Abstract
Background
Asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome (ACOS) is associated with rapid decline in lung function, poorer health-related quality-of-life outcomes, and frequent exacerbations, compared to COPD alone. Although the numbers of patients with ACOS have increased, there is little established evidence regarding diagnostic criteria and treatment options. Thus, the aim of our study was to clarify the clinical, physiological, and radiological features of patients with ACOS.
Methods
We examined a total of 100 patients with COPD and 40 patients with ACOS, who were selected based on clinical criteria. All patients underwent baseline testing, including a COPD assessment test, pulmonary function tests, and multidetector row computed tomography imaging. Percentage of low attenuation volume, percentage of wall area, and percentage of total cross-sectional area of pulmonary vessels less than 5 mm2 (%CSA <5) were determined using multidetector row computed tomography. ACOS patients were administered a fixed dose of budesonide/formoterol (160/4.5 μg, two inhalations; twice daily) for 12 weeks, after which the ACOS patients underwent multidetector row computed tomography to measure the same parameters.
Results
At baseline, the ACOS patients and COPD patients had a similar degree of airflow limitation, vital capacity, and residual volume. ACOS patients had higher COPD assessment test scores, percentage of wall area, and %CSA <5 than COPD patients. Compared to baseline, budesonide/formoterol treatment significantly increased the forced expiratory volume in 1 second and decreased the degree of airway wall thickness (percentage of wall area) as well as pulmonary microvascular density (%CSA <5) in ACOS patients.
Conclusion
Our results suggest that ACOS is characterized by an airway lesion–dominant phenotype, in contrast to COPD. Higher %CSA <5 might be a characteristic feature of ACOS.
Keywords: budesonide/formoterol, cross-sectional area (CSA), pulmonary vessels, percentage of wall area (WA%)
Introduction
Asthma and COPD are two major obstructive airway diseases. Although their pathogeneses differ in origin, they share similar physiological features and may clinically co-exist as so-called asthma–COPD overlap syndrome (ACOS). Patients with ACOS experience more rapid decline in lung function, frequent exacerbations, have poorer health-related quality-of-life (HRQoL) outcomes, and require a large amount of medical resources compared to patients with asthma or COPD alone.1–5 The number of patients with ACOS is expected to increase as asthma patients become older and with an increased recognition of this syndrome by physicians. However, there is little established evidence regarding its diagnosis and treatment, as ACOS patients have been excluded from clinical trials for both asthma and COPD. After the Science Committees of both the Global Initiative for Asthma and Global Initiative for Chronic Obstructive Lung Disease provided a consensus document describing ACOS, this outcast syndrome has become an active area of research.6
Asthma and COPD do not have independent or exclusive pathological features; their features can overlap. Subepithelial hypervascularity and angiogenesis have been reported to be involved in the structural remodeling of the airway wall in asthma and are related to pulmonary function and bronchial hyperresponsiveness.7,8 Patients with ACOS have an asthmatic component and therefore may manifest to some degree the same clinical and pathophysiological features as patients with asthma. It was reported that inhaled corticosteroids (ICS) play a role in the downregulation of angiogenic remodeling of the airways in asthma.9–11 However, ICS treatment for COPD is controversial, although several large, randomized controlled trials have demonstrated that ICS treatment for severe to very severe COPD patients is associated with reduction in the number of clinical exacerbations.12–14 Bronchodilators are key drugs for patients with COPD, whereas bronchodilators used alone are not recommended for treating patients with asthma.5 ICS/long-acting β-agonist (LABA) use may be very beneficial for ACOS patients, even during early-stage disease; therefore, understanding the clinical differences between ACOS and COPD patients is very important.
As we previously reported, the cross-sectional area (CSA) of small pulmonary vessels has been proposed for use as a radiological parameter that reflects alterations in pulmonary vessels,15 and airway wall thickness is easily measured by computed tomography (CT).16
We performed this study based on the hypothesis that patients with ACOS might have more airway lesions and have more subepithelial vascularity than patients with COPD and therefore benefit from treatment with ICS/LABA. Thus, the aim of this study was to evaluate the clinical, physiological, and radiological differences between ACOS and COPD.
Methods
Subjects
Our study subjects included 243 consecutive patients who were diagnosed with or were suspected of having clinically stable COPD at Chiba University Hospital from July 2010 to July 2014. All patients had a history of smoking. Asthma was diagnosed based on a post-bronchodilator increase in forced expiratory volume in 1 second (FEV1), a bronchodilator response, diurnal variations in peak expiratory flow (PEF) recordings, or moderate to severe bronchial hyperreactivity, together with a clinical history compatible with asthma based on British Guidelines on Asthma Management.17 COPD was diagnosed based on smoking history, physical examination, respiratory symptoms, and spirometric results based on the Global Initiative for Chronic Obstructive Lung Disease criteria.18 A diagnosis of ACOS was made when a patient fulfilled the diagnostic criteria for both asthma and COPD. We also retrospectively confirmed that all the ACOS patients who were selected by our criteria fulfilled recent ACOS criteria determined by a joint statement released by the Global Initiative for Asthma and the Global Initiative for Chronic Obstructive Lung Disease.6 No patient had experienced any acute exacerbations for at least 1 month prior to entry in this study.
We excluded patients for (1) any obvious abnormal lung parenchymal lesions, including interstitial pneumonia; (2) lung cancer; (3) congestive heart failure; (4) infectious disease including lung tuberculosis; (5) other obstructive lung diseases such as obliterative bronchiolitis or diffuse panbronchiolitis; and (6) any cognitive disorder.
After patient selection, a total of 100 COPD patients and 40 ACOS patients were included in this study (Figure 1). The proportion with ACOS among all COPD patients was 28.6%, which was quite similar to the previously reported prevalence.19 Of the ACOS patients, 20 treatment-naïve patients were administered budesonide/formoterol therapy. They underwent a COPD assessment test (CAT), pulmonary function tests (PFTs), and multidetector row CT (MDCT) imaging at pre- and posttreatment on the same day. They also provided self-reported PEF rate results. ACOS exacerbation was defined as a short course of treatment with prednisolone (up to 2 weeks) alone or in combination with an antibiotic or an acute admission to the hospital because of ACOS.
Figure 1.
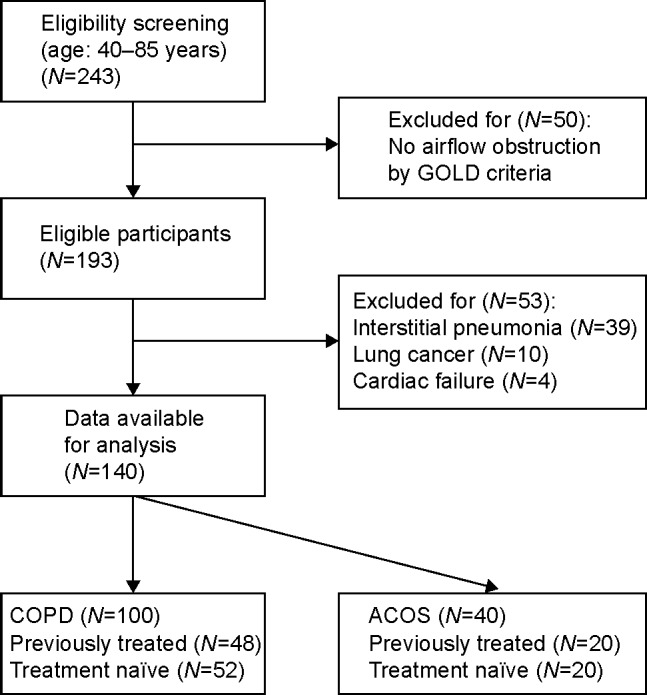
Flow chart for selecting study participants.
Abbreviations: ACOS, asthma–chronic obstructive pulmonary disease overlap syndrome; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
This study was approved by the Ethics Committee of Chiba University (approval no 857 and G22036). Written informed consent was obtained from all participants.
Pulmonary function tests
After the patients inhaled a short-acting β-agonist (SABA), PFTs were conducted using a CHSTAC-8900 (Chest MI Corp, Tokyo, Japan), according to the American Thoracic Society and European Respiratory Society guidelines.18 Total lung volume and diffusing capacity of the lungs for carbon monoxide (DLCO) were measured by helium dilution and the single-breath method, respectively. Percentage of predicted forced expiratory volume in 1 second (FEV1%predicted) and percentage of the DLCO per liter of lung volume predicted value (DLCO/VA [%predicted]) were determined based on Japanese Respiratory Society guidelines.20
MDCT scanning
All patients underwent 64-MDCT scanning (Aquillion ONE; Toshiba Medical, Tokyo, Japan) while at full inspiration. No contrast medium was used. MDCT scan parameters were collimation of 0.5 mm; 120 kV; 200 mA (automatic exposure control system); gantry rotation time of 0.5 seconds; and beam pitch of 0.83. All images were reconstructed using standard reconstruction algorithms with a slice thickness of 0.5 mm and a reconstruction interval of 0.5 mm. The voxel size was 0.63×0.63×0.5 mm.
CT measurements of airway luminal area, wall area, and low attenuation volume
Reconstruction images were transferred to a commercial workstation (Aze, AZE Ltd., Tokyo, Japan). A three-dimensional bronchial pathway was automatically reconstructed from transverse, sagittal, and coronal images. The bronchial pathway was converted to a curved multiplane reconstruction of the bronchial long axis, from which a bronchial short-axis image was generated perpendicular to the long axis. The short-axis image was used for automated determinations using the full-width at half-maximum principle. We determined the area of the bronchiolar lumen (Ai), and the percentage of wall area (WA%) was determined by 100% × WA/(WA + Ai). The outline of the airway wall was manually corrected when the computer-generated outline was obviously out of contour. Fourth (subsegmental) bronchial generations were identified, and mean Ai and WA% values were obtained for each generation. The apical bronchus of the upper lobe was evaluated on an inspiratory image. A change in Ai between inspiration and expiration was determined by 100% × (inspiratory Ai − expiratory Ai)/inspiratory Ai. Based on a threshold of −960 Hounsfield units, total lung volume and low attenuation volume (LAV) were also obtained. LAV% was determined by: 100% × LAV/total lung volume. Values were confirmed independently by two pulmonologists (TS and YM) who were blinded to all clinical information.
CT measurements of small pulmonary vessels
For measurements of the CSA of small pulmonary vessels, three MDCT images were selected: level at 1 cm above the upper margin of the aortic arch (upper lung fields), 1 cm below the carina (middle lung fields), and 1 cm below the right inferior pulmonary vein (lower lung fields). MDCT images were analyzed using Image J software, Version 1.44 (imagej.nih.gov/ij/download/).
CSA measurements were made using the following steps. The threshold technique was adopted for a lung field with attenuation between −500 and −1,024 Hounsfield units. Segmented images were converted to binary images with a window level of −720 Hounsfield units. Pulmonary vessels were displayed in black. The range of circularity was set from 0.9 to 1.0 using the “Analyze Particles” function incorporated in ImageJ software. Percentage of total cross-sectional area of pulmonary vessels less than 5 mm2 (%CSA <5) was determined at the subsegmental level.21 CSAs on the three selected CT slices were summed, and the average of these values was determined. These data were confirmed independently by two pulmonologists (TS and YM) who were blinded to all clinical information.
Budesonide/formoterol treatment
A fixed dose of budesonide/formoterol (Symbicort®) of 160/4.5 μg, two inhalations twice daily, was administered to 20 treatment-naïve ACOS patients. Prior to their inclusion in this study, none of these patients had taken any medications for asthma or COPD, including a corticosteroid(oral or inhaled) and other bronchodilators. Patients were allowed to use single maintenance and reliever therapy with budesonide/formoterol to control minor asthma attacks.
Self-reported diurnal variations in PEF rates
The 20 ACOS patients were provided with peak flow meters (Mini-Wright Peak Flow Meter®; Clement Clarke International, Harlow, UK) to self-record their PEF results and a diary for keeping a record of these readings. All these patients were fully trained on the correct use of this equipment, and any of their questions were answered. They were instructed to record PEF while erect five times per day (upon waking up in the morning, between 9 and 11 am, between 2 and 4 pm, between 6 and 8 am, and at bedtime) before and at 3 months after starting budesonide/formoterol therapy. Diurnal variations in PEF were determined by: (day’s highest minus day’s lowest)/(mean of day’s highest and lowest); these results were averaged over 1 week.22
Statistical analysis
Results are given as the mean ± standard deviation. The results of PFTs, CATs, and radiological assessments of the ACOS and COPD patients were compared using the Mann–Whitney U-test. For ACOS patients, the results of PFTs, CATs, and radiological assessments performed before treatment were compared with the results of the same tests performed after 3 months of therapy with budesonide/formoterol, using the nonparametric Wilcoxon rank sum test. The level of significance was set at P<0.05. All statistical analyses were performed using JMP 10.0 software (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
The general characteristics of 40 stable ACOS patients and 100 stable COPD patients are shown in Table 1. There were no differences between these groups for sex, age, smoking history, or body mass index values. As expected, a response to a SABA was greater for patients with ACOS than for those with COPD (COPD vs ACOS: 3.54%±4.80% vs 11.6%±9.3%, P<0.001). The distributions of FEV1%predicted results were not significantly different between these groups (COPD vs ACOS: 58.0%±17.3% vs 58.1%±17.6%, P=0.676). There were also no significant differences in vital capacity results and their ratios of residual volume to total lung capacity. These results indicated that both groups were well balanced with regard to pulmonary function, except for reversible airway responses to a bronchodilator. It was noteworthy that COPD patients had lower DLCO/VA (%predicted) values as compared to those with ACOS (COPD vs ACOS: 70.7%±19.9% vs 79.9%±23.6%, P<0.05).
Table 1.
Patients characteristics
| COPD (N=100) |
ACOS (N=40) |
P-value | |
|---|---|---|---|
| Age, years | 69.7±8.3 | 67.2±10.3 | 0.413 |
| Body mass index, kg/m2 | 22.8±3.3 | 22.9±4.7 | 0.970 |
| Smoking, pack years | 52.8±34.4 | 49.4±44.0 | 0.412 |
| Male sex, % | 87.0 | 85.0 | |
| Previously treated, N (%) | 48 (48.0) | 20 (50.0) | |
| LAMA use, N (%) | 10 (10.0) | 4 (10.0) | |
| LABA use, N (%) | 32 (32.0) | 14 (35.0) | |
| ICS/LABA use, N (%) | 6 (6.0) | 2 (5.0) | |
| Post-bronchodilator | |||
| VC, %predicted | 88.7±16.5 | 88.0±16.0 | 0.976 |
| FEV1, L | 1.55±0.49 | 1.60±0.54 | 0.607 |
| FEV1 %predicted | 58.0±17.3 | 58.1±17.6 | 0.676 |
| FEV1/FVC | 53.6±11.0 | 52.1±11.1 | 0.399 |
| RV, L | 2.44±0.67 | 2.49±0.69 | 0.622 |
| TLC, L | 5.59±1.07 | 5.56±1.11 | 0.944 |
| RV/TLC, %predicted | 119.3±19.3 | 124.6±21.7 | 0.229 |
| DLCO/VA, %predicted | 70.7±19.9 | 79.9±23.6 | 0.044 |
| Response to β2-agonist | |||
| ΔFEV1, mL | 46.0±62.1 | 137.8±107.6 | <0.001 |
| % change in FEV1, % | 3.54±4.80 | 11.6±9.0 | <0.001 |
Note: Data are expressed as mean ± standard deviation.
Abbreviations: ACOS, asthma-chronic obstructive pulmonary disease overlap syndrome; COPD, chronic obstructive pulmonary disease; DLCO/VA, diffusing capacity for carbon monoxide per liter of lung volume; FEV1, forced expiratory volume in 1 second; ΔFEV1, increases in forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroids; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; RV, residual volume; TLC, total lung capacity; VC, vital capacity.
Regarding HRQoL assessments, ACOS patients had higher CAT scores compared to those with COPD (COPD vs ACOS: 9.8±6.8 vs 12.3±7.2, P=0.046; Figure 2). This finding was in agreement with a previous report that found worse QOL outcomes for ACOS patients as determined by the St George’s Respiratory Questionnaire.23
Figure 2.
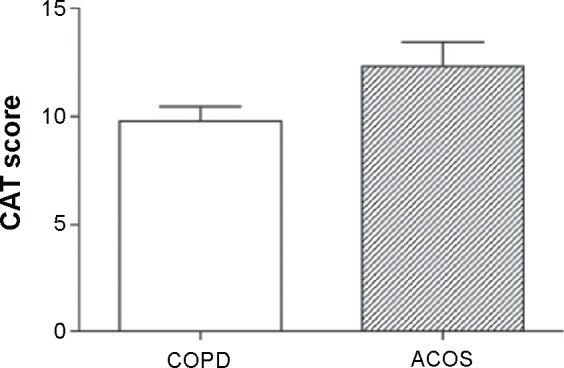
Comparison of CAT scores between COPD and ACOS patients.
Notes: Results for CAT scores were compared between ACOS and COPD patients by Mann–Whitney U-tests. Bars indicate standard errors.
Abbreviations: ACOS, asthma–chronic obstructive pulmonary disease overlap syndrome; CAT, chronic obstructive pulmonary disease assessment test; COPD, chronic obstructive pulmonary disease.
Radiological features of ACOS compared with COPD
As with COPD, it is well known that asthma involves small airway inflammation and remodeling. Recent evidence showed that wall thickness of the proximal airways as detected by CT scanning (WA%) predicted small airway impairment and showed good correlations with FEV1 and residual volume results.24 The CT metric of %CSA <5 has been used to evaluate pulmonary vessel alterations and perfusion.25 In addition, we previously reported that %CSA <5 was negatively correlated with LAV% results for COPD patients.15 Thus, we focused on these two MDCT variables (WA% and %CSA <5) and compared them between ACOS and COPD patients. There was no significant difference in LAV% results between COPD and ACOS patients (ACOS vs COPD: 4.5%±8.2% vs 6.2%±9.3%, P=0.963). However, ACOS patients had significantly higher WA% values (ACOS vs COPD: 79.1%±4.0% vs 76.9%±3.4%, P=0.001) and higher %CSA <5 results (ACOS vs COPD: 0.861±0.249 vs 0.731±0.265, P=0.011) than those with COPD (Figure 3). These findings were characteristic of ACOS and had not been previously reported.
Figure 3.
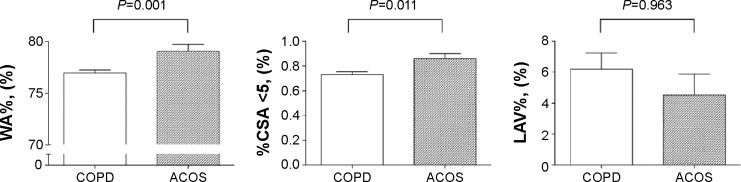
Comparison of radiological variable results between COPD and ACOS patients.
Notes: Results for radiological variables were compared between ACOS and COPD patients by Mann–Whitney U-tests. Bars indicate standard errors.
Abbreviations: ACOS, asthma–chronic obstructive pulmonary disease overlap syndrome; COPD, chronic obstructive pulmonary disease; %CSA <5, percentage of total cross-sectional area of pulmonary vessels less than 5 mm2; LAV%, percentage of low attenuation volume; WA%, percentage of wall area.
Budesonide/formoterol treatment effects for ACOS patients
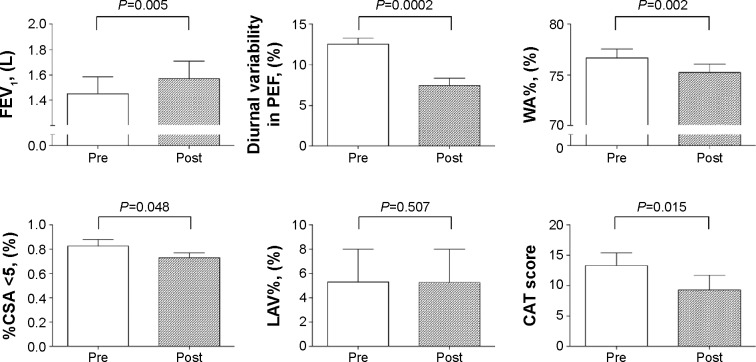
The general characteristics of patients treated with budesonide/formoterol are shown in Table 2. All the patients had been treatment-naïve until the initiation of budesonide/formoterol treatment in this study. None of our study patients experienced any acute exacerbations during the study period (up to 12 weeks after initiating budesonide/formoterol treatment). Figure 4 shows the longitudinal changes in clinical outcomes for patients with ACOS. During the 12-week study period, budesonide/formoterol treatment resulted in significantly increased FEV1 (1.44±0.58 L vs 1.57±0.58 L, P=0.005) and self-reported diurnal variations in PEF readings (12.5±3.3 L vs 7.5±4.0 L, P=0.0002).
Table 2.
Characteristics of the ACOS patients treated with budesonide/formoterol
| Age, years | 67.7±10.8 |
| Body mass index, kg/m2 | 22.8±3.7 |
| Smoking, pack years | 52.8±36.2 |
| Male sex, % | 70.0 |
| CAT score | 13.4±8.0 |
| Post-bronchodilator | |
| VC, %predicted | 89.4±13.1 |
| FEV1, L | 1.45±0.58 |
| FEV1 %predicted | 57.2±17.5 |
| FEV1/FVC | 51.9±10.7 |
| RV, L | 2.59±1.21 |
| TLC, L | 5.74±2.48 |
| RV/TLC, %predicted | 125.4±17.6 |
| DLCO/VA, %predicted | 78.3±26.6 |
Note: Data are expressed as mean ± standard deviation.
Abbreviations: ACOS, asthma–chronic obstructive pulmonary disease overlap syndrome; CAT, chronic obstructive pulmonary disease assessment test; DLCO/VA, diffusing capacity for carbon monoxide per liter of lung volume; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; RV, residual volume; TLC, total lung capacity; VC, vital capacity.
Figure 4.
Changes in clinical variables after treatment with inhaled budesonide/formoterol.
Notes: Results for pulmonary function parameters, radiological parameters, and CAT scores were compared between pretreatment and 3 months after therapy with budesonide/formoterol using nonparametric Wilcoxon rank sum tests. Bars indicate standard errors.
Abbreviations: CAT, chronic obstructive pulmonary disease assessment test; %CSA <5, percentage of total cross-sectional area of pulmonary vessels less than 5 mm2; FEV1, forced expiratory volume in 1 second; LAV%, percentage of low attenuation volume; PEF, peak expiratory flow; Post, 3 months after treatment with budesonide/formoterol; Pre, pretreatment with budesonide/formoterol; WA%, percentage of wall area.
Regarding CT image findings, budesonide/formoterol treatment resulted in slightly reduced WA% values (76.6±4.1 L vs 72.9±4.2 L, P=0.002) and %CSA <5 results (0.83±0.23 L vs 0.73±0.17 L, P=0.048), but had no effects on LAV% results over time (5.3±11.8 L vs 5.2±12.0 L, P=0.507). In conjunction with improvements in airflow, total CAT scores were significantly reduced in response to this treatment (13.4±8.0 L vs 9.3±9.5 L, P=0.015). During the 12-week study period, no adverse effects of any grade were recorded that were due to budesonide/formoterol, including pneumonia.
Discussion
Patients with ACOS had higher diffusing capacity, greater airway wall thickness, higher pulmonary microvascular density, and worse HRQoL than patients with COPD, despite being of similar age and having comparable FEV1 values. To the best of our knowledge, this is the first study to demonstrate the functional and radiological characteristics of patients with ACOS, who were selected based on clinical criteria.
The novel finding of this study was that there were radiological differences between COPD and ACOS on MDCT imaging. The patient groups in this study were well balanced with regard to their demographic characteristics, and both groups had similar degrees of emphysematous changes (LAV%); that is, similar degrees of parenchymal destruction. Airway wall thickness (WA%) results were significantly higher in those with ACOS and, interestingly, these patients showed reductions in this variable in response to budesonide/formoterol treatment. In contrast, budesonide/formoterol treatment did not have any effect on emphysematous changes (LAV%). These results suggest that ACOS is an airway lesion–dominant disease as compared to COPD. Moreover, airway remodeling in ACOS exhibited considerable reversibility in response to ICS/LABA, similar to asthma.
Another interesting finding of this study was that ACOS patients had higher %CSA <5 results than did COPD patients. The CT metric of %CSA <5 was a radiological metric recently introduced to reflect pulmonary microvascular density, which is useful for evaluating pulmonary perfusion.25 In COPD patients, a decrease in %CSA <5 preceded emphysematous changes on MDCT images and was positively correlated with FEV1 results. This was in accordance with the well-known hypothesis that depleting vascular endothelial growth factor induces the apoptosis of parenchymal epithelial and endothelial cells and results in emphysematous changes in COPD.26–29 In contrast, air way inflammation in asthma is accompanied by vascular endothelial growth factor oversecretion and an increase in subepithelial microvessels.30 Leakage from these immature microvessels enhances airway inflammation and hypersensitivity. For these situations, ICS/LABA could mitigate the remodeling of both the airways and the pulmonary vasculature by suppressing inflammation, in addition to reducing local vascular constriction.
Indeed, it has not yet been shown that %CSA <5 directly reflects peribronchial perfusion. Nevertheless, it is interesting that ACOS patients, who had a reversible asthma phenotype, had higher %CSA <5 results than those with COPD. A decrease in %CSA <5 in parallel with WA% suggests that treatment with ICS/LABA has potential for resolving the remodeling of the pulmonary microcirculation.
In asthma, the airway wall contains a high percentage of a subepithelial vasculature bed (up to 20%) and reducing this may have a considerable impact on airway wall thickness.9 Thus, it is possible that reducing this abnormal microvascular bed may have resulted in increasing the airway diameter that resulted in improved FEV1 results in ACOS patients.
Our study had several limitations. First, our sample size was small because this study was conducted at a single institute. However, our data were accurate and reproducible, because all parameters were assessed using the same equipment. Second, this was an observational study with a single arm.
A confirmation study with a larger sample size of ACOS patients who are selected based on clinical criteria is warranted. It will also be important to determine the partner drug for an ICS, either a LABA or a long-acting muscarinic antagonist. In this context, our pilot study results should aid in promoting this disease concept for conducting a larger scale randomized clinical trial.
Conclusion
ACOS patients had augmented clinical features, including more impaired HRQoL, higher diffusing capacity, greater airway wall thickness (WA%), and higher pulmonary micro-vascular density (%CSA <5) than COPD patients.
Acknowledgments
We wish to thank Mrs Chieko Handa (Chiba University) and Mrs Junko Takeuchi (Chiba University) for technical assistance. This work was supported in part by a grant to the Respiratory Failure Research Group from the Ministry of Health, Labor and Welfare of Japan. Dr Tatsumi reports grants from Boehringer Ingelheim Japan, Inc., Pfizer, Inc., and Astellas, Inc. outside the submitted work.
Footnotes
Author contributions
Conceived and designed the paper: TS, YT, and KT. Collected the data: TS, NK, YM, JI, YK, and KT. Analyzed the data: TS and YT. Wrote the paper: TS, YT, and KT. All authors read and approved the final manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
- 1.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2000;160(11):1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 2.Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48(3):279–285. doi: 10.3109/02770903.2011.555576. [DOI] [PubMed] [Google Scholar]
- 3.Miravitlles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107(7):1053–1060. doi: 10.1016/j.rmed.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Rhee CK, Yoon HK, Yoo KH, et al. Medical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthma. COPD. 2014;11(2):163–170. doi: 10.3109/15412555.2013.831061. [DOI] [PubMed] [Google Scholar]
- 5.Chung JW, Kong KA, Lee JH, Lee SJ, Ryu YJ, Chang JH. Characteristics and self-rated health of overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2014;9:795–804. doi: 10.2147/COPD.S61093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Initiative for Asthma and Global Initiative for Chronic Obstructive Lung Disease Asthma COPD and Asthma–COPD Overlap Syndrome (ACOS) 2015. [Accessed March 10, 2015]. Available from: http://www.ginasthma.org/local/uploads/files/AsthmaCOPDOverlap.pdf.
- 7.Detoraki A, Granata F, Staibano S, Rossi FW, Marone G, Genovese A. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy. 2010;65(8):946–958. doi: 10.1111/j.1398-9995.2010.02372.x. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156(1):229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- 9.Feltis BN, Wignarajah D, Reid DW, Ward C, Harding R, Walters EH. Effects of inhaled fluticasone on angiogenesis and vascular endothelial growth factor in asthma. Thorax. 2007;62(4):314–319. doi: 10.1136/thx.2006.069229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino M, Nakamura Y, Sim JJ, et al. Inhaled corticosteroid reduced lamina reticularis of the basement membrane by modulation of insulin-like growth factor (IGF)-I expression in bronchial asthma. Clin Exp Allergy. 1998;28(5):568–577. doi: 10.1046/j.1365-2222.1998.00277.x. [DOI] [PubMed] [Google Scholar]
- 11.Orsida BE, Li X, Hickey B, Thien F, Wilson JW, Walters EH. Vascularity in asthmatic airways: relation to inhaled steroid dose. Thorax. 1999;54(4):289–295. doi: 10.1136/thx.54.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calverley P, Pauwels R, Vestbo J, et al. Trial of Inhaled STeroids ANd Long-Acting Beta2 Agonists Study Group Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 13.Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(1):74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- 14.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuura Y, Kawata N, Yanagawa N, et al. Quantitative assessment of cross-sectional area of small pulmonary vessels in patients with COPD using inspiratory and expiratory MDCT. Eur J Radiol. 2013;82(10):1804–1810. doi: 10.1016/j.ejrad.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Yahaba M, Kawata N, Iesato K, et al. The effects of emphysema on airway disease: correlations between multi-detector CT and pulmonary function tests in smokers. Eur J Radiol. 2014;83(6):1022–1028. doi: 10.1016/j.ejrad.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 17.British Thoracic Society Scottish Intercollegiate Guidelines Network British guideline on the management of asthma. Thorax. 2009;63(4):1–121. doi: 10.1136/thx.2008.097741. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society/European Respiratory Society Task Force . Standards for the Diagnosis and Management of Patients with COPD Version 1.2. New York: American Thoracic Society; 2004. [Google Scholar]
- 19.Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64(8):728–735. doi: 10.1136/thx.2008.108027. [DOI] [PubMed] [Google Scholar]
- 20.Committee of Respiratory Physiology in Japanese Respiratory Society. [Guideline of respiratory function tests-spirometry, flow-volume curve, diffusion capacity of the lung] Nihon Kokyuki Gakkai Zasshi. 2004:1–56. Japanese. [PubMed] [Google Scholar]
- 21.Coche E, Pawlak S, Dechambre S, Maldague B. Peripheral pulmonary arteries: identification at multi-slice spiral CT with 3D reconstruction. Eur Radiol. 2003;13(4):815–822. doi: 10.1007/s00330-002-1734-2. [DOI] [PubMed] [Google Scholar]
- 22.Global Initiative for Asthma Global Strategy for Asthma Management and Prevention 2014 (Revision) 2015. [Accessed March 10, 2015]. Available from: http://www.ginasthma.org/local/uploads/files/GINA_Report_2014_Aug12.pdf.
- 23.Hardin M, Silverman EK, Barr RG, et al. COPDGene Investigators The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171(2):142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka S, Yamashiro T, Matsushita S, et al. Usefulness of coronal reconstruction CT images for quantitative evaluation of the cross-sectional area of small pulmonary vessels. Acad Radiol. 2014;21(11):1411–1415. doi: 10.1016/j.acra.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106(11):1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med. 2001;163(3 pt 1):737–744. doi: 10.1164/ajrccm.163.3.2002117. [DOI] [PubMed] [Google Scholar]
- 28.Barr RG, Mesia-Vela S, Austin JH, et al. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med. 2007;176(12):1200–1207. doi: 10.1164/rccm.200707-980OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shigeta A, Tada Y, Wang JY, et al. CD40 amplifies Fas-mediated apoptosis: a mechanism contributing to emphysema. Am J Physiol Lung Cell Mol Physiol. 2012;303(2):141–151. doi: 10.1152/ajplung.00337.2011. [DOI] [PubMed] [Google Scholar]
- 30.Zanini A, Chetta A, Imperatori AS, Spanevello A, Olivieri D. The role of the bronchial microvasculature in the airway remodelling in asthma and COPD. Respir Res. 2010;11:132. doi: 10.1186/1465-9921-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]