mTBI patient was observed to ‘normalize’ with time, coincident with the resolution of the patient’s mTBI symptoms (Figure 1). This finding is intriguing as it suggests that breath-hold fMRI could possibly serve to inform objective guidelines for optimizing treatment and estimate the possible consequences of repeated concussions [25, 26] Importantly, a biomarker with this capacity could likely guide 'return to play/duty' decisions in athletes, military personnel and other at-risk individuals with known mTBI history. This encourages the continued development of the breath-hold fMRI technique for future clinical trials in patients with mTBI.
Acknowledgments
This research was carried out in whole at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. This work was also supported, in part, by NIH-K23MH086619.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Mannix R, O'Brien MJ, Meehan WP., 3rd The epidemiology of outpatient visits for minor head injury: 2005 to 2009. Neurosurgery. 2013;73(1):129–134. doi: 10.1227/01.neu.0000429846.14579.41. discussion 134. [DOI] [PubMed] [Google Scholar]
- 2.Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, Onate JA, Kelly JP. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. Journal of the American Medical Association. 2003;290(19):2549–2555. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- 3.Iverson GL, Gaetz M, Lovell MR, Collins MW. Cumulative effects of concussion in amateur athletes. Brain Injury. 2004;18(5):433–443. doi: 10.1080/02699050310001617352. [DOI] [PubMed] [Google Scholar]
- 4.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57(4):719–726. doi: 10.1093/neurosurgery/57.4.719. discussion 719-26. [DOI] [PubMed] [Google Scholar]
- 5.Bigler ED. Neuroimaging Biomarkers in Mild Traumatic Brain Injury (mTBI) Neuropsychology Review. 2013;23(3):169–209. doi: 10.1007/s11065-013-9237-2. [DOI] [PubMed] [Google Scholar]
- 6.Len TK, Neary JP, Asmundson GJ, Goodman DG, Bjornson B, Bhambhani YN. Cerebrovascular reactivity impairment after sport-induced concussion. Medicine and Science in Sports and Exercise. 2011;43(12):2241–2248. doi: 10.1249/MSS.0b013e3182249539. [DOI] [PubMed] [Google Scholar]
- 7.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 8.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical; 1988. [Google Scholar]
- 9.Gold S, Christian B, Arndt S, Zeien G, Cizadlo T, Johnson DL, Flaum M, Andreasen NC. Functional MRI statistical software packages: a comparative analysis. Human Brain Mapping. 1998;6(2):73–84. doi: 10.1002/(SICI)1097-0193(1998)6:2<73::AID-HBM1>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conklin J, Fierstra J, Crawley AP, Han JS, Poublanc J, Mandell DM, Silver FL, Tymianski M, Fisher JA, Mikulis DJ. Impaired cerebrovascular reactivity with steal phenomenon is associated with increased diffusion in white matter of patients with Moyamoya disease. Stroke. 2010;41(8):1610–1616. doi: 10.1161/STROKEAHA.110.579540. [DOI] [PubMed] [Google Scholar]
- 11.Han JS, Mikulis DJ, Mardimae A, Kassner A, Poublanc J, Crawley AP, deVeber GA, Fisher JA, Logan WJ. Measurement of cerebrovascular reactivity in pediatric patients with cerebral vasculopathy using blood oxygen level-dependent MRI. Stroke. 2011;42(5):1261–1269. doi: 10.1161/STROKEAHA.110.603225. [DOI] [PubMed] [Google Scholar]
- 12.Krainik A, Hund-Georgiadis M, Zysset S, von Cramon DY. Regional impairment of cerebrovascular reactivity and BOLD signal in adults after stroke. Stroke. 2005;36(6):1146–1152. doi: 10.1161/01.STR.0000166178.40973.a7. [DOI] [PubMed] [Google Scholar]
- 13.Ziyeh S, Rick J, Reinhard M, Hetzel A, Mader I, Speck O. Blood oxygen level-dependent MRI of cerebral CO2 reactivity in severe carotid stenosis and occlusion. Stroke. 2005;36(4):751–756. doi: 10.1161/01.STR.0000157593.03470.3d. [DOI] [PubMed] [Google Scholar]
- 14.Chang TY, Liu HL, Lee TH, Kuan WC, Chang CH, Wu HC, Wu TC, Chang YJ. Change in cerebral perfusion after carotid angioplasty with stenting is related to cerebral vasoreactivity: a study using dynamic susceptibility-weighted contrast-enhanced MR imaging and functional MR imaging with a breath-holding paradigm. American Journal of Neuroradiology. 2009;30(7):1330–1336. doi: 10.3174/ajnr.A1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pillai JJ, Zaca D. Comparison of BOLD cerebrovascular reactivity mapping and DSC MR perfusion imaging for prediction of neurovascular uncoupling potential in brain tumors. Technology in Cancer Research and Treatment. 2012;11(4):361–374. doi: 10.7785/tcrt.2012.500284. [DOI] [PubMed] [Google Scholar]
- 16.Zaca D, Jovicich J, Nadar SR, Voyvodic JT, Pillai JJ. Cerebrovascular reactivity mapping in patients with low grade gliomas undergoing presurgical sensorimotor mapping with BOLD fMRI. Journal of Magnetic Resonance Imaging. 2013 doi: 10.1002/jmri.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505(7482):223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Gelderen P, de Zwart JA, Duyn JH. Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magnetic Resonance in Medicine. 2008;59(4):788–795. doi: 10.1002/mrm.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tancredi FB, Gauthier CJ, Madjar C, Bolar DS, Fisher JA, Wang DJ, Hoge RD. Comparison of pulsed and pseudocontinuous arterial spin-labeling for measuring CO2 -induced cerebrovascular reactivity. Journal of Magnetic Resonance Imaging. 2012;36(2):312–321. doi: 10.1002/jmri.23658. [DOI] [PubMed] [Google Scholar]
- 20.Tancredi FB, Hoge RD. Comparison of cerebral vascular reactivity measures obtained using breath-holding and CO2 inhalation. Journal of Cerebral Blood Flow and Metabolism. 2013;33(7):1066–1074. doi: 10.1038/jcbfm.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katzenberger RJ, Loewen CA, Wassarman DR, Petersen AJ, Ganetzky B, Wassarman DA. A Drosophila model of closed head traumatic brain injury. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(44):E4152–E4159. doi: 10.1073/pnas.1316895110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keightley ML, Saluja RS, Chen JK, Gagnon I, Leonard G, Petrides M, Ptito A. An fMRI Study of Working Memory in Youth Following Sports-related Concussion: Is it Still Working? Journal of Neurotrauma. 2013 doi: 10.1089/neu.2013.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vakhtin AA, Calhoun VD, Jung RE, Prestopnik JL, Taylor PA, Ford CC. Changes in intrinsic functional brain networks following blast-induced mild traumatic brain injury. Brain Injury. 2013;27(11):1304–1310. doi: 10.3109/02699052.2013.823561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang C, Glover GH. Relationship between respiration, end-tidal CO2, and BOLD signals in resting-state fMRI. NeuroImage. 2009;47(4):1381–1393. doi: 10.1016/j.neuroimage.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korngold C, Farrell HM, Fozdar M. The national football league and chronic traumatic encephalopathy: legal implications. The Journal of the American Academy of Psychiatry and the Law. 2013;41(3):430–436. [PubMed] [Google Scholar]
- 26.Longhi L, Saatman KE, Fujimoto S, Raghupathi R, Meaney DF, Davis J, McMillan BSA, Conte V, Laurer HL, Stein S, Stocchetti N, McIntosh TK. Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery. 2005;56(2):364–374. doi: 10.1227/01.neu.0000149008.73513.44. discussion 364-74. [DOI] [PubMed] [Google Scholar]