Abstract
Sonochemistry has become a well-known technique for fabricating nanomaterials. Since one of the advantages of nanomaterials is that they have higher chemical activities compared with particles in the bulk form, efforts are being made to produce nano organic compounds with enhanced biological activities that could be exploited in the medical area. This study uses the sonication technique to prepare nano Vitamin B12 and nano Penicillin, and demonstrates their enhanced biological and pharmacological activity. The size and morphology of the nano Penicillin and nano Vitamin B12 were investigated using electron microscopy as well as dynamic light scattering techniques. The sizes of Penicillin and Vitamin B12 nanoparticles (NPs) were found to be 70 and 120–180 nm, respectively. The bactericidal effect of nano Penicillin was studied and found to be higher than that of the bulk form. Reducing the size of Vitamin B12 resulted in their enhanced antioxidative activity as observed using the electron paramagnetic spectroscopy technique. The penetration depth of these organic NPs can be detected by an optical iterative method. It is believed that nano organic drugs fabrication will have a great impact on the medical field.
Keywords: sonochemistry, antibacterial, antioxidant, Vitamin B12, Penicillin, nanomaterials
Introduction
In recent years, sonochemistry has become a well-known technique for the fabrication of nanomaterials.1–4 Sonochemistry deals with sonic waves, their properties, and their effect on chemical systems. Previous to the sonication process, the bulk solution contains big aggregates of the material molecules, and those aggregates can be broken into molecules by the ultrasonic radiation. When ultrasonic waves (20 KHz–1 MHz) are applied to molecules in solution, they cause a chemical reaction (as shown in Figure 1). This happens due to a phenomenon called acoustic cavitation (the middle part in Figure 1). There are few theories explaining the effect of sonic radiation on the breakdown of chemical bonds. The consensus is that this phenomenon happens when a bubble, which was created in the liquid, grows and then collapses. It grows because the solute and/or the solvent vapors diffuse into the volume of the bubble, and it collapses when it gets to its maximum size. Upon the collapse of the bubble, chemical bonds can be broken/formed due to the development of very high local temperatures (>5,000°C).1 In the preparation of nano Penicillin and nano Vitamin B12, the material molecules are naturally drawn to the bubble surface, creating a shell of the material molecules around the bubble. During implosion, the molecules shell collapses into the bubble center and thereby creates a nanoparticle (NP) that contains many small molecules (a magnification of the NP is presented in the last phase of the process described in Figure 1).5
Figure 1.
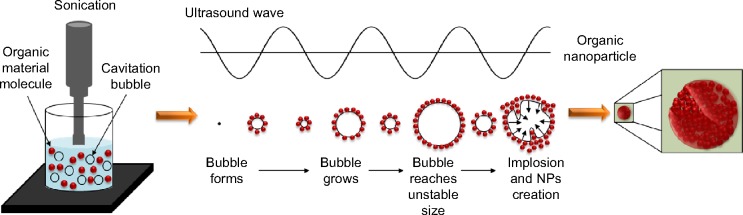
The acoustic cavitation phenomenon that occurs under ultrasonic radiation and the NP creation.
Notes: The acoustic cavitation phenomenon happens when a bubble, which was created in the liquid, grows and then collapses. The bubble grows because the solute and/or the solvent vapors diffuse into the volume of the bubble, and it collapses when it gets to its maximum size. In the preparation of nano Penicillin and nano Vitamin B12, the material molecules are naturally drawn to the bubble surface, creating a shell of the material molecules around the bubble. During implosion, the molecules shell collapses into the bubble center and thereby creates a nanoparticle that contains many small molecules (a magnification of the nanoparticle is presented in the last phase of the process).
Abbreviation: NP, nanoparticle.
Nanomaterials have an important advantage compared with their bulk counterparts. It is well known that when the particle size decreases, the proportion of the surface area increases, which predicts greater activity per given mass compared with larger particles.6 Therefore, the advantage of NPs is that they have higher activity than particles in the bulk form.7–10 An additional advantage of small size is the enhanced penetration through semisolid matrix such as skin and the underlying tissues. We have recently synthesized NPs of salts soluble in water such as NaCl, KI, KBr, and CuSO4. The salts’ nanocrystals were prepared by sonicating an aqueous solution of the mentioned salts.11 This method was later extended to organic molecules where solutions of the organic compounds were sonicated under ambient conditions and NPs of the organic compounds were prepared. Using the sonication technique, several organic nanomaterials were synthesized,12 such as enzyme (amylase) NPs,13,14 tannic acid NPs, or even RNA NPs.15,16
This study focuses on two organic materials, Penicillin and Vitamin B12. In addition to these two organic materials, other materials like tannic acid and more,12–17 were synthesized in a similar way by us and others. However, the synthesis of NPs from Penicillin and Vitamin B12 is first being reported here. Penicillin, which was discovered in 1928, is still widely used to treat bacterial infections. However, its bactericidal effect is limited because of the bacterial resistance to antibiotics.18 Vitamin B12 is known for its antioxidative properties19 and for its role as an efficient intracellular scavenger. By synthesizing NPs of Vitamin B12 and Penicillin, their biological and chemical activity would increase as well as their penetration depth within the skin and the underlying tissue. Recently, we showed that organic NPs (ONPs) can be detected within tissues17 by a novel optical iterative technique,17,20 and their skin penetration ability indeed increased with their size reduction. Several articles suggesting the conjugation or encapsulation of antibiotics/Vitamin B12 with/into NPs21–25 have been published; however, the formation of nanoantibiotics was reported only in the case of Nystatin26 following wet milling of the bulk form material for 4 hours. To the best of our knowledge, the production of Vitamin B12 NPs has not been yet reported.
This article is organized as follows: the next section describes the materials that were used in this work and the methods for fabricating and characterizing the nanoparticles. The size characterization, antibacterial and antioxidant activity, and the chemical structure of the particles examination results are presented and discussed in the following section. Finally, in the last section, the conclusion of the research work is presented in detail.
Material and methods
Fabrication of nanoparticles
Nano Vitamin B12 and nano Penicillin G were prepared using the sonochemistry technique.1,27 The bulk aqueous solutions of 36.9 μM and 1.7 mM Vitamin B12 (Teva Pharmaceuticals Industries Ltd., Petach Tikva, Israel) and Penicillin G (Sigma-Aldrich Co, St Louis, MO, USA), respectively, were prepared as follows: Penicillin G solution was prepared by dissolving the powder in DDW (double-distilled water, 18 Ω), and Vitamin B12 was obtained as a solution and diluted using DDW to the appropriate concentration. The prepared solutions were then irradiated with a high-intensity ultrasonic horn (Ti-horn, 20 KHz, 100 W/cm2) operating at 25% efficiency for up to 10 minutes.
Size characterization
In order to measure the NPs’ size and morphology, transmission electron microscopy (TEM) and dynamic light scattering (DLS) measurements were conducted.
The 2D imaging of the various samples was performed using TEM (JEM-1400; JEOL, Tokyo, Japan). Measurements were performed by drying a drop of product solution placed on a charged copper grid.
DLS analyses were performed with a Zetasizer Nano ZS series (Malvern Instruments, Malvern, UK) under a 633 nm He–Ne laser. The estimated average particle diameter in terms of intensity was measured by the instrument. Sample solutions were poured into a 1 mL disposable cuvette up to a height of 15 mm. The cuvette was placed in the sample holder, and the instrument was run automatically. The average value of three scans was calculated and evaluated. Since the sonochemical process may affect the chemical nature of the molecule, the bulk form and the modified products were characterized using UV-Vis spectroscopy (Cary 100 scan Varian UV/Vis spectrophotometer; Varian Inc., Palo Alto, CA, USA), Fourier transform infrared (FTIR) spectroscopy, and Raman spectroscopy. Nuclear magnetic resonance spectroscopy was considered; however, it was not used because of the complicated sample preparations and the possibility that use of heavy water in this preparation may affect our result.
Activity characterization
Antioxidant activity
Antioxidative activity of bulk form and modified Vitamin B12 was measured using the electron paramagnetic resonance (EPR) spectroscopy. EPR is a very sensitive technique for determining reactive oxygen species (ROS). To detect •OH, we used the EPR-spin trapping technique coupled with the spin traps 5,5-dimethyl-1-pyrroline N-oxide (DMPO, 0.02 M) (Sigma-Aldrich Co). The antioxidant activity was determined by measuring the amount of hydroxyl radical produced via the Fenton reaction before and after the addition of Vitamin B12 or nano Vitamin B12 (H2O2 + FeCl2 + 4H2O + DMPO + DDW/Vitamin B12 [74 fM]). Fenton reagents with Vitamin B12 were drawn by a syringe into a gas-permeable Teflon capillary (Zeus Industries, Raritan, NJ, USA) and inserted into a narrow quartz tube that was kept open at both ends. The tube was then placed in the EPR cavity, and the spectra were recorded on a Bruker EPR 100d X-band spectrometer (Bruker Corporation, Billerica, MA, USA). The conditions of the EPR measurement were as follows: frequency − 9.74 GHz; microwave power − 20 mW; scan width − 65 G; resolution − 1,024; receiver gain − 2×105; conversion time − 8 ms; time constant − 655 ms; sweep time − 84 s; scans − 2; modulation frequency − 100 kHz.
Purification and characterization of the spin traps
DMPO was purified in the dark in DDW, with activated charcoal. After about 30–60 minutes, the solution was filtered and its concentration was determined spectrophotometrically using ε227 nm =8.0 Mm−1 × cm−1. The solution was stored at −20°C for no longer than 2 weeks.
The EPR measurements for each suspension sample were performed at least three times, and the averaged value is plotted in the figures. After acquisition, the spectra were processed using the Bruker WIN-EPR software version 2.11 (Bruker Corporation) for baseline correction. The peak intensity was calculated by double integration of the peak signals, and the intensity was expressed in arbitrary units.
Antibacterial activity
Clinical isolate of Staphylcoccus aureus (S. aureus B5467161) was obtained from the clinical laboratories of Assaf HaRofeh Medical Center (Zrifin, Israel).
To examine the antibacterial activity of Penicillin and Penicillin NPs, the agar diffusion test28 and minimum inhibitory concentrations (MIC)29 were conducted on S. aureus B5467161 (161).
For the determination of bulk form and nanoantibiotic activity, cultures of representative bacteria strains were grown aerobically overnight at 37°C on plates containing Brain Heart Infusion Agar. Colonies were transferred into nutrient broth (NB) to obtain an optical density of A660 =0.1 (A660 refers to absorbance at 660 nm). They were then grown aerobically (37°C) in a rotary shaker (180 rpm) till the logarithmic stage (A660 = ~0.4) was reached. The cells were then diluted at 1:100 and spread uniformly on agar plates containing Nutrient agar. To test bactericidal activity, 10 μL drops of Penicillin containing an equal concentration of 6 μg (for both the bulk form and the nanoantibiotics solution) were placed on the surface of the plates, which were then incubated aerobically for 24 hours at 37°C.
For the MIC tests, serial twofold dilutions of bulk Penicillin and Penicillin NPs were made in test tubes containing 1 mL of NB medium. The inoculum’s density of bacterial isolate had a final concentration of ~1×106 CFU/mL. The results were examined following overnight incubation (37°C).
Chemical structure characterization
Penicillin chemical structure characterization
Ultrasonic irradiation was applied in order to form Penicillin NPs, and, therefore, it is necessary to examine whether the ultrasonic process damages the chemical structure of Penicillin molecules. FTIR spectroscopy was performed for the characterization of bulk Penicillin as well as for the sample irradiated sonochemically for 5 minutes. The FTIR spectra were recorded using the Avarter model FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) in the range of 4,000–1,000 cm−1. The concentration of the Penicillin used for the FTIR measurements was 0.6 mg/mL.
Vitamin B12 chemical structure characterization
In order to examine how the chemical structure of Vitamin B12 is affected following ultrasonic radiation, the Raman spectroscopy technique was used. Raman spectroscopy was performed for the characterization of bulk form and nano Vitamin B12. Raman spectra were collected using the Renishaw inVia Raman microscope (Renishaw inVia Raman, Gloucestershire, UK) equipped with a Leica DM2500 M analysis microscope (Leica Microsystems, Wetzlar, Germany), and sample focusing was done by a 50× (NA 0.75) lens. All the samples were irradiated by a 514 nm excitation wavelength. The concentration of the Vitamin B12 used for the Raman spectroscopy was 0.05 mg/mL.
Results and discussion
Characterization of bulk form and nano Penicillin
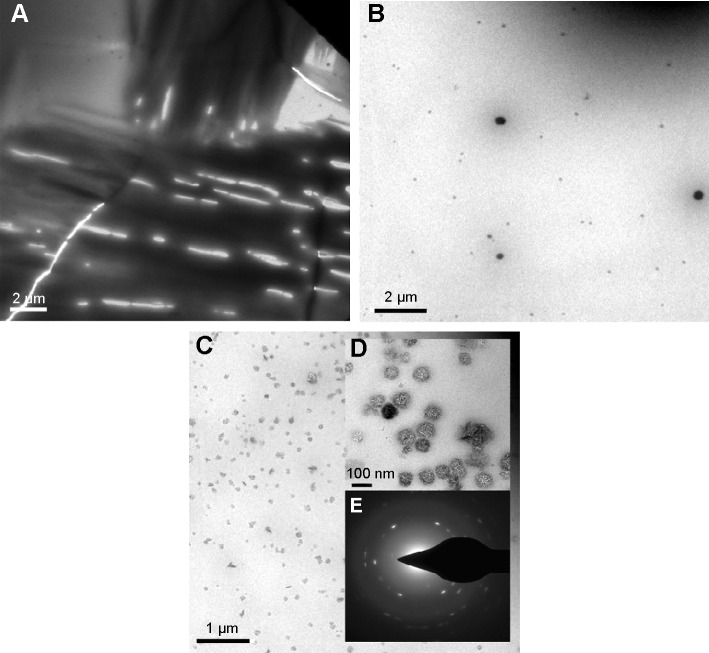
The morphology and size of both unmodified and nano Penicillin were studied using transmission microscopy analysis. The results of the TEM measurements are shown in (Figure 2). The bulk Penicillin (before the sonochemical reaction) created a thin layer of the compound that virtually covered the entire grid area (Figure 2A). Following the application of ultrasound (US), the formation of Penicillin NPs can be observed clearly in Figure 2B–D. It is also observed that the sonication time had an effect on particle size, namely, as the sonication time increases, the particle size decreases. After 5 minutes of sonication, a clear formation of particles is observed, with larger aggregates of Penicillin still present (Figure 2B). After 10 minutes of sonication (Figure 2C), the amount of particles increased significantly, and their size decreased (Figure 2C). An insert (Figure 2D) shows higher magnification of Penicillin NPs obtained after 10 minutes of sonication. As shown in the figure, the most prominent property of the particles is their uniformity and almost perfect spherical shape; the size of the NPs was measured to be ~70 nm.
Figure 2.
TEM images of Penicillin before and after the ultrasonic radiation.
Notes: (A) TEM image of Penicillin bulk form, (B) TEM image of Penicillin NPs after 5-minute sonication, (C) TEM images of Penicillin NPs after 10-minute sonication, (D) an enlargement of TEM image of Penicillin NPs after 10-minute sonication, and (E) diffraction lines of individual Penicillin nanocrystal.
Abbreviations: TEM, transmission electron microscopy; NPs, nanoparticles.
Figure 2E depicts the existence of diffraction lines in the nanosized Penicillin, showing its crystalline nature. The activity of Penicillin nanocrystals will be discussed below.
DLS is an important tool for characterizing the size of NPs in solution (biological size of the NPs). In fact, the DLS measurement requires a larger number of particles (several orders of magnitude greater) compared with TEM, and thus provides much better statistics. However, it is important to remember that in some cases, the hydrodynamic size is affected by the hydrophilicity/hydrophobicity nature of the substance, and therefore the size measured by DLS appears larger than that of the TEM. The size of the nano Penicillin, upon 5 minutes of ultrasonic radiation, as measured by the DLS technique, was found to be 150±15 nm. The big size difference in nano Penicillin that was recorded in TEM and DLS measurements may be a result of aggregation that occurred between the NPs. However, it is important to note that in spite of this aggregation, we produced nanosized particles that differ from aggregation of molecules, which can reach to micrometer-size particles.
Occurrence of NPs was also observed in bulk Penicillin; however, their concentration was very low, and most of the Penicillin appeared to be like an amorphous film (as can be noticed in the TEM image [Figure 2A]).
Measurements of Penicillin antibacterial activity
The antibacterial activity of the Penicillin NPs, as opposed to that of the bulk material, was examined with both the agar diffusion test and the determination of MIC.
The diameters of the inhibition zones received in the agar diffusion test can be seen in Table 1. The experiment was conducted for the bacteria with Penicillin NPs at different sonication times. As can be observed, S. aureus 161 inhibition zone sizes have grown with the sonication time of the Penicillin NPs, while the Penicillin solution (control) has given the lowest diameter. These results indicate that Penicillin NPs can achieve a better antibacterial effect.
Table 1.
Inhibition zone diameter for the different Penicillin NPs
| Bacteria | Inhibition zone diameter (mm)
|
||
|---|---|---|---|
| Control | 3 minute | 5 minute | |
| S. aureus B5467161 | 21.75±0.4 | 24.5±0.5 | 24.5±1.2 |
Notes: The inhibition zone diameter for bulk Penicillin and Penicillin following 3- and 5-minute sonication (average ± standard deviation).
Abbreviations: NPs, nanoparticles; S. aureus, Staphylcoccus aureus.
The results of the MIC experiments presented in Table 2 further prove the higher antibacterial activity of nano Penicillin. The MIC of the nanoantibiotics was up to 1 units/mL, while the MIC of the unmodified (bulk form) antibiotics was higher than 33 units/mL. These results clearly demonstrate a higher antibacterial activity of the sonicated drug in comparison with the control sample. Two possible explanations can be proposed for the observed higher activity: 1) increased surface area of the smaller particles; 2) sonochemical irradiation promotes the formation of Penicillin nanocrystals (as seen from the TEM diffraction lines, Figure 2E). Organic nanocrystals first appeared in the cosmetic market in the year 2000 with the product “Juvedical.”30 It was demonstrated that compared with the original molecules, the nanocrystal formulation possessed 500 times higher bioactivity.31 The US-induced formation of Penicillin nanocrystals might explain the higher antibacterial activity of the nano Penicillin described in this article.
Table 2.
MIC for the different Penicillin NPs
| Bacteria | MIC
|
||
|---|---|---|---|
| Control | 3 minute | 5 minute | |
| S. aureus B5467161 | >33 units/mL | 1 unit/mL | 0.2 units/mL |
Notes: MIC results for bulk Penicillin and Penicillin following 3- and 5-minute sonication.
Abbreviations: MIC, minimum inhibition concentration; NPs, nanoparticles; S. aureus, Staphylcoccus aureus.
We also verified that the specific antibacterial activity of Penicillin is not changed due to sonication. It is generally accepted that gram-negative bacteria are less vulnerable to a Penicillin attack because their peptidoglycans are shielded by the outer membranes. Therefore, we tested the antibacterial activity of bulk form and nano Penicillin on Escherichia coli (gram-negative bacteria). Both Penicillin and nano Penicillin had no effect on E. coli viability, proving that the specific action of the antibiotic is retained in the nano form.
The agar diffusion test and MIC were used for Penicillin NPs after 3, 5, and 10 minutes of sonication. The results achieved from those tests for Penicillin NPs after 10 minutes did not present better antibacterial activity in comparison with the NPs after 5 minutes of sonication. Those results indicate that in spite of the increased amount and decreased size in nano Penicillin after 10 minutes of sonication, the antibacterial activity was not enhanced any more than what was obtained for Penicillin after 5-minute sonication. Hence, the examination of Penicillin NPs after more than 10-minute sonication was not performed.
Confirmation of unchanged chemical structure of bulk form and nano Penicillin
The chemical structure of bulk Penicillin in comparison with nano Penicillin was examined by FTIR spectroscopy. The representative vibrational peaks for Penicillin, presented in Figure 3, were found to be unchanged, and no new peaks were detected. The spectra show no change in the chemical structure of Penicillin upon 5-minute ultrasonic irradiation. The FTIR characterizations of bulk form and nano Penicillin (solid and dashed lines, respectively, in Figure 3) show that the Penicillin molecules did not undergo chemical changes for the duration of the ultrasonic radiation.
Figure 3.
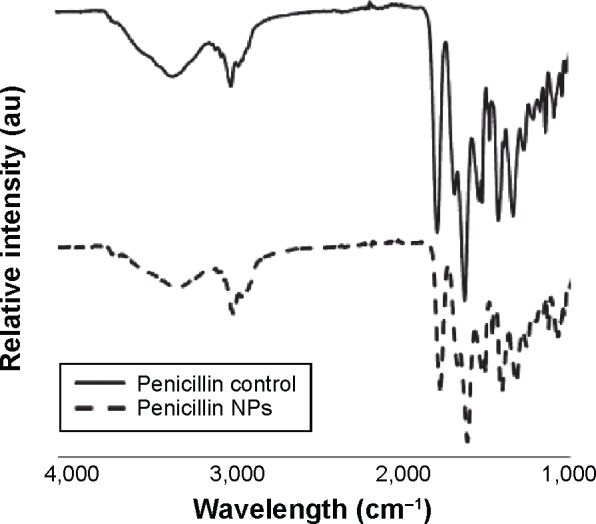
Comparison of FTIR spectra of Penicillin before and after ultrasonic radiation.
Notes: FTIR spectrum of bulk Penicillin (solid line) and 5-minute ultrasonicated Penicillin (dashed line). The spectra show no change in the chemical structure of Penicillin upon 5-minute ultrasonic irradiation.
Abbreviations: FTIR, Fourier transform infrared spectroscopy; NPs, nanoparticles.
Characterization of bulk form and nano Vitamin B12
Figure 4 describes the hydrodynamic particle size of Vitamin B12. For Vitamin B12, the hydrodynamic particle size significantly decreased with the sonication time. The hydrodynamic particle size of the unmodified Vitamin B12 was 847 nm ±127 nm, while that of the sonicated sample was reduced tô179 nm ±27 nm and ~124 nm ±19 nm following sonication of 3 and 5 minutes, respectively.
Figure 4.
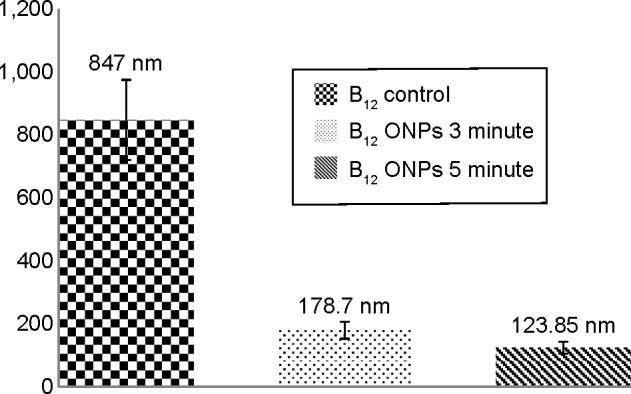
A comparison of size determination for Vitamin B12 by DLS measurements.
Notes: Size determination of bulk Vitamin B12 and Vitamin B12 upon 3- and 5-minute ultrasonic radiation.
Abbreviations: ONPs, organic nanoparticles; DLS, dynamic light scattering.
A very similar reduction in size (a factor of 4) was also observed in the TEM images of Vitamin B12 solution. Figure 5A shows the image of Vitamin B12 in the bulk form, and Figure 5B presents the sonochemically prepared Vitamin B12. It can be observed that Vitamin B12 in bulk form (Figure 5A) created big lumps. However, following sonication (Figure 5B), nanosized Vitamin B12 (segments of 100 nm×20 nm) are easily observed.
Figure 5.
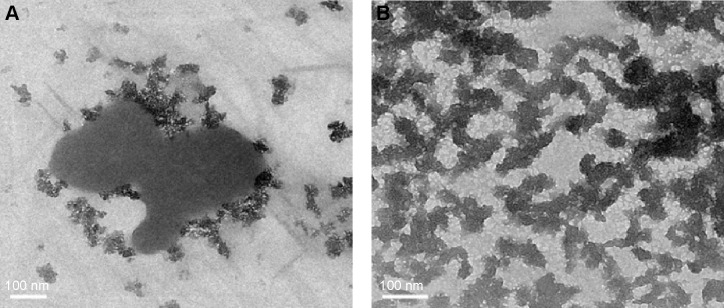
TEM images of Vitamin B12 before and after sonication.
Notes: (A) TEM image of Vitamin B12 bulk form. (B) TEM image of Vitamin B12 NPs upon 5-minute ultrasonic radiation.
Abbreviations: TEM, transmission electron microscopy; NPs, nanoparticles.
To verify that the sonication process did not alter the molecular structure of Vitamin B12, we measured the UV-Vis absorption spectra of Vitamin B12 before and after sonication. Figure 6 presents the absorption spectra of Vitamin B12 before the sonochemical synthesis (dashed line) and after 3 and 5 minutes of US operation (solid line and dots, respectively). As can be observed (Figure 6), the sonochemical process did not alter Vitamin B12 absorption bands, indicating that there is no change in the molecule structure following sonication.
Figure 6.
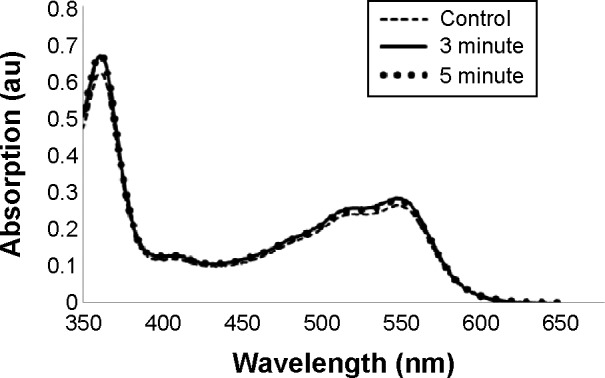
The absorption spectra of Vitamin B12 before and following ultrasonic radiation.
Notes: The absorption spectra of bulk Vitamin B12 (control, dashed line), Vitamin B12 following 3-minute sonication (3 minute, solid line), and Vitamin B12 after 5-minute sonication (5 minute, dots).
Measurements of Vitamin B12 antioxidation activity
Significant attention has been paid to antioxidants that play an important role in preventing ROS-induced damage. Vitamin B12 is known for its antioxidative properties.32 By reducing the size of Vitamin B12, it is expected to increase its antioxidative capability. The antioxidative properties of bulk form and nano Vitamin B12 were measured using the EPR technique, as described in “Material and methods” section. The results in Figure 7A present the typical spectra produced by the OH radicals’ formation from Fenton reaction. Figure 7B and C depicts the quartet indicative of hydroxyl radicals generated in a Fenton reaction in the presence of the bulk form of Vitamin B12 and Vitamin B12 NPs upon 5 minutes of ultrasonic radiation respectively. As can be seen from the decrease in the intensity of the quartet in Figure 7C, relative to that in Figure 7B, the antioxidative capability of Vitamin B12 NPs is greater than that of the bulk form.
Figure 7.
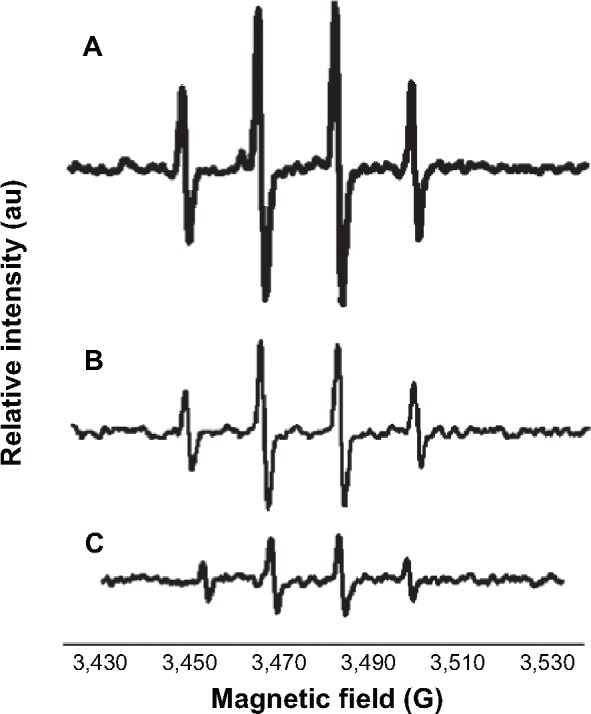
Hydroxyl radical formation via Fenton reaction in the presence of Vitamin B12 bulk form and NPs.
Notes: (A) The formation of hydroxyl radical via Fenton reaction. (B) The formation of hydroxyl radical in the presence of bulk Vitamin B12. (C) The formation of hydroxyl radical in the presence of nano Vitamin B12 obtained after 5-minute sonication.
The amount of DMPO-trapped radicals, as presented in Figure 8, shows that the produced ROS amount for Vitamin B12 NPs is approximately twice as low as for the bulk form. These results indicate that sonication of Vitamin B12 created a greater antioxidant activity in comparison with Vitamin B12 in the bulk form.
Figure 8.
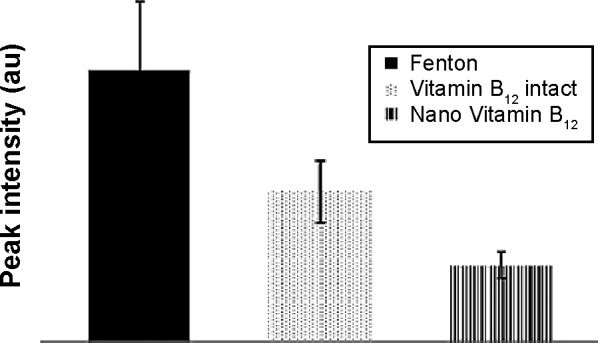
A comparison of hydroxyl radicals’ production.
Notes: The production of hydroxyl radicals (calculated by the integrated areas of DMPO spin adduct) via Fenton reaction in comparison with the production of hydroxyl radicals (calculated by the integrated areas of DMPO spin adduct) via Fenton reaction in the presence of bulk form and nano Vitamin B12 obtained after 5-minute sonication.
Abbreviation: DMPO, 5,5-dimethyl-1-pyrroline N-oxide.
The enhanced antioxidant activity of nanosized Vitamin B12 originates in the increased surface area of the smaller particles produced due to the US radiation.
Confirmation of unchanged chemical structure of bulk form and nano Vitamin B12
In order to confirm the unchanged chemical structure of Vitamin B12 upon ultrasonic radiation, Raman spectroscopy was applied. The important characteristic peaks of Vitamin B12 were identified by Raman measurements, before and after sonication, and are shown in Figure 9A and B, respectively. It can be observed that the characteristic peaks have not been changed. Moreover, the formation of new peaks was not observed, proving that the sonication process does not change the chemical nature of the Vitamin B12 molecule.
Figure 9.
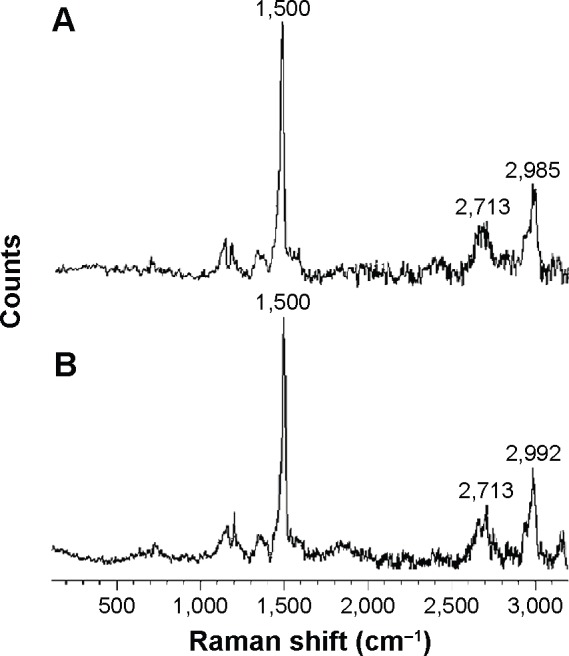
A comparison of Raman spectra of Vitamin B12 before and after ultrasonic radiation.
Notes: (A) Raman spectrum of bulk Vitamin B12. (B) Raman spectrum of Vitamin B12 upon 5-minute ultrasonic radiation. The results indicate the confirmation of the chemical structure of bulk form and nano Vitamin B12.
Since nano organic compounds tend to recombine in an aqueous solution, thus losing their enhanced activity, their stability/“shelf life” was examined by measuring their specific activity. Both Penicillin and Vitamin B12 NPs were stored at 5°C for several days, during which DLS measurements were conducted to examine their stability. The results indicated that the Penicillin and Vitamin B12 NPs stability was maintained during this time. In order to receive a more stable product, it is suggested that they be immersed in a nonaqueous cream (based on oils, fatty acids, Glycerol, Glycol, etc) following the US radiation.
Conclusion
In the present study, we have demonstrated that a relatively very short, one-step, sonochemical reaction1,27 of two organic drugs, Penicillin and Vitamin B12, resulted in the formation of NPs, exhibiting a stronger biological activity compared with the bulk form. The reaction was performed in a water solution of the bulk material without the need for adaptive materials or purification steps. The size and morphology of the nano Penicillin and Vitamin B12 were investigated using electron microscopy as well as DLS techniques. The bactericidal effect of nano Penicillin was studied and found to be higher than that of the bulk form. Reducing the size of Vitamin B12 resulted in its enhanced antioxidative activity as observed using the EPR spectroscopy technique.
No change in the chemical nature of the molecules was observed following sonication. It is evident that the formation of the nano drugs gave new life to the compounds, preserving their specificity while enhancing their activity. This study clearly shows that US enhances or even restores the activity of drugs. The NPs of Penicillin and Vitamin B12, in an aqueous solution, were left uniformly suspended without aggregation for several days. It is also possible to dry those solutions by lyophilization and then redissolve them, as with many medications today. In addition to the increased biological activity of nano Penicillin and Vitamin B12, it can be assumed that their penetration into tissues and cellular sites is higher than that of the unmodified drugs. The increased penetration ability of the ONPs can be detected by an iterative optical technique that was developed in our lab.17,20 On the basis of the presented results, we would recommend the use of ultrasonic radiation for a duration of 5 minutes for the preparation of Penicillin and Vitamin B12 NPs-based drugs. We believe that nano organic drugs fabrication would have a great impact in medicine.
Acknowledgments
The authors are grateful to Prof Lellouche’s Lab, Chemistry Department, Bar-Ilan University, for their assistance with the FTIR and Raman spectroscopy measurements, and to Dr Gruzman’s Lab, Chemistry Department, Bar-Ilan University, for the use of their lyophilizer in the sample preparation process for FTIR and Raman spectroscopy.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gedanken A. Using sonochemistry for the fabrication of nanomaterials. Ultrason Sonochem. 2004;11(2):47–55. doi: 10.1016/j.ultsonch.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 2.Zheng YY, Zhu TJ, Zhao XB, Tu JP, Cao GS. Sonochemical synthesis of nanocrystalline Bi2Te3 thermoelectric compounds. Mater Lett. 2005;59(23):2886–2888. [Google Scholar]
- 3.Bang JH, Suslick KS. Sonochemical synthesis of nanosized hollow hematite. J Am Chem Soc. 2007;129(8):2242–2243. doi: 10.1021/ja0676657. [DOI] [PubMed] [Google Scholar]
- 4.Suslick K, Fang M, Hyeon T, Mdleleni M. Sonochemistry and Sonoluminescence. New York, NY: Springer; 1999. Applications of sonochemistry to materials synthesis. [Google Scholar]
- 5.Shimanovich U, Lipovsky A, Eliaz D, et al. Tetracycline nanoparticles as antibacterial and gene-silencing agents. Adv Healthc Mater. 2014 Nov 25; doi: 10.1002/adhm.201400631. Epub. [DOI] [PubMed] [Google Scholar]
- 6.Ansari AA, Khan MN, Alhoshan M, Aldwayyan AS, Alsalhi MS. Nanostructured materials: classification, properties, fabrication, characterization and their applications in biomedical sciences. In: Kestell AE, DeLorey GT, editors. Nanoparticles: Properties, Classification, Characterization, and Fabrication. Hauppauge, NY: Nova Science Publishers, Inc; 2010. [Google Scholar]
- 7.Leutwyler WK, Bürgi SL, Burgl H. Semiconductor clusters, nanocrystals, and quantum dots. Science. 1996;271(5251):933–937. [Google Scholar]
- 8.Gelperina S, Kisich K, Iseman MD, Heifets L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care. 2005;172(12):1487–1490. doi: 10.1164/rccm.200504-613PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui Y, Wei Q, Park H, Lieber CM. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science. 2001;293(5533):1289–1292. doi: 10.1126/science.1062711. [DOI] [PubMed] [Google Scholar]
- 10.Fubini B. Surface reactivity in the pathogenic response to particulates. Environ Health Perspect. 1997;105(Suppl 5):1013. doi: 10.1289/ehp.97105s51013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiel S, Grinberg O, Perkas N, Charmet J, Kepner H, Gedanken A. Forming nanoparticles of water-soluble ionic molecules and embedding them into polymer and glass substrates. Beilstein J Nanotechnol. 2012;3(1):267–276. doi: 10.3762/bjnano.3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grinberg O, Shimanovich U, Gedanken A. Encapsulating bioactive materials in sonochemically produced micro- and nano-spheres. J Mater Chem B. 2013;1(5):595–605. doi: 10.1039/c2tb00006g. [DOI] [PubMed] [Google Scholar]
- 13.Meridor D, Gedanken A. Forming nanoparticles of α-amylase and embedding them into solid surfaces. J Mol Catal B-Enzym. 2013;90:43–48. [Google Scholar]
- 14.Meridor D, Gedanken A. Preparation of enzyme nanoparticles and studying the catalytic activity of the immobilized nanoparticles on polyethylene films. Ultrason Sonochem. 2013;20(1):425–431. doi: 10.1016/j.ultsonch.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Perelshtein I, Ruderman E, Francesko A, Fernandes MM, Tzanov T, Gedanken A. Tannic acid NPs – synthesis and immobilization onto a solid surface in a one-step process and their antibacterial and anti-inflammatory properties. Ultrason Sonochem. 2014;21(6):1916–1920. doi: 10.1016/j.ultsonch.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Benisvy-Aharonovich E, Shimanovich U, Kronfeld N, et al. Pre-miRNA expressing plasmid delivery for anti-cancer therapy. Med Chem Commun. 2014;5(4):459–462. [Google Scholar]
- 17.Yariv I, Rahamim G, Shliselberg E, et al. Detecting nanoparticles in tissue using an optical iterative technique. Biomed Opt Express. 2014;5(11):3871–3881. doi: 10.1364/BOE.5.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 19.Moreira ES, Brasch NE, Yun J. Vitamin B12 protects against superoxide-induced cell injury in human aortic endothelial cells. Free Radic Biol Med. 2011;51(4):876–883. doi: 10.1016/j.freeradbiomed.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yariv I, Barnea Y, Genzel E, Duadi H, Fixler D. Detecting concentrations of milk components by an iterative optical technique. J Biophotonics. 2015 Feb 26; doi: 10.1002/jbio.201400144. Epub. [DOI] [PubMed] [Google Scholar]
- 21.Salman H, Gamazo C, de Smidt PC, Russell-Jones G, Irache J. Evaluation of Bioadhesive capacity and immunoadjuvant properties of vitamin B12 -Gantrez nanoparticles. Pharm Res. 2008;25(12):2859–2868. doi: 10.1007/s11095-008-9657-5. [DOI] [PubMed] [Google Scholar]
- 22.de Britto D, de Moura MR, Aouada FA, Mattoso LHC, Assis OBG. N,N,N-trimethyl chitosan nanoparticles as a vitamin carrier system. Food Hydrocolloid. 2012;27(2):487–493. [Google Scholar]
- 23.Pinto-Alphandary H, Andremont A, Couvreur P. Targeted delivery of antibiotics using liposomes and nanoparticles: research and applications. Int J Antimicrob Agents. 2000;13(3):155–168. doi: 10.1016/s0924-8579(99)00121-1. [DOI] [PubMed] [Google Scholar]
- 24.Saha B, Bhattacharya J, Mukherjee A, et al. In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics. Nanoscale Res Lett. 2007;2(12):614–622. [Google Scholar]
- 25.Turos E, Shim J-Y, Wang Y, et al. Antibiotic-conjugated polyacrylate nanoparticles: New opportunities for development of anti-MRSA agents. Bioorg Med Chem Lett. 2007;17(1):53–56. doi: 10.1016/j.bmcl.2006.09.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melkoumov A, Goupil M, Louhichi F, Raymond M, de Repentigny L, Leclair G. Nystatin nanosizing enhances in vitro and in vivo antifungal activity against Candida albicans. J Antimicrob Chemother. 2013;68(9):2099–2105. doi: 10.1093/jac/dkt137. [DOI] [PubMed] [Google Scholar]
- 27.Gedanken A. Sonochemistry and its application to nanochemistry. Curr Sci India. 2003;85(12):1720–1722. [Google Scholar]
- 28.Clinical and Laboratory Standards Institute M100-S17: Performance Standards for Antimicrobial susceptibility testing; 17th Informational Supplement; January 2007; Wayne, PA. [Google Scholar]
- 29.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemoth. 2001;48(Suppl 1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto J, Annapragada A, Decuzzi P, Ferrari M. Antibiological barrier nanovector technology for cancer applications. Expert Opin Drug Deliv. 2007;4(4):359–369. doi: 10.1517/17425247.4.4.359. [DOI] [PubMed] [Google Scholar]
- 31.Petersen R, Inventor, US Patents, assignee Nanocrystals for use in topical cosmetic formulations and method of production thereof. 20100047297 A12010 United States patent US.
- 32.Manzanares W, Hardy G. Vitamin B12: the forgotten micronutrient for critical care. Curr Opin Clin Nutr Metab Care. 2010;13(6):662–668. doi: 10.1097/MCO.0b013e32833dfaec. [DOI] [PubMed] [Google Scholar]