Abstract
Objective
Increased systemic inflammation is associated with stress-related psychopathology. Specifically, levels of the pro-inflammatory marker C-reactive protein (CRP) are increased in individuals with posttraumatic stress disorder (PTSD). Furthermore, single nucleotide polymorphisms (SNPs) in the CRP gene are associated with CRP levels, risk for cardiovascular disease and obesity. We examined whether polymorphisms within the CRP gene and increased CRP levels are associated with PTSD symptoms and fear physiology in a highly traumatized civilian population.
Method
Cross-sectional data were collected from subjects recruited from an inner-city public hospital that serves a primarily African American, low socioeconomic population. Participants were interviewed to determine PTSD symptom severity, and a fear-potentiated startle (FPS) paradigm was administered to assess fear-related phenotypes of PTSD.
Results
One SNP within the CRP gene, rs1130864, was significantly associated with increased PTSD symptoms (N=2692; p=0.004), including ‘being overly alert’ as the most significant individual symptom (N=2698; p =1.5×10−5). Additionally CRP genotype was also associated with the odds of PTSD diagnosis (N=2692; p=0.004). This SNP was also associated with increased CRP levels (N=137; p=0.007), and high CRP levels (>3mg/L) were positively associated with PTSD symptoms (N=187; p=0.032) and FPS to a safety signal (N=135; p<0.005).
Conclusions
Together, these data indicate that genetic variability in the CRP gene is associated with increased CRP levels and PTSD symptoms, including hyperarousal symptoms. Increased CRP levels were also associated with exacerbated fear-related psychophysiology and PTSD symptoms and diagnosis. These findings offer a potential mechanism by which an increased pro-inflammatory state may lead to heightened PTSD symptoms.
Keywords: PTSD, c-reactive protein, stress, inflammation, psychophysiology, startle
Introduction
Posttraumatic stress disorder (PTSD) is an anxiety disorder characterized by avoidance, re-experiencing, and hyperarousal symptoms following exposure to a traumatic life event. However, a diagnosis of PTSD has been linked to high rates of illness (1) and inflammatory disease (2), including cardiovascular (3) and metabolic disease (4). Individuals with PTSD show elevated levels of the inflammatory cytokines IL-6, IL-1β, and IL-2 (5–7), augmented nuclear factor-κB (NF-κB) gene expression (8) and activity (9), as well as altered immune cell sensitivity to glucocorticoids (10). Furthermore, peripheral levels of inflammatory molecules correlate with PTSD symptomology (11). These data linking PTSD to a pro-inflammatory state further support the notion that PTSD is associated with chronic inflammation, in a manner similar to depression (12).
More recently, increased levels of the pro-inflammatory marker C-reactive protein (CRP) have been described in individuals with PTSD (13, 14). The directionality of the change in CRP levels in PTSD is equivocal, as other studies have shown decreased levels of CRP in individuals with PTSD (15) or even a lack of association between PTSD and CRP levels (11, 16). However, a prospective study recently showed that increased baseline CRP prior to deployment was a significant predictor for PTSD, suggesting that increased inflammation is a risk factor for PTSD (17). Genetic variation among individuals that increase CRP levels could also server a vulnerability factor for PTSD, as genetic traits significantly account for inter-individual variability in CRP levels (18). Single nucleotide polymorphisms (SNPs) are present in the CRP gene that influence CRP levels (19) and confer increased individual vulnerability to cardiovascular disease (20).
In the current study, we assessed whether particular SNPs that tag the CRP gene at moderate levels of linkage disequilibrium (rs3093068, rs1205, rs3093067, rs1130864, rs3093066, rs3091244) are associated with greater serum levels of CRP, PTSD symptoms, and increased odds ratio for likelihood of PTSD diagnosis. Furthermore, we sought to determine whether increased CRP levels are associated with PTSD in a highly traumatized urban population where the lifetime prevalence of PTSD is very high (21). We hypothesized that CRP levels in highly traumatized individuals would be associated positively with PTSD symptoms. We also assessed whether CRP levels would be associated with fear-potentiated startle, a robust psychophysiological phenotype that is increased in PTSD (22). These data will elucidate whether CRP genetic variance is associated with heightened inflammation indicated by CRP as well as augmented PTSD symptoms in trauma exposed individuals. Together, these data offer a potential mechanism by which increased pro-inflammatory states may modulate neurophysiology and heighten PTSD symptoms.
Methods
Participants
Study participants were recruited from primary care clinics at Grady Memorial Hospital in Atlanta, GA, serving a primarily African American, low socioeconomic status (SES), inner-city population (21). Study participants were English-speaking men and women between the ages of 18 and 65 years who provided written informed consent. All study procedures were reviewed and approved by the Emory Institutional Review Board and the Grady Hospital Research Oversight Committee.
All participants (N=2692) of the current study underwent a screening interview wherein DNA was collected into Oragene saliva kits (DNAGenotek, Ottawa, Ontario, Canada). Demographic information was also collected with a locally developed Demographics Form to assess for subject gender, age, self-identified race, education, and income (21). Lifetime trauma history was determined by the 14-item Traumatic Events Inventory (TEI), which assesses for experiencing and witnessing traumatic events separately and has been used to describe our sample population previously (21). Lastly, the PTSD Symptom Scale (PSS) was used to measure PTSD symptoms (23).
A sub-group of participants (N=187), who were available for much more in-depth interviews and phenotyping and who represented a cross-section of the entire cohort, returned to participate in structured clinical interviews, phlebotomy, and physiological measures. Within this subgroup, in addition to previously mentioned measures, the Clinician Administered PTSD Scale (CAPS) was also assessed as a diagnostic instrument to measure the categorical presence of current PTSD, as well as current PTSD continuous symptoms (24). On the morning of the interview, blood samples were collected at the Clinical Interactions Network within the Atlanta Clinical and Translational Science Institute for assessment of CRP levels. Of these subjects, 135 were assessed using the Fear-Potentiated Startle Paradigm (22) to assess neurophysiology as described below. Importantly, some participants declined to answer some questions, the thus the total number of participants listed for sub-analysis may be lower than the total number listed above.
CRP Genotyping and Analyses
DNA from all participants was extracted using the Qiagen M48 automated extraction system. All DNA for genotyping was quantified by gel electrophoresis using Quantity One (BioRad, Hercules, CA) and then normalized to 400 ng. Using the Illumina Human Omni1-Quad BeadChip (Illumina Inc.), genome-wide SNP genotyping was performed according to instructions by the manufacturer.
For these analyses, we analyzed six targeted tagging SNPs that were available from Illumina’s Omni1-Quad platform and that lay within the CRP gene, as previously described (25–28): rs3093068, rs1205, rs3093067, rs1130864, rs3093066, rs3091244. Maximum r2 in our sample of traumatized individuals between each CRP SNP is described in Supplementary Table 1. Quality control and association analyses via linear (or logistic) regression were conducted using PLINK software version 1.07 (29). The genotype call rate for all SNPs was greater than 99%, and all of the SNPs in CRP were in Hardy Weinberg Equilibrium (p>0.1). Subjects were included in the PSS analyses if they experienced >1 lifetime adult or childhood trauma exposures to examine main effects in subjects with substantial trauma exposure (N=2692). All subjects, regardless of trauma level, were included in the CRP analyses (N=137), since we were not directly examining PTSD symptoms. Except where noted, we tested for association under a dominant model, where the ‘risk’ allele carriers were coded as 1 and the ‘resilient’ (major allele homozygote) group coded as 0. A Bonferroni threshold of p<0.0083 was used for correction for multiple testing. As a secondary analysis, we also tested for association under an additive model (Supplementary Material). Using PLINK, we verified key results with permutation tests (10,000) to ensure that our results did not depend on distributional assumptions. In each permutation, trait values were randomly shuffled across individuals, the association test was re-performed, and the t-statistic was recorded. Permutation P values were then estimated as the proportion of permutations for which the t-statistic exceeded the original in magnitude. To verify our findings from the continuous outcome measure of PTSD symptoms, we also conducted a logistic regression analysis using categorical PTSD diagnosis as an outcome measure using a dominant model.
We had genome-wide data on a subset of our subjects, and thus had available principal components to infer axes of ancestry. Prior to principal component analysis (PCA), we used PLINK to prune the data in windows of 50 base pairs, removing one SNP from each pair of SNPs with r2>0.05 to obtain a set of roughly independent SNPs. We then used the top ten principal components as covariates in secondary analyses for PTSD outcomes (30, 31). Given the smaller sample size for the CRP level analyses, we only used the top two principal components as covariates to prevent model over-fitting.
As many more biological and genetic factors are thought to be associated with intermediate phenotypes than with the complete syndrome, we separately assessed 17 specific PTSD symptoms examined within DSM-IV criteria for PTSD via the PSS (N=2692). Specifically, we examined the effect of the dominant model of SNP rs1130864 controlling for the principal components as above, for each symptom in question with a Bonferroni-corrected threshold of p<0.0029.
CRP protein levels
Serum samples were stored at −80C until the time of CRP assay. Serum CRP was measured using an immunoturbidometric assay from Sekisui Diagnostics (www.sekisuidiagnostics.com) on the Beckman AU480 chemistry analyzer, with an inter-assay coefficient of variation (CV) of 5.2% and an intra-assay CV of 3.1%. Individuals with CRP levels >20 mg/L were excluded from the current analysis. CRP levels averaged 5.14±4.77 mg/L and ranged from 0.03 to 18.84 mg/L. As previously described (13), a cutpoint was used >3 mg/L was used to distinguish those individuals with low versus high CRP levels.
Fear-Potentiated Startle Paradigm
Based on our previous work (22), the fear-potentiated startle protocol consisted of an initial habituation phase wherein conditioned stimuli (CS) were presented without any reinforcement. The conditioning phase of acquisition consisted of three blocks with four trials of each type of CS (reinforced conditioned stimulus, CS+; non-reinforced conditioned stimulus, CS−; noise probe alone, NA) for 12 trials per block and a total of 36 trials. CSs were different colored shapes presented on a computer monitor for six seconds each. The unconditioned stimulus (US; aversive stimulus) was a 250-msec air blast of 140-psi intensity to the larynx that we have shown in our previous studies produces a robust fear-potentiated startle response (22). The air blast was delivered from a compressed air tank via a polyethylene tub and controlled by a switch. The inter-trial intervals throughout the acquisition phase were randomized to be between nine and 22 seconds in duration.
Startle response data were acquired using the electromyography (EMG) module of the BIOPAC MP150 for Windows (Biopac Systems, Goleta, California). The acquired data were filtered, rectified, and smoothed using the MindWare software suite (MindWare Technologies, Gahanna, Ohio) and exported for statistical analyses. The EMG signal was sampled at a frequency of 1kHz and filtered with low- and high-frequency cutoffs at 28 and 500 Hz, respectively. The maximum amplitude of the eye-blink muscle contraction 20 to 200 msec after presentation of the startle probe was used as a measure of the acoustic startle response. The eye-blink component of the acoustic startle response was measured by EMG recordings of the right orbicularis oculi muscle with two 5-mm Ag/AgCl electrodes filled with electrolyte gel as previously described (22). One electrode was positioned 1 cm below the pupil of the right eye, and the other was placed 1 cm below the lateral canthus. Impedance levels were less than 6 kOhms for each participant. The startle probe was a 108-dB [A] SPL, 40-msec burst of broadband noise with near instantaneous rise time, delivered binaurally through headphones.
FPS was assessed by comparing average startle magnitude on the CS trials to the average startle magnitude to the NA trials using a mixed-model ANOVA with trial type and block as within-subjects factors. Fear acquisition was measured using a difference score by subtracting startle magnitude to the NA trials from startle magnitude in the presence of a CS in each conditioning block. Analyses assessing the effects of CRP levels on FPS were conducted in 135 individuals from the larger sample who had both CRP and FPS data. Analyses assessing the effects of rs1130864 SNP on FPS were conducted in 188 individuals whose data genetic and FPS was available.
Statistical Analyses
Bivariate correlation and linear regression were used to assess the association between CRP levels and total current PTSD symptoms and FPS. Chi-square analysis and the calculation of odds ratios and 95% confidence intervals [OR(95%CI)] were used to assess differences between low and high CRP levels on categorical variables (PTSD diagnosis) and analyses of variance (ANOVA) for continuous variables (PTSD symptoms, FPS). Analyses controlled for potential confounders that have previously been associated with CRP levels, such as age and sex (32). The data was analyzed using SPSS (v.20) and summarized as mean ± SD (standard deviation). A p ≤ 0.05 was considered significant and corrections for multiple comparisons were done using the Bonferroni method when necessary.
Results
Demographic characteristics
Female subjects (70.3%) outnumbered male subjects (29.7%) and the self-identified race of our sample was predominantly African- American (92.9%) in the larger CRP genotype cohort. The majority of the subjects (69.1%) was unemployed and had received a high school diploma (35.6%). The socioeconomic status of the majority of subjects is very low with 86.5% of the sample having a mean monthly household income of less than $2,000 and 24.8% of the sample having a mean monthly household income of less than $250. Finally, the mean ± SD total number of traumas experienced and witnessed in the sample was 4.72 ± 3.11 traumas. The demographic information for the cohorts used for the CRP genotype, CRP peptide, and FPS analyses are summarized in Table 1.
Table 1.
Demographic characteristics of samples in frequency (n), percentage (%), and mean ± SD for the overall sample and the subsample used for the CRP peptide analyses, including those participating in the fear physiology study.
| Demographics. | Genetic Sample (n=2692) | CRP Peptide Sample (n=187) | Startle Sample (n=135) | |||
|---|---|---|---|---|---|---|
| Sex | n | % | n | % | n | % |
| Female | 1892 | 70.3 | 107 | 57.4 | 63 | 47.0 |
| Male | 800 | 29.7 | 80 | 42.6 | 72 | 53.0 |
| Race | ||||||
| African America/Black | 2500 | 92.9 | 168 | 89.9 | 124 | 91.8 |
| Hispanic or Latino | 21 | 0.80 | 1 | 0.80 | 0 | 0 |
| Asian | 5 | 0.20 | 1 | 0.80 | 0 | 0 |
| Caucasian or White | 99 | 3.70 | 11 | 5.40 | 7 | 5.2 |
| Mixed | 35 | 1.30 | 3 | 1.60 | 3 | 2.2 |
| Other | 32 | 1.20 | 3 | 1.60 | 1 | 0.7 |
| Employment | ||||||
| Unemployed | 1860 | 69.1 | 149 | 79.8 | 106 | 79.1 |
| Education | ||||||
| < 12the Grade | 608 | 22.6 | 52 | 27.9 | 29 | 21.6 |
| 12th or High School Grad | 958 | 35.6 | 64 | 34.1 | 51 | 37.3 |
| GED | 129 | 4.80 | 18 | 9.30 | 11 | 8.2 |
| Some College or Tech School | 641 | 23.8 | 36 | 19.4 | 32 | 23.9 |
| Tech School Grad | 113 | 4.20 | 10 | 5.40 | 4 | 3.0 |
| College Grad | 205 | 7.60 | 6 | 3.10 | 7 | 5.2 |
| Grad School | 38 | 1.40 | 2 | 0.80 | 1 | 0.7 |
| Income | ||||||
| $0 – 249 | 669 | 24.8 | 54 | 28.5 | 43 | 32.1 |
| $250 – 499 | 262 | 9.70 | 25 | 13.8 | 21 | 15.3 |
| $500 – 999 | 719 | 26.7 | 53 | 28.5 | 36 | 26.7 |
| $1000 – 1999 | 678 | 25.2 | 44 | 23.6 | 25 | 18.3 |
| $2000 or more | 364 | 13.5 | 11 | 5.70 | 10 | 7.6 |
| Mean ± SD | Mean | SD | Mean | SD | Mean | SD |
| Age | 39.3 | 12.5 | 39.4 | 14.5 | 41.7 | 11.0 |
| PTSD Symptom Severity (PSS) | 13.0 | 12.4 | 15.3 | 11.9 | 16.1 | 12.7 |
| Total Traumas Experienced/Witnessed | 4.72 | 3.11 | 5.22 | 4.10 | 5.66 | 3.75 |
CRP genotype, CRP levels, and PTSD symptoms
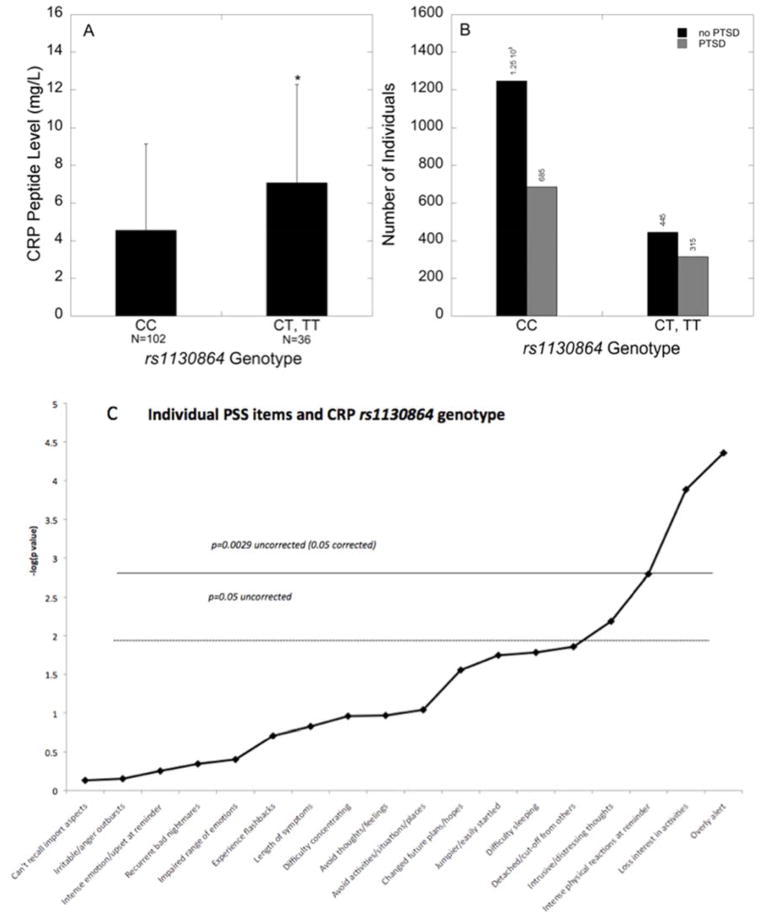
The examination of the six SNPs spanning the CRP gene yielded a single SNP that survived multiple testing when assessing SNP effects on the number of PTSD symptoms and CRP levels. Using a dominant model, the SNP rs1130864 was significantly associated with overall PTSD symptoms (N=2692, t=2.86, p=0.004, permutation p=0.004; Table 2) and CRP levels (N=137, t=2.77, p=0.007, permutation p=0.0006; Table 2). The main effects of genotype on PTSD symptoms (N=2692, t=2.65, p=0.008) and CRP levels (N=129, t=2.77, p=0.007) were maintained when controlling for population stratification (using principal components) in the dominant model. Traumatized subjects with the ‘CC’ genotype had an average PSS value of 14.1, whereas those with one or two ‘T’ alleles had an average PSS value of 15.7. Additionally, individuals with the ‘CC’ genotype had lower serum CRP compared to those carrying the ‘T’ allele (p<0.005, Figure 1A).
Table 2.
Main effects (Dominant Models) of CRP SNPs on PTSD symptoms (N>2600) and on CRP peptide levels (N=137).
| PTSD symptoms | CRP peptide levels | |||||
|---|---|---|---|---|---|---|
| CRP SNP | n | t | p-value | n | t | p-value |
| rs1205 | 2692 | −0.11 | 0.91 | 137 | −0.68 | 0.50 |
| rs1130864 | 2692 | −0.11 | 0.004 | 137 | 2.73 | 0.007 |
| rs3091244 | 2686 | 0.39 | 0.70 | 137 | −2.76 | 0.007 |
| rs3093066 | 2691 | 0.66 | 0.51 | 137 | −2.05 | 0.04 |
| rs3093067 | 2692 | 1.31 | 0.19 | 137 | 0.55 | 0.59 |
| rs3093068 | 2686 | −2.31 | 0.02 | 137 | 0.55 | 0.58 |
Figure 1. CRP genotype (rs1130864) is associated with CRP levels, PTSD diagnosis, and hypervigilance symptoms.
(A) Mean ± SD serum CRP levels in traumatized individuals of each rs1130864 genotype. Individuals with the ‘CC’ genotype have lower serum CRP compared to carriers of the ‘T’ allele (n=137, t=2.73, p=0.007). (B) Number of individuals categorized by PTSD diagnosis (Yes vs. No) in traumatized subjects of each rs1130864 genotype (dominant model, n=2547, t=2.893, p=0.004). (C) Bonferroni-corrected results showing the association between rs1130864 and the 17 different symptom questions on the PSS, covarying for the top 10 principal components. The most robust effect was with the hypervigilance symptom of being overly alert (n=2698, t=4.33, p=1.52×10−05).
Additionally, using a dominant model, the rs1130864 SNP was also associated with a categorical diagnosis of PTSD (N=2692, t=2.893, p=0.004; Figure 1B), and remained so after co-varying for principal components (t=2.802, p=0.005). Thus, individuals carrying the ‘T’ allele had increased odds for PTSD [OR(95%CI)=1.29 (1.09,1.53)] compared to individuals with the ‘CC’ genotype, with 41% of traumatized ‘T’ carriers having a PTSD diagnosis compared to 35% of those with the ‘CC’ genotype. Note that these effects of SNP rs1130864 genotype on PTSD symptoms (N=2571, t=2.705, p=0.007), CRP levels (N=117, t=2.263, p=0.026), and PTSD diagnosis (N=2571, t=2.805, p=0.005) survived after co-varying for principal components, sex, age, education, current employment, income, and total trauma experienced/witnessed using a dominant model.
After examining the effects of SNP rs1130864 on 17 symptoms measured on the PSS co-varying for principal components, three symptoms survived Bonferroni correction (p<0.0029); specifically the hypervigilance symptom of being overly alert, loss of interest in activities, and intense physical reactions at reminder (Figure 1C). The most robust effect was with the hypervigilance symptom of being overly alert (N=2698, t=4.33, p=1.5×10−5), as there was a 19% increase in the hypervigilance symptom in carriers of the ‘T’ allele compared to individuals with the ‘CC’ genotype.
CRP levels, CRP genotype, and fear physiology
Figure 2A shows a diagram of the fear conditioning experiment; while the CS+ was reinforced with the air blast (danger signal), the CS− was never paired with the aversive air blast thus providing a learned safety signal. Results showed that peripheral levels of CRP were positively correlated with FPS to a safety signal (r=0.186; p<0.05). A linear regression analysis controlling for age, race and PTSD symptoms showed that CRP still independently predicted FPS (β=0.20, t=2.24, p<0.05). However, adding sex to the model reduced the effect of CRP to trend levels (p=0.075), with sex also sharing some of the variance in startle response (p=0.068). When sex and CRP levels were included in the regression as an interaction term, it was significantly predictive of increased startle (β=0.24, t=2.74, p=0.007). Follow-up analyses separately within each sex indicated that CRP was associated with startle response in women more than men (β=0.22, p=0.10 and β=0.02, p=0.88 respectively, although neither were statistically significant). Furthermore, individuals with high levels of CRP (>3mg/L) had higher FPS to a safety signal (Figure 2B; F1,134=, 8.60, p<0.005), suggesting an impaired ability to control fearful responses, compared to those with lower levels of CRP. This main effect of high CRP levels on FPS to a safety signal was still significant after covarying for age, sex, race, and PTSD symptoms (F1,124=, 8.60, p=0.006). Given the above interaction with sex, we repeated this analysis separately for males and females and found that high CRP was associated with higher FPS to the safety signal in females (F1,58=, 5.86, p=0.019), but not males (F1,65=, 1.03, p=0.31). Lastly, the rs1130864 SNP was not associated with FPS to a danger (F1,187=, 0.29, p=0.59) or safety signal (F1,187=, 0.013, p=0.91) in a sub-sample of 188 individuals (data not shown).
Figure 2. CRP levels are associated with increased fear-potentiated startle.
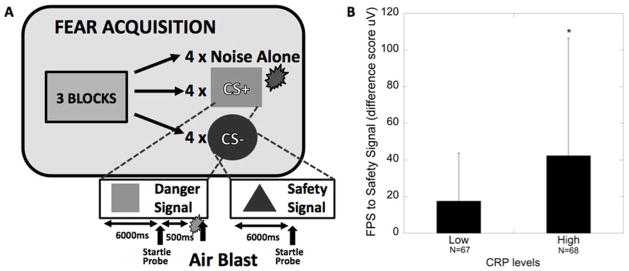
(A) Diagram of the fear-potentiated startle experiment; CS+=reinforced conditioned stimulus (danger signal), CS−=non-reinforced conditioned stimulus (safety signal); (B) Mean ± SD fear-potentiated startle (FPS) to the safety signal (CS-) in traumatized individuals with low and high levels of CRP (n=135).
CRP levels and PTSD
Peripheral levels of CRP were positively associated with total frequency of current PTSD symptoms as determined by the CAPS (r=0.179; p=0.018), and not significantly associated with age (r=0.043; p=0.49) or body mass index (BMI; r=0.026; p=0.77). High levels of CRP were associated with greater PTSD symptoms (Figure 3A; F1,173=3.71, p=0.05) and a diagnosis of PTSD as determined by the CAPS in traumatized subjects (Figure 3B; χ2=4.58; p=0.032; n=187). In addition, individuals with high levels of CRP had increased odds for PTSD [OR (95%CI)=2.24(1.06,4.74)] compared to ndividuals with low levels CRP (p=0.035).
Figure 3. CRP levels are associated with increased PTSD symptomology and a greater proportion of PTSD diagnosis.
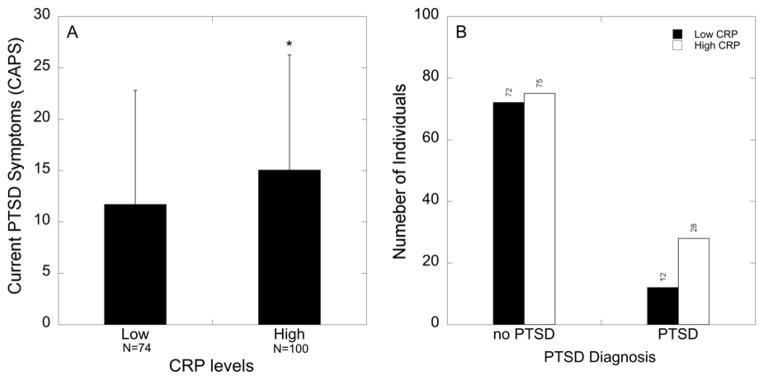
(A) Mean ± SD PTSD symptoms in traumatized individuals with low (<3 mg/L) and high levels (>3 mg/L) of CRP. Individuals with high CRP levels have increased PTSD symptoms (p=0.05; n=174). (B) Number of individuals broken down by PTSD diagnosis (Yes vs. No) and CRP levels (low vs. high) in traumatized subjects (χ2=4.578; p=0.032; n=187).
Discussion
The current results indicate that genetic variation with the CRP gene in traumatized individuals augments CRP levels and confers vulnerability to increased hypervigilance. While CRP SNPs are linked to increased susceptibility to cardiovascular and metabolic disease, and increased CRP levels, this is the first demonstration that a SNP within the CRP gene (rs1130864) is associated with PTSD symptomology and CRP levels in traumatized individuals. Furthermore, we demonstrated that elevated levels of CRP are associated with increased psychophysiological hyperarousal as determined by increased fear-potentiated startle, exacerbated PTSD symptoms, and greater odds of a PTSD diagnosis. These observations are consistent with previous reports linking a pro-inflammatory state with psychopathology (12), including PTSD (5, 6, 9).
Our study is the first to describe a genetic locus within the CRP gene, the rs1130864 SNP, which is associated with increased susceptibility to a PTSD diagnosis and greater PTSD symptomology. The rs1130864 SNP has previously been associated with increased CRP levels (26), a result that we replicate in our sample. While both the rs1130864 and rs3091244 SNPs were the only two loci that were associated with CRP levels, only the rs1130864 SNP survived multiple test correction for effects on CRP levels and PTSD symptoms. The data indicate that while the rates of PTSD were high in both rs1130864 allelic groups (CC vs. T) in our population, paralleling the high overall rates of trauma in our inner city population (21), the differences between groups were largely based on three symptoms, highlighting the significant effects of the rs1130864 genotype on PTSD symptoms. Specifically, an analysis of the effects of rs1130864 on individual PTSD symptoms in trauma-exposed individuals yielded a strong association with being ‘overly alert’, suggesting that this genetic locus confers individual vulnerability to hypervigilance by influencing CRP levels. Furthermore, a recent study suggests that PTSD in women and increased inflammation is correlated with intrusive re-experiencing, a hallmark symptom of PTSD (33). Increased alertness and re-experiencing in PTSD may in fact continually activate the stress axis and the immune system, and thus dysregulate cytokine-glucocorticoid negative feedback (34) and alter fear physiology (35) in PTSD.
Higher levels of CRP in the current study were associated with impaired inhibition of fear-potentiated startle (FPS) to a safety signal, which is a biomarker for PTSD (35) and is related to the severity of current PTSD symptoms (36), and more specifically, hyperarousal symptoms (22). This effect of CRP on fear physiology was independent of PTSD symptoms, indicating that inflammation may increase vulnerability to a heightened fear response. The association between CRP level and FPS was stronger in women than men, supporting recent evidence for heightened FPS post-puberty in females compared to males (37) and the role of menstrual cycle phase in modulating FPS in females (38). This sex difference in the CRP/FPS relationship also corroborates previous findings of higher vulnerability to PTSD in females (39, 40) and data indicating sex differences in immune function that leave women more susceptible to increased inflammation (41, 42). Further studies are necessary to elucidate the effects of increased inflammation and female gonadal hormones on fear psychophysiology.
The rs1130864 SNP that was associated with both CRP levels and PTSD symptoms was not associated with FPS. The lack of a genotype effect on FPS should be considered preliminary as it could be due to our in our small sample that underwent FPS. While self-reported fear has been linked to increased CRP levels in healthy individuals (43), there are no reports in humans or in rodents that describe the effects of inflammation on the psychophysiology of fear responses. It is important to note that exaggerated neurobiological sensitivity to threat characteristic of anxiety disorders, including PTSD, can lead to increased activity of the stress and immune axes, and thus promotes a state of chronic inflammation (44). These data taken together with our finding that increased FPS is associated with greater serum CRP levels, provide a rationale for assessing the role of inflammation in fear responses and hypervigilance in future research.
Our data corroborate previous reports describing heightened CRP levels in individuals with PTSD (13, 14), while others have shown decreased levels of CRP in individuals with PTSD (15) or even a lack of association between PTSD and CRP levels (11, 16). The inconsistencies between these reports may be related to small sample sizes (16), distinct study and ethnic populations (45), the presence of uncontrolled confounders (13), and the use of control groups with high levels of infection (15). The positive association between CRP levels and PTSD symptoms in the current study was described in a highly traumatized inner-city population consisting primarily of African American subjects (21). Individuals in this population have higher prevalence of PTSD (21), and also have increased risk for cardiovascular and metabolic disease (4, 46), suggesting that increased inflammation in this population of highly traumatized individuals may serve as an underlying mechanism by which psychopathology and illness manifest together. Indeed, even though the current analyses could not account for co-morbid physical illness in the study sample, CRP levels were high (average 5.14±4.77), suggesting that some traumatized individuals recruited from primary care settings also are suffering from physical illness. Future studies from clinical populations should analyze both illness comorbidity as well as the potential confound of obesity through measures such as body mass index (BMI). Additionally, although our study is the largest to date examining CRP genotype on PTSD related phenotypes, the number of subjects for whom we had CRP levels limited such that we are not powered to examine mediation between CRP genotype, CRP levels, fear physiology and PTSD symptoms.
The current finding describes a SNP within the CRP gene that is associated with increased susceptibility to a PTSD diagnosis and greater PTSD symptomology in our inner city, primarily African American population. This finding joins a group of previous reports within this growing cohort linking distinct candidate genes to increased risk for psychopathology and altered psychophysiology. Genetic loci within genes critical for the neuroendocrine regulation of the stress axis have been associated with increased risk for psychopathology. Namely, a SNP within the corticotropin-releasing hormone (CRH) receptor 1 (CRHR1) gene is associated with increased depressive symptoms in individuals who have experienced childhood trauma (47). Polymorphisms within the FKBP5 gene, an important regulator of glucocorticoid negative feedback, increase risk for PTSD symptoms in childhood-trauma exposed individuals (48). Additionally, a polymorphism in the receptor gene (ADCYAP1R1) for pituitary adenylate cyclase-activating polypeptide (PACAP), a peptide implicated in stress-related behavior and physiology (49–51), is associated with PTSD in a sex-dependent manner (52). The ADCYAP1R1 SNP is predictive of PTSD diagnosis and symptoms, as well as dysregulated fear discrimination in females (52). In complement to this finding, we also found that a polymorphism within the testosterone metabolic pathway, SRD5A2, was associated with PTSD only in males (53). Results from these retrospective candidate gene studies, together with the current data, suggest SNPs within genes that regulate neuroendoimmunological function confer increased risk for psychopathology, and PTSD in particular, in individuals exposed to psychosocial stressors. In the future, we aim to assess whether these genetic loci confer increased risk for PTSD in a prospective manner, and to utilize genome-wide association study approaches.
In summary, the current data support the notion that increased CRP levels are associated with exacerbated PTSD symptoms in subjects recruited from a hospital that serves a primarily African American, low SES, highly traumatized civilian population (21). The findings are limited as the study was cross-sectional in nature, thus not allowing for the determination of causality. Additionally, the current study is not able to account for potential confounders of the association between CRP and PTSD, including smoking status (54), cardiovascular disease (20), and obesity (55), as these measures were not obtained in the large cohort. However, our genetic data linking the rs1130864 SNP in the CRP gene to increased CRP levels and increased PTSD symptoms suggest that heightened CRP levels due to a genetic trait increase PTSD symptoms in trauma-exposed individuals in a manner independent of other experiential factors. The notion that baseline CRP levels influence PTSD symptoms are corroborated by recent prospective data indicating that baseline CRP levels are predictive of PTSD following deployment in a military cohort (17).
Lastly, while the homogeneity of the study population is a strength of the current study, it could also serve as a limitation, as the results may not be generalizable to other samples. Furthermore, our inner-city hospital sample is not a population-based sample, and thus selection biases could be present in the small subset of individuals who participated in CRP peptide level and FPS analyses and limits our study, as these individuals self-selected to participate further. However, prior initial studies from this cohort demonstrating genetic associations with PTSD and depression (48, 52) have replicated in a variety of other populations of varied trauma, race, and socioeconomic status (56, 57). Additionally, high rates of trauma exposure (21) and inflammation (58) are prevalent in urban, low SES, African American communities and may lead to adverse health outcomes via dysregulation of the stress-immune system as evidenced by findings also indicating that African-Americans (58) and individuals of low SES (59) have increased levels of CRP. Our data suggest a possible therapeutic target for alleviating the symptoms associated with PTSD in this population, similar to what has been described in some types of depression (60).
Supplementary Material
Acknowledgments
The current study was supported by MH096764, MH071537 (KJR), MH070129 (TJ), MH082256 (CFG), the Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01RR00039), NARSAD (CFG, TJ), The Burroughs Welcome Fund (KJR), the Howard Hughes Medical Institute (KJR), the Atlanta Clinical Translational Science Institute, the NIH National Centers for Research Resources (M01 RR00039), and the Emory University General Clinical Research Center at Grady Hospital. This study would not have been possible without the research expertise and technical assistance of Allen Graham, Angelo Brown, and all the staff, volunteers, and participants of the Grady Trauma Project.
Footnotes
Conflict of Interest
All authors have no conflict of interests.
References
- 1.Boscarino JA. Diseases among men 20 years after exposure to severe stress: implications for clinical research and medical care. Psychosom Med. 1997;59:605–614. doi: 10.1097/00006842-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Annals of the New York Academy of Sciences. 2004;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- 3.Boscarino JA, Chang J. Electrocardiogram abnormalities among men with stress-related psychiatric disorders: implications for coronary heart disease and clinical research. Ann Behav Med. 1999;21:227–234. doi: 10.1007/BF02884839. [DOI] [PubMed] [Google Scholar]
- 4.Weiss T, Skelton K, Phifer J, Jovanovic T, Gillespie CF, Smith A, Umpierrez G, Bradley B, Ressler KJ. Posttraumatic stress disorder is a risk factor for metabolic syndrome in an impoverished urban population. Gen Hosp Psychiatry. 2011;33:135–142. doi: 10.1016/j.genhosppsych.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R, Bosmans E. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biological psychiatry. 1999;45:833–839. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 6.Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A, Valevski A, Weizman A. Elevated levels of serum interleukin-1 beta in combat-related posttraumatic stress disorder. Biological psychiatry. 1997;42:345–348. doi: 10.1016/S0006-3223(96)00375-7. [DOI] [PubMed] [Google Scholar]
- 7.Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:700–708. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, Neylan T. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis Markers. 2011;30:123–132. doi: 10.3233/DMA-2011-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun. 2012;26:13–17. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- 10.Yehuda R, Golier JA, Yang RK, Tischler L. Enhanced sensitivity to glucocorticoids in peripheral mononuclear leukocytes in posttraumatic stress disorder. Biological psychiatry. 2004;55:1110–1116. doi: 10.1016/j.biopsych.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 11.von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. Journal of psychiatric research. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spitzer C, Barnow S, Volzke H, Wallaschofski H, John U, Freyberger HJ, Lowe B, Grabe HJ. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. Journal of psychiatric research. 2010;44:15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Miller RJ, Sutherland AG, Hutchison JD, Alexander DA. C-reactive protein and interleukin 6 receptor in post-traumatic stress disorder: a pilot study. Cytokine. 2001;13:253–255. doi: 10.1006/cyto.2000.0825. [DOI] [PubMed] [Google Scholar]
- 15.Sondergaard HP, Hansson LO, Theorell T. The inflammatory markers C-reactive protein and serum amyloid A in refugees with and without posttraumatic stress disorder. Clinica chimica acta; international journal of clinical chemistry. 2004;342:93–98. doi: 10.1016/j.cccn.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 16.McCanlies EC, Araia SK, Joseph PN, Mnatsakanova A, Andrew ME, Burchfiel CM, Violanti JM. C-reactive protein, interleukin-6, and posttraumatic stress disorder symptomology in urban police officers. Cytokine. 2011;55:74–78. doi: 10.1016/j.cyto.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O’Connor DT, Baker DG. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71:423–431. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pankow JS, Folsom AR, Cushman M, Borecki IB, Hopkins PN, Eckfeldt JH, Tracy RP. Familial and genetic determinants of systemic markers of inflammation: the NHLBI family heart study. Atherosclerosis. 2001;154:681–689. doi: 10.1016/s0021-9150(00)00586-4. [DOI] [PubMed] [Google Scholar]
- 19.Kathiresan S, Larson MG, Vasan RS, Guo CY, Gona P, Keaney JF, Jr, Wilson PW, Newton-Cheh C, Musone SL, Camargo AL, Drake JA, Levy D, O’Donnell CJ, Hirschhorn JN, Benjamin EJ. Contribution of clinical correlates and 13 C-reactive protein gene polymorphisms to interindividual variability in serum C-reactive protein level. Circulation. 2006;113:1415–1423. doi: 10.1161/CIRCULATIONAHA.105.591271. [DOI] [PubMed] [Google Scholar]
- 20.Lange LA, Carlson CS, Hindorff LA, Lange EM, Walston J, Durda JP, Cushman M, Bis JC, Zeng D, Lin D, Kuller LH, Nickerson DA, Psaty BM, Tracy RP, Reiner AP. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. Jama. 2006;296:2703–2711. doi: 10.1001/jama.296.22.2703. [DOI] [PubMed] [Google Scholar]
- 21.Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress. 1993:459–473. [Google Scholar]
- 24.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 25.Almeida OP, Norman PE, Allcock R, van Bockxmeer F, Hankey GJ, Jamrozik K, Flicker L. Polymorphisms of the CRP gene inhibit inflammatory response and increase susceptibility to depression: the Health in Men Study. Int J Epidemiol. 2009;38:1049–1059. doi: 10.1093/ije/dyp199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong H, Qian YS, Tang XF, Zhang J, Gao PJ, Zhang Y, Zhu DL. C-reactive protein (CRP) gene polymorphisms, CRP levels and risk of incident essential hypertension: findings from an observational cohort of Han Chinese. Hypertens Res. 2012;35:1019–1023. doi: 10.1038/hr.2012.89. [DOI] [PubMed] [Google Scholar]
- 27.Hung AM, Crawford DC, Griffin MR, Brown-Gentry K, Lipkowitz MS, Siew ED, Cavanaugh K, Lewis JB, Ikizler TA. CRP polymorphisms and progression of chronic kidney disease in African Americans. Clin J Am Soc Nephrol. 2010;5:24–33. doi: 10.2215/CJN.01900309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kettunen T, Eklund C, Kahonen M, Jula A, Paiva H, Lyytikainen LP, Hurme M, Lehtimaki T. Polymorphism in the C-reactive protein (CRP) gene affects CRP levels in plasma and one early marker of atherosclerosis in men: The Health 2000 Survey. Scand J Clin Lab Invest. 2011;71:353–361. doi: 10.3109/00365513.2011.568123. [DOI] [PubMed] [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 31.Lin S, Zhoa H. Handbook on Analyzing Human Genetic Data: Computational Approaches and Software. New York: Springer; 2009. [Google Scholar]
- 32.Delongui F, Kallaur AP, Oliveira SR, Bonametti AM, Grion CM, Morimoto HK, Simao AN, Magalhaes GG, Reiche EM. Serum levels of high sensitive C reactive protein in healthy adults from southern Brazil. J Clin Lab Anal. 2013;27:207–210. doi: 10.1002/jcla.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heath NM, Chesney SA, Gerhart JI, Goldsmith RE, Luborsky JL, Stevens NR, Hobfoll SE. Interpersonal violence, PTSD, and inflammation: Potential psychogenic pathways to higher C-reactive protein levels. Cytokine. 2013;63:172–178. doi: 10.1016/j.cyto.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, Duncan EJ. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry research. 2009;167:151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz A, Grillon C, Avenevoli S, Cui L, Merikangas KR. Developmental investigation of fear-potentiated startle across puberty. Biological psychology. 2014;97:15–21. doi: 10.1016/j.biopsycho.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armbruster D, Strobel A, Kirschbaum C, Brocke B. The impact of sex and menstrual cycle on the acoustic startle response. Behavioural brain research. 2014 doi: 10.1016/j.bbr.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Breslau N. Gender differences in trauma and posttraumatic stress disorder. J Gend Specif Med. 2002;5:34–40. [PubMed] [Google Scholar]
- 40.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26:537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neuroscience and biobehavioral reviews. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 43.Melamed S, Shirom A, Toker S, Berliner S, Shapira I. Association of fear of terror with low-grade inflammation among apparently healthy employed adults. Psychosom Med. 2004;66:484–491. doi: 10.1097/01.psy.0000130963.52755.b9. [DOI] [PubMed] [Google Scholar]
- 44.O’Donovan A, Slavich GM, Epel ES, Neylan TC. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neuroscience and biobehavioral reviews. 2013;37:96–108. doi: 10.1016/j.neubiorev.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee CC, You NC, Song Y, Hsu YH, Manson J, Nathan L, Tinker L, Liu S. Relation of genetic variation in the gene coding for C-reactive protein with its plasma protein concentrations: findings from the Women’s Health Initiative Observational Cohort. Clin Chem. 2009;55:351–360. doi: 10.1373/clinchem.2008.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spann SJ, Gillespie CF, Davis JS, Brown A, Schwartz A, Wingo A, Habib L, Ressler KJ. The association between childhood trauma and lipid levels in an adult low-income, minority population. Gen Hosp Psychiatry. 2013 doi: 10.1016/j.genhosppsych.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Archives of general psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashimoto H, Shintani N, Baba A. New insights into the central PACAPergic system from the phenotypes in PACAP- and PACAP receptor-knockout mice. Annals of the New York Academy of Sciences. 2006;1070:75–89. doi: 10.1196/annals.1317.038. [DOI] [PubMed] [Google Scholar]
- 51.Ghzili H, Grumolato L, Thouennon E, Tanguy Y, Turquier V, Vaudry H, Anouar Y. Role of PACAP in the physiology and pathology of the sympathoadrenal system. Frontiers in neuroendocrinology. 2008;29:128–141. doi: 10.1016/j.yfrne.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillespie CF, Almli LM, Smith AK, Bradley B, Kerley K, Crain DF, Mercer KB, Weiss T, Phifer J, Tang Y, Cubells JF, Binder EB, Conneely KN, Ressler KJ. Sex dependent influence of a functional polymorphism in steroid 5-alpha-reductase type 2 (SRD5A2) on post-traumatic stress symptoms. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:283–292. doi: 10.1002/ajmg.b.32147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 55.Sanip Z, Ariffin FD, Al-Tahami BA, Sulaiman WA, Rasool AH. Obesity indices and metabolic markers are related to hs-CRP and adiponectin levels in overweight and obese females. Obes Res Clin Pract. 2013;7:e235–320. doi: 10.1016/j.orcp.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Boscarino JA, Kirchner HL, Hoffman SN, Erlich PM. Predicting PTSD using the New York Risk Score with genotype data: potential clinical and research opportunities. Neuropsychiatr Dis Treat. 2013;9:517–527. doi: 10.2147/NDT.S42422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, Cao C, Wang R, Qing Y, Zhang J, Zhang XY. PAC1 receptor (ADCYAP1R1) genotype is associated with PTSD’s emotional numbing symptoms in Chinese earthquake survivors. Journal of affective disorders. 2013;150:156–159. doi: 10.1016/j.jad.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 58.McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom Med. 2006;68:376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- 59.Owen N, Poulton T, Hay FC, Mohamed-Ali V, Steptoe A. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain Behav Immun. 2003;17:286–295. doi: 10.1016/s0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 60.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.