Abstract
Natural and man-made mass disasters directly or indirectly affect huge populations, who need basic infrastructural help and support to survive. However, despite the potentially negative impact on survival chances, these health care issues are often neglected by the authorities.
Treatment of both acute and chronic kidney diseases (CKDs) is especially problematic after disasters, because they almost always require complex technology and equipment, whereas specific drugs may be difficult to acquire for the treatment of the chronic kidney patients. Since many crush victims in spite of being rescued alive from under the rubble die afterward due to lack of dialysis possibilities, the terminology of ‘renal disaster’ was introduced after the Armenian earthquake. It should be remembered that apart from crush syndrome, multiple aetiologies of acute kidney injury (AKI) may be at play in disaster circumstances.
The term ‘seismonephrology’ (or earthquake nephrology) was introduced to describe the need to treat not only a large number of AKI cases, but the management of patients with CKD not yet on renal replacement, as well as of patients on haemodialysis or peritoneal dialysis and transplanted patients. This wording was later replaced by ‘disaster nephrology’, because besides earthquakes, many other disasters such as hurricanes, tsunamis or wars may have a negative impact on the ultimate outcome of kidney patients.
Disaster nephrology describes the handling of the many medical and logistic problems in treating kidney patients in difficult circumstances and also to avoid post-disaster chaos, which can be made possible by preparing medical and logistic scenarios. Learning and applying the basic principles of disaster nephrology is vital to minimize the risk of death both in AKI and CKD patients.
Keywords: acute tubular necrosis, AKI, chronic renal failure, dialysis, rhabdomyolysis
Mass disasters and their consequences
Disasters: inevitable dark side of life
Mass disasters cause widespread and severe damage, injury and loss of life or property, resulting in severe disruption of daily activities and infrastructure. The number of victims often overwhelms the local system and dictates the need for external help. These disasters can either be natural, such as earthquakes, volcanic eruptions, tsunamis and hurricanes, or man-made, such as technological disasters, terrorist attacks and wars. Until recently, most of the disasters were natural in origin; however, lately, man-made disasters, especially wars, have become more and more frequent.
Irrespective of the origin, the toll of disaster victims is growing (http://earthquake.usgs.gov/earthquakes/world/world_deaths.php) due to a dramatic rise in global population density with financially deprived people being forced to live in the most endangered areas. Because of poor building materials and lack of appropriate construction standards, casualty rates are much higher in the emerging world, so that massive demolition can occur with earthquakes of even relatively low magnitude [1].
After the Haiti earthquake, 230 000 people died, and another 300 000 were injured, with an earthquake magnitude of 7.0 on the Richter's scale (http://news.bbc.co.uk/2/hi/americas/8507531.stm; http://earthquake.usgs.gov/earthquakes/pager/events/us/2010rja6/index.html.). According to the detailed statistics on another mass disaster, the Marmara-Turkey 1999 earthquake, nearly 16 000 000 people were somehow affected by the disaster. This figure includes displaced populations, those who lost their jobs as a result of economic collapse and deprivation and those who lost properties [2]. The same source reported that >130 000 buildings collapsed entirely, making 600 000 people homeless [2]. Man-made disasters cause a dramatic number of fatalities as well; during the Iraq war, nearly 500 000 people died [3].
Health care issues after disasters
The above-mentioned statistics underline that to survive in the early period after mass disasters, a huge population needs basic infrastructural help and support, which includes but is not limited to housing, water and electricity supply, sanitation, nutrition, security, transportation and communication. This hyperacute need of support should in a later period be followed by long-lasting measures, such as constructing permanent housing, organizing education, refurbishing the transportation and communication systems and improving economics to achieve pre-disaster living standards.
Although being the most important priority for the medical community, only a minority of the overall affected population suffers from direct trauma and related effects. For example, during the Marmara earthquake ‘only’ ∼17 000 victims died and another 43 000 were injured [2]. At first glance, this is a very high number but it turns out to be a small segment when compared with the overall affected population (16 000 000 people). Even less attention is paid to the ‘renal’ victims after disasters, mainly because they constitute a relatively minor group among all injured. In addition, there is some reluctance to engage in presumably complex/labour-intensive therapeutic measures. As a result, authorities understandably tend to neglect some of these health care issues, particularly in kidney patients. On the other hand, the nephrological community has no excuse to ignore or minimize these problems, as kidney patients can only survive with appropriate therapy. Thus, nephrologists should be aware of the extent and types of kidney problems linked to disasters.
Nephrological problems after disasters
In the aftermath of disasters, the acute nephrological problems are not the only ones encountered; traumatic injuries, many de novo acute medical problems and difficulties for delivering treatment for pre-existing chronic diseases may be overwhelming (Table 1). Within the context of this review article, we will only focus on ‘nephrological problems after mass disasters’.
Table 1.
Overall medical problems after mass disasters
| I. Non-nephrological Acute
Acute cases
|
aInclude, but are not limited to.
ATN: acute tubular necrosis; AKI: acute kidney injury; CKD: chronic kidney disease; PD: peritoneal dialysis.
Problems in the management of acute nephrological problems
These problems arise because of traumatic or non-traumatic aetiologies. Among the traumatic causes, the most important one is crush syndrome-related acute kidney injury (AKI).
Crush syndrome
Literally, the word ‘crush’ means ‘to press or squeeze something that it is damaged or injured hard so as to make it lose its shape or its configuration’. Although crush injury refers only to the traumatic cause, the term ‘crush syndrome’ indicates systemic manifestations following muscle crush injury due to direct traumatic impact or ischaemia-reperfusion injury [4]. Manifestations may include tense, oedematous and painful muscles; hypovolaemic shock; AKI; hyperkalaemia; acidosis; arrhythmias, cardiac and respiratory failure; infections and psychological trauma [5–7].
The underlying pathogenetic mechanism in the crush syndrome is rhabdomyolysis, defined as damage to striated muscle cells by either traumatic or non-traumatic causes resulting in the release of intracellular components into the systemic circulation, ultimately triggering many clinical and laboratory abnormalities [8]. In mass disasters, most, if not all, cases of crush syndrome are caused by traumatic crush injury.
Evolution of the concepts: ‘renal disaster’, ‘seismonephrology’ and ‘disaster nephrology’
The first description of the crush syndrome appeared in the modern medical literature after the Messina earthquake in 1909 [9]. Trauma-related crush syndrome was first recognized as a single/broad pathophysiologic entity in 1941. During the bombing of London, Bywaters and Beall described in detail the clinical picture in four crush cases [5]. Three of them were oliguric and all produced dark brownish urine. All four patients died; histopathological examination of the kidneys revealed pigmented casts, polymorphonuclear invasion and acute tubular necrosis (ATN).
Despite this publication, disaster-related crush syndrome went further unnoticed until the occurrence of one of the deadliest earthquakes of all times in 1976. The Tangshan-China earthquake caused >240 000 deaths and 165 000 injured. Three important observations were made in the context of this catastrophe: (i) the incidence of crush syndrome ranged from 2 to 5% among injured victims, (ii) crush patients, whose general condition seems satisfying, can nevertheless suddenly die due to hyperkalaemia and (iii) irrespective of the severity of muscular compression, any patient may develop crush syndrome; hence, all crush cases should be observed closely for any signs of incipient AKI [10]. All these observations were confirmed in several later earthquakes [6, 11–13].
Two interesting observations in, respectively, 1979 and 1982 had an important impact on the subsequent therapeutic approach toward disaster-related crush syndrome. In 1979, seven victims who were entrapped following total collapse of a building were extricated from under the rubble after 12 h and received the first intravenous infusion with a minimal delay of 6 h after rescue. All these cases developed AKI within the first day of rescue despite 5–10 L of saline per day of volume replacement [14]. In contrast, in 1982, eight patients were trapped under the rubble in a similar incident, and in seven of them, intravenous fluids were started promptly at the site of the building collapse even before complete extrication. Within 2 h of release, they were evacuated to a hospital, where forced volume treatment with alkaline solutions was continued. None of them developed AKI. The remaining patient was buried under the rubble for 5.5 h and following release accidentally received only 2 L of intravenous fluid until he reached a trauma centre after a delay of 24 h. By that time, he already had developed established AKI for which he required dialysis support for one month [15]. These observations underlined the vital importance of early fluid administration to crush victims, if possible even before extrication.
In 1988, the Armenian earthquake, another milestone disaster, resulted in ∼150 000 deaths. The rescue teams, which arrived in the disaster area with a substantial delay, were confronted with ∼600 casualties, who had been rescued from under the rubble and who had subsequently developed AKI, after which many of them had died, because dialysis was unavailable [16]. As a matter of fact, this event was at the origin of the term ‘renal disaster’. Extensive support from outside Armenia was not effective [17], because no organized international support structure was available at that time [18]. Subsequently, the Renal Disaster Relief Task Force (RDRTF) of the International Society of Nephrology was founded with the intention to offer structured help if an area was affected by a mass disaster and had to cope with a large number of crush casualties [19, 20].
Of the 639 registered crush syndrome patients in the 1999 Marmara earthquake, 477 required dialysis support and >5000 dialysis treatments were applied [21, 22]. Among all other well-documented subsequent severe earthquakes, the highest number of crush-related AKI patients was reported after the Kobe disaster in Japan in 1995, with 202 cases [23].
All these reports underlined that disaster-related crush syndrome was more frequent than previously suspected; therefore, the terminology of ‘seismo-nephrology’ (or ‘earthquake nephrology’) was introduced by Vanholder et al. in 2000 [24]. More recently, several other disasters confirmed the importance of disaster-related crush and of its timely and appropriate treatment [13, 25–28].
Because many other types of disasters, including hurricanes [29–31], cyclones [32], nuclear accidents [33], tsunamis [34] or wars [35, 36] besides earthquakes may affect the outcome of kidney patients, a more appropriate term such as ‘disaster nephrology’ should be used, referring to an ‘area of nephrology dealing with the problems of acute and chronic kidney patients during and subsequent to disasters’.
Medical aspects of crush syndrome
Several publications on clinical [6, 27, 37, 38], laboratory [8, 23, 39], prognostic [27, 28, 40] and therapeutic [21] features of crush syndrome casualties after mass disasters have been published. Moreover, a recent comprehensive guideline [41] and highlights from this guideline [42] provided ample recommendations for the diagnosis and treatment of crushed patients. For reasons of space, we will here only summarize the key elements, which are specific to this problem.
Rhabdomyolysis is the triggering event in crush syndrome. Pathogenesis of rhabdomyolysis following trauma involves the following mechanisms, alone or in combination: [7, 8, 43] (i) increased permeability of the sarcolemma of striated muscle after crush injury, (ii) sustained increments in the sarcoplasmic calcium concentration and (iii) inadequate supply of adenosine triphosphate (ATP). All these factors cause an imbalance between energy consumption and energy production, resulting in muscle cell necrosis. Another contributing mechanism is ischaemia-reperfusion injury resulting in the invasion into the damaged muscles of inflammatory cells, generating reactive oxygen species and diverse cytokines (Figure 1).
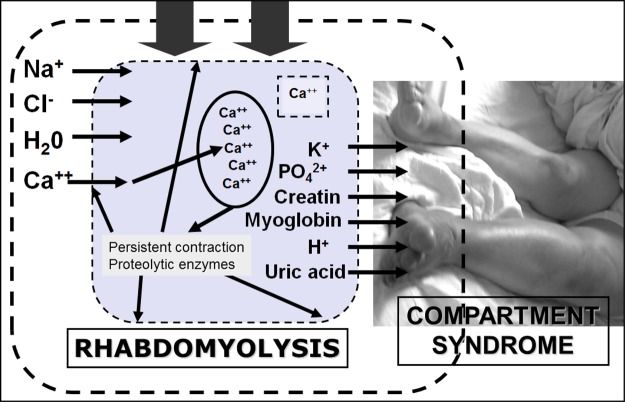
Fig. 1.
Pathogenesis of pressure-induced rhabdomyolysis. When muscles are compressed, permeability of the sarcolemma increases and substances present in the extracellular environment such as calcium, sodium and water move to the intracellular milieu, whereas substances highly concentrated in the muscle cells such as potassium and myoglobin seep out of the muscle cells into the extracellular space. Increased free calcium triggers muscle contraction and depletes ATP stores; mitochondrial damage occurs resulting in oxidative stress; proteases, phospholipases and other enzymes are activated, resulting in myofibril and membrane phospholipid damage. The next step is myocyte lysis and release of toxic intracellular constituents into the extracellular microenvironment, resulting in microvascular damage, producing capillary leak and causing compartmental syndrome. Increased pressure on the capillaries occludes microcirculation and depletes myoglobin oxygen content, resulting in more cell lysis. Most of the damage occurs only after the blood flow into the damaged tissue has been restored due to decompression (reperfusion injury) (adapted from [44]).
Also, the pathogenesis of crush syndrome-related AKI after rhabdomyolysis is multifactorial (Figure 2) [8, 43, 45–47]. The most important factor is hypovolaemia, leading to renal hypoperfusion and ischaemia, meaning that AKI at the beginning is often prerenal; however, if not treated properly, ATN develops. In addition, myoglobinuria, free radical production renal hypoperfusion due to the negative inotropic effect of hyperkalaemia and hypocalcaemia can play a role in the pathogenesis of AKI (Figure 2).
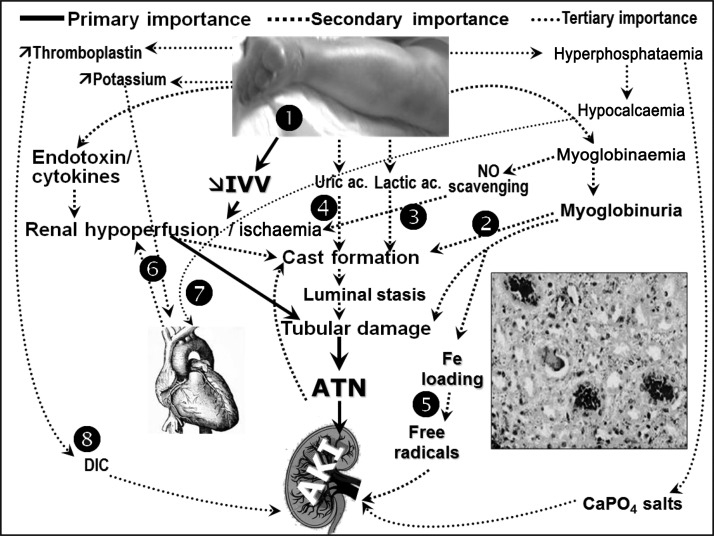
Fig. 2.
Pathogenesis of AKI related to the crush syndrome. 1. Muscle necrosis causes dramatic fluid third spacing, leading to intravascular volume depletion, renal hypoperfusion and ischaemia. 2. Myoglobinuria causes intratubular cast formation, which contributes to AKI. 3. Scavenging of nitric oxide by myoglobin and activation of the inflammatory pathways due to severe muscle injury, can aggravate renal hypoperfusion and tissue injury. 4. Nucleosides released from disintegrating cell nuclei, and metabolized to uric acid, may contribute to cast formation and tubular obstruction. 5. Degradation of intratubular myoglobin causes release of free iron, which catalyses free radical production enhancing ischaemic damage. 6. Hyperkalaemia depresses cardiac output potentiating renal hypoperfusion. 7. Hyperphosphataemia may contribute to hypocalcaemia, which can further depress myocardial contractility, and may result in the precipitation of CaPO4 salts that induce inflammation of the kidney tissue. 8. Damaged muscles can release tissue thromboplastin, triggering disseminated intravascular coagulation (adapted from [44]). IVV: intravascular volume; ATN: acute tubular necrosis; AKI: acute kidney injury; DIC: disseminated intravascular coagulation; NO: nitric oxide.
A dirty-brownish discolouration of the urine as a result of myoglobinuria and increased serum levels of substances released from the injured muscles such as creatinine, phosphate, potassium and muscle enzymes are important laboratory findings. Among these, an increased serum creatine phosphokinase by more than 5-fold the upper normal limit is an important diagnostic parameter [48–50]. Hyperkalaemia is very frequent and one of the major causes of mortality [12].
In disaster victims, crush syndrome is the second most common cause of death next to asphyxia [51]; the mortality rate in dialysed patients has been reported to be as high as 41% [23]. Mortality figures in more recent disasters such as the Marmara [21], Taiwan [52], Iran [28] and Pakistan [37] earthquakes were however lower (∼15–20%).
In the crushed disaster victims, the first goal is preventing development of crush syndrome, which can best be accomplished by early and massive fluid administration [13, 41, 45, 53]. However, extensive uncontrolled fluid resuscitation can result in hypervolaemia and related complications especially in elderly patients, and in those in whom the start of treatment is considerably delayed [1]. Therefore, fluid resuscitation should be individualized considering several medical and logistic variables, i.e. demographic characteristics, scale of the disaster, environmental conditions, time spent under the rubble, length of extrication procedure, volume status and urine flow [1, 41, 54]. Since crush syndrome can be avoided by intensive fluid management [13, 55], patients with crush injury should never be abandoned untreated, even when dialysis is unavailable [56, 57].
Even in the case of ideal treatment, these patients may suffer from several physical and psychological complications, as has been described in an illustrative case after the Marmara earthquake (Figure 3).
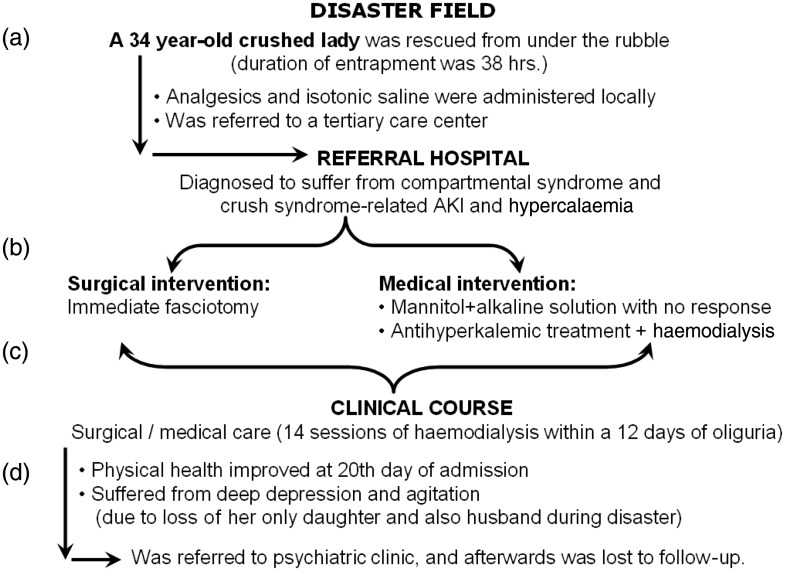
Fig. 3.
Clinical course of a typical case of crush syndrome patient after the 1999 Marmara Earthquake-Turkey: (a) at the disaster field, fluid resuscitation was initiated with a certain delay and the amount of applied fluid was inadequate; thus, crush-related AKI could not be prevented; (b) at the referral hospital, immediate fasciotomy was performed. Although this patient showed an uneventful course, routine fasciotomies are not recommended for treating compartmental syndrome because of high risk of sepsis in disaster victims; (c) very frequent haemodialysis was performed, mainly for treating hyperkalaemia; (d) patient suffered from serious psychological trauma, because of loss of the family members, and finally was lost to follow-up. Both of the latter observations are very frequent among victims of mass disasters.
AKI from other causes than the crush syndrome
As most disaster victims suffer from multiple trauma to various parts of the body, traumatic bleeding can lead to hypovolaemic shock and result in prerenal, and subsequently intrinsic AKI. Penetrating trauma to the kidneys or pelvic region can disrupt the integrity of the urinary system and cause postrenal AKI [58]. Injured victims frequently suffer from non-traumatic AKI as well. Antibiotics, contrast agents and nonsteroidal anti-inflammatory drugs are frequently used in trauma victims and may result in AKI by various pathogenetic mechanisms [59–61]. Importantly, most of these patients are hospitalized in surgery or orthopaedic wards, where nephrotoxic agents tend to be used with less consideration of their renal effects, as was the case in the Marmara earthquake [62]. In at least one-third of trauma patients, nephrotoxic drugs contribute to the induction of AKI [63].
Blood transfusions are frequently needed and are another important potential cause of non-traumatic AKI. During the Armenian earthquake, six patients with previous AKI suffered from severe transfusion reactions after their renal function had recovered; all of them became oliguric again and two died [64]. During the Marmara earthquake, ∼3000 units of blood were transfused to crush victims. Although no transfusion-related complications were described, this was probably because of underreporting due to chaos or to avoiding complaints for malpractice.
Lastly, sepsis is frequent in disaster victims [65, 66] and may contribute to the pathogenesis of AKI [6].
Problems in the treatment of chronic kidney patients
Chronic kidney disease patients not on renal replacement
To the best of our knowledge, there are no reports focusing on the outcome after disasters of non-dialysed chronic kidney disease (CKD) patients. Diabetes, hypertension and vascular disease are among the main causes of CKD in many countries, and a large number of CKD patients suffer from serious cardiovascular disease, deterioration of blood pressure [67–69] and inadequate glycaemic control [70–72]. Increased cardiovascular morbidity and mortality are frequent problems after disasters [73–75] and by extension should be considered as potential complicating factors in the CKD population. Underlying causes are difficulties to maintain an appropriate diet and to obtain medication, next to the stressful conditions in which these patients have to live. Therefore, disasters certainly generate medical and therapeutic problems in CKD patients, even if they are not yet on dialysis.
Chronic haemodialysis patients
Delivery of dialysis may create serious problems because (i) civil infrastructure (i.e. tap water and electricity delivery, communication) may be destroyed, (ii) dialysis units may be destroyed or their functioning impaired, (iii) disposables may be damaged, while their transport may be hampered or even impossible and (iv) dialysis personnel may be injured or in the impossibility to deliver efficient work [53, 76, 77].
There are limited data about the effect of these drawbacks on the outcome of chronic dialysis patients after mass disasters. Following the Kobe earthquake in Japan, dialysis centres in the region were seriously damaged and treatment of many haemodialysis patients was temporarily carried out in other units [78]. In addition, many patients had died, partly because of their inability to reach operative haemodialysis units, and also because of heart failure and pneumonia [48]. Following the Marmara earthquake, we retrospectively analysed the features of chronic haemodialysis practice and found that, in the units close to the epicentre, both the number of dialysis patients and the number of sessions had declined significantly [79]. Many patients temporarily or permanently left their dialysis units. Similar observations were made in the aftermath of Hurricane Katrina in the USA in 2005 [29]; overall, 48% of the dialysis clinics within the affected area had to close for 10 days or more. This figure reached up to ∼70% in some states [30], so that many patients missed dialysis sessions increasing their risk of hospitalization [31].
Peritoneal dialysis (PD) patients
The major problem for PD patients is the lack of dialysis material due to transportation problems [80]. All these logistic problems caused hypervolaemia in a number of PD patients after the Marmara earthquake [81]. In addition, probably due to unhygienic conditions, peritonitis rate increased. Importantly, automated peritoneal dialysis (APD) patients, who were connected to their machines at the moment of the disaster, were in the impossible situation of not being able to disconnect immediately, and all of them experienced important psychiatric problems later on [81].
Renal transplant recipients
There are no reports describing the outcome of renal transplant recipients after mass disasters. It can be speculated that, due to unhygienic conditions, risk of infections should significantly increase in these immunosuppressed patients. Also, problems in obtaining medication in chaotic circumstances can increase the risk of graft rejection. Inherent panic, confusion and depression may result in non-adherence, hence further contributing to rejection probability.
In order to minimize the adverse effects of disasters on chronic kidney patients, educational programmes are of vital importance. Courses should focus on (i) how to react in case of nonhygienic conditions; specifically, how to disconnect if APD or haemodialysis is applied at the moment of disaster and also (ii) how to determine the reserve of medical material and drugs to be stored at home in anticipation of problems in delivery if a disaster would occur [41, 78, 80, 81].
Logistic problems and their management
The term logistics refers to ‘the procurement, maintenance, distribution and replacement of personnel and material’, in order to have ‘the right measure, at the right place, at the right time’ [41]. Although usually not necessary in routine practice, logistic preparations are vital in anticipation of mass disasters, because of increased patient load, limited resources and considerable chaos even in countries experienced in disaster management [48, 71, 82, 83]. This is even more critical for kidney patients, because they almost always require complex technology and equipment and also specific drugs for their treatment [41].
In addition to general logistic preparations (which includes, but is not limited to, constructing disaster-resistant infrastructure, buildings, roads, hospitals, schools; developing disaster scenarios; educating rescue teams and all other parties involved in intervention about disaster circumstances), renal preparations include the following:
(i) Nephrology units in and around disaster prone areas should develop their own detailed disaster preparedness plans to cope with increased need of dialysis [41]. This preparation can be done ad hoc for potentially predictable disasters, such as hurricanes, volcano eruptions or even tsunamis; however, it is very problematic for unpredictable disasters, such as earthquakes [41]. Anyway, mapping of facilities where chronic dialysis patients can be relocated in the aftermath of disasters and planning for mobilization of extra staff can be of substantial value, once a disaster occurs.
Abdominal trauma as well as pulmonary and cardiac complications make PD less appropriate in crush victims. Also, low clearance rate of PD may not cope with the high catabolism, a typical finding in crush syndrome [18, 22], underlining that PD is not an ideal treatment modality for crush victims. However, if there is no possibility for haemodialysis and continuous renal replacement therapy (CRRT), PD may be an alternative. Crush patients on PD should be closely monitored for hyperkalaemia, and if necessary, aggressive antihyperkalemic therapy should be administered for this complication. In brief, PD can only be used as a temporary rescue when haemodialysis is not available. In the Marmara earthquake experience, of the 477 dialysed victims, only 8 patients were treated with PD, 4 of whom were switched to intermittent haemodialysis or CRRT later on [22].
(ii) The exact need for medical consumables to treat kidney patients should be defined in advance for both stockpiling of supplies and the organization of acute help from outside the damaged area. However, since the amount of needed medical material reaches gigantic proportions, while drugs and medical devices are prone to expire after some time, stockpiling of the medical material may not be a realistic solution [53]. On the other hand, calling for national/international help instantly, immediately after the disaster, can be very useful to cope with the problems. This was the case after the 1999 Marmara-Turkey [76], 2003 Bam-Iran [28], 2005 Kashmir-Pakistan [37] and lastly 2010 Haiti earthquake [26]. Collaboration of RDRTF and Médecins Sans Frontières has yielded very effective support after these calamities.
Dialysis infrastructure of the countries, faced by the disaster, can be seriously damaged; or, alternatively, this may have been inadequate even before the disaster. Haiti is a typical example for the latter; there was only one functioning dialysis machine in the whole country before the earthquake. RDRTF always leaves behind dialysis machines and disposables upon return after an earthquake.
Theoretically, smaller portable machines may be advantageous for providing dialysis services in acute circumstances, because they minimize the transportation problems and can more easily be transported to the affected area. As an example, sorbent systems allowing a dialysate regeneration (i.e. the well-known ‘REcirculating DialYsis’ (REDY) technique, which provides opportunity to conduct dialysis by using just 6 L of dialysis fluid), had been used in this regard in the aftermath of the Armenian earthquake and were credited with their easy transportation, simplicity and minimum dialysate requirements [17]. On the other hand, insufficient uraemic toxin clearance in highly catabolic crush syndrome patients and its high price limit wide use of this technique during mass disasters.
(iii) Since the number of patients who are in need of dialysis increases considerably after mass disasters, the local staff may be insufficient to deal with the increased patient load; the personnel of nonfunctioning units should be redistributed to the units that remain functional [29, 53].
Thus, in order to decrease the extent of post-disaster chaos, it is vital to implement a renal disaster relief response programme [84].
Implementation of a renal disaster relief response programme
Preparations before the disaster
Renal disaster relief strategies include an advanced plan of measures to be taken following a disaster. This plan should focus on composing renal disaster response teams, which should include coordinators of operations, assessment team members, rescuers and medical personnel [85]. Advance knowledge is needed about locations, structural and functional features and capacities of local dialysis facilities and also referral hospitals for deploying an effective response immediately after the disaster [41]. Also, educational programmes targeting the public, rescue teams, medical and para-medical personnel as well as the kidney patients should be developed and implemented in advance, describing how to survive and also how one can help others to survive in the case of a disaster [85–87]. Finally, a disaster response scenario for collaboration with external rescue organizations should be prepared (Figure 4) [85].
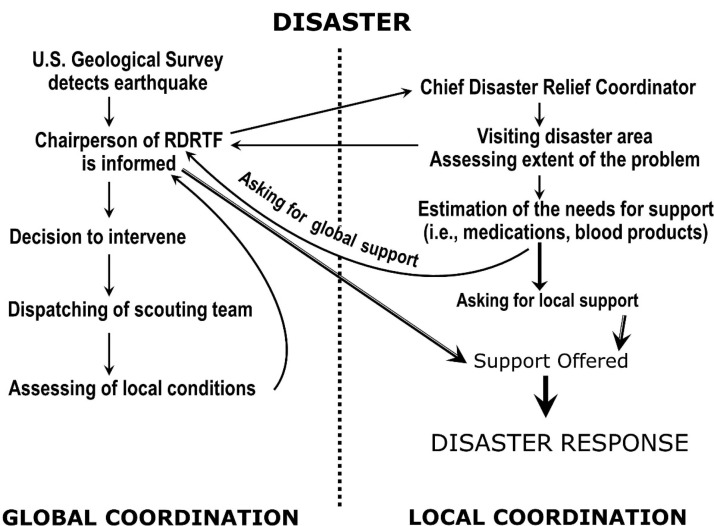
Fig. 4.
Principal steps in global and local coordination of renal-disaster relief efforts (reproduced from [85] with permission) (the figure is for viewing only and, for any reuse, permission must be obtained from Oxford University Press).
Measures to be taken in the aftermath of the disaster
(i) The chairperson of the RDRTF and local authorities should be contacted as soon as possible. If necessary, the chairman assigns a local chief disaster relief coordinator, dispatches a nephrological assessment team and offers support (Figure 4). The local coordinator visits the disaster field to assess the extent of damage and asks for national and international support, if the disaster cannot be coped with locally.
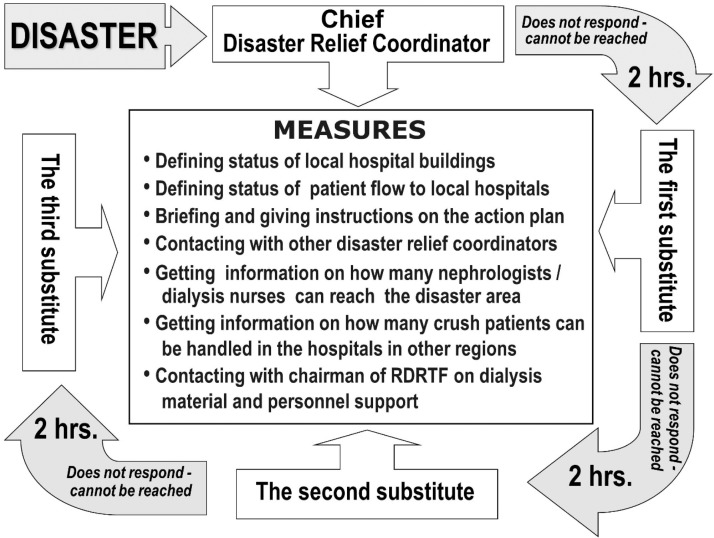
(ii) Previously developed action plans (as described above) should be implemented as early as possible, under the guidance of a formerly identified coordinator. In the case, this person is disabled or not reachable, a step-by-step order of alternative assignment should be followed in selecting the next in-line substitute when needed (Figure 5) [85].
Fig. 5.
Application of the action plan during the acute phase of major disasters. Measures to be taken by the responsible relief coordinator are summarized at the centrum. The substitutes should try to contact the chief disaster relief coordinator and each other even before the 2 h limit shown, so that they know each other's status and availability as early as possible (reproduced from [85] with permission) (the figure is for viewing only and, for any reuse, permission must be obtained from Oxford University Press). RDRTF: renal disaster relief task force.
Conclusions
After disasters (and by extension ‘renal disasters’) not only many new AKI patients emerge, but also predialysis CKD and dialysis patients as well as transplant recipients may suffer from serious problems, which can adversely affect their ultimate outcome. Pragmatic help and support from national and international sources may be useful for the local responders to cope with the dimensions of the problem. On the other hand, help campaigns are not always useful and may even be harmful for the local people [88–90]. Therefore, following termination of the intervention, and after all teams have returned home, a formal debriefing session should be organized to evaluate the positive aspects of the intervention as well as the encountered problems, for the sake of avoiding similar mistakes in the next disaster responses [32, 34, 85, 91]. Since medical applications during disasters differ considerably from routine medical practice, organizing CME courses and making preparations for future disasters is vital to decrease the extent of post-disaster chaos and minimize the risk of death both in AKI and CKD patients.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Gibney RT, Sever MS, Vanholder RC. Disaster nephrology: crush injury and beyond. Kidney Int 2014; 85: 1049–1057 [DOI] [PubMed] [Google Scholar]
- 2.Reports of Statistics Institution of the Turkish Prime Ministry. Crisis Center of the Turkish Prime Ministry, Ankara, 1997, pp: 3–15.
- 3.Hagopian A, Flaxman AD, Takaro TK, et al. Mortality in Iraq associated with the 2003–2011 war and occupation: findings from a national cluster sample survey by the university collaborative Iraq Mortality Study. PLoS Med 2013; 10: e1001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slater MS, Mullins RJ. Rhabdomyolysis and myoglobinuric renal failure in trauma and surgical patients: a review. J Am Coll Surg 1998; 186: 693–716 [DOI] [PubMed] [Google Scholar]
- 5.Bywaters EG, Beall D. Crush injuries with impairment of renal function. 1941. J Am Soc Nephrol 1998; 9: 322–332 [DOI] [PubMed] [Google Scholar]
- 6.Sever MS, Erek E, Vanholder R, et al. Clinical findings in the renal victims of a catastrophic disaster: the Marmara earthquake. Nephrol Dial Transplant 2002; 17: 1942–1949 [DOI] [PubMed] [Google Scholar]
- 7.Better OS. The crush syndrome revisited (1940–1990). Nephron 1990; 55: 97–103 [DOI] [PubMed] [Google Scholar]
- 8.Vanholder R, Sever MS, Erek E, et al. Rhabdomyolysis. J Am Soc Nephrol 2000; 11: 1553–1561 [DOI] [PubMed] [Google Scholar]
- 9.Better OS. History of the crush syndrome: from the earthquakes of Messina, Sicily 1909 to Spitak, Armenia 1988. Am J Nephrol 1997; 17: 392–394 [DOI] [PubMed] [Google Scholar]
- 10.Sheng ZY. Medical support in the Tangshan earthquake: a review of the management of mass casualties and certain major injuries. J Trauma 1987; 27: 1130–1135 [PubMed] [Google Scholar]
- 11.Sever MS, Erek E, Vanholder R, et al. The Marmara earthquake: epidemiological analysis of the victims with nephrological problems. Kidney Int 2001; 60: 1114–1123 [DOI] [PubMed] [Google Scholar]
- 12.Sever MS, Erek E, Vanholder R, et al. Serum potassium in the crush syndrome victims of the Marmara disaster. Clin Nephrol 2003; 59: 326–333 [DOI] [PubMed] [Google Scholar]
- 13.Gunal AI, Celiker H, Dogukan A, et al. Early and vigorous fluid resuscitation prevents acute renal failure in the crush victims of catastrophic earthquakes. J Am Soc Nephrol 2004; 15: 1862–1867 [DOI] [PubMed] [Google Scholar]
- 14.Ron D, Taitelman U, Michaelson M, et al. Prevention of acute renal failure in traumatic rhabdomyolysis. Arch Intern Med 1984; 144: 277–280 [PubMed] [Google Scholar]
- 15.Reis ND, Michaelson M. Crush injury to the lower limbs. Treatment of the local injury. J Bone Joint Surg Am 1986; 68: 414–418 [PubMed] [Google Scholar]
- 16.Collins AJ. Kidney dialysis treatment for victims of the Armenian earthquake. N Engl J Med 1989; 320: 1291–1292 [DOI] [PubMed] [Google Scholar]
- 17.Tattersall JE, Richards NT, McCann M, et al. Acute haemodialysis during the Armenian earthquake disaster. Injury 1990; 21: 25–28 [DOI] [PubMed] [Google Scholar]
- 18.Eknoyan G. Acute renal failure in the Armenian earthquake. Ren Fail 1992; 14: 241–244 [DOI] [PubMed] [Google Scholar]
- 19.Solez K, Bihari D, Collins AJ, et al. International dialysis aid in earthquakes and other disasters. Kidney Int 1993; 44: 479–483 [DOI] [PubMed] [Google Scholar]
- 20.Lameire N, Mehta R, Vanholder R, et al. The organization and interventions of the ISN Renal Disaster Relief Task Force. Adv Ren Replace Ther 2003; 10: 93–99 [DOI] [PubMed] [Google Scholar]
- 21.Sever MS, Erek E, Vanholder R, et al. Treatment modalities and outcome of the renal victims of the Marmara earthquake. Nephron 2002; 92: 64–71 [DOI] [PubMed] [Google Scholar]
- 22.Sever MS, Erek E, Vanholder R, et al. Renal replacement therapies in the aftermath of the catastrophic Marmara earthquake. Kidney Int 2002; 62: 2264–2271 [DOI] [PubMed] [Google Scholar]
- 23.Oda J, Tanaka H, Yoshioka T, et al. Analysis of 372 patients with crush syndrome caused by the Hanshin-Awaji earthquake. J Trauma 1997; 42: 470–475 [DOI] [PubMed] [Google Scholar]
- 24.Vanholder R, Sever MS, Erek E, et al. Acute renal failure related to the crush syndrome: towards an era of seismo-nephrology? Nephrol Dial Transplant 2000; 15: 1517–1521 [DOI] [PubMed] [Google Scholar]
- 25.Vanholder R, Borniche D, Claus S, et al. When the Earth trembles in the Americas: the experience of Haiti and Chile 2010. Nephron Clin Pract 2011; 117: c184–c197 [DOI] [PubMed] [Google Scholar]
- 26.Vanholder R, Gibney N, Luyckx VA, et al. Renal disaster relief task force in Haiti earthquake. Lancet 2010; 375: 1162–1163 [DOI] [PubMed] [Google Scholar]
- 27.van der Tol A, Hussain A, Sever MS, et al. Impact of local circumstances on outcome of renal casualties in major disasters. Nephrol Dial Transplant 2009; 24: 907–912 [DOI] [PubMed] [Google Scholar]
- 28.Hatamizadeh P, Najafi I, Vanholder R, et al. Epidemiologic aspects of the Bam earthquake in Iran: the nephrologic perspective. Am J Kidney Dis 2006; 47: 428–438 [DOI] [PubMed] [Google Scholar]
- 29.Kopp JB, Ball LK, Cohen A, et al. Kidney patient care in disasters: lessons from the hurricanes and earthquake of 2005. Clin J Am Soc Nephrol 2007; 2: 814–824 [DOI] [PubMed] [Google Scholar]
- 30.Kutner NG, Muntner P, Huang Y, et al. Effect of Hurricane Katrina on the mortality of dialysis patients. Kidney Int 2009; 76: 760–766 [DOI] [PubMed] [Google Scholar]
- 31.Anderson AH, Cohen AJ, Kutner NG, et al. Missed dialysis sessions and hospitalization in hemodialysis patients after Hurricane Katrina. Kidney Int 2009; 75: 1202–1208 [DOI] [PubMed] [Google Scholar]
- 32.Johnson DW, Hayes B, Gray NA, et al. Renal services disaster planning: lessons learnt from the 2011 Queensland floods and North Queensland cyclone experiences. Nephrology (Carlton) 2013; 18: 41–46 [DOI] [PubMed] [Google Scholar]
- 33.Kamei D, Kuno T, Sato S, et al. Impact of the Fukushima Daiichi Nuclear Power Plant accident on hemodialysis facilities: an evaluation of radioactive contaminants in water used for hemodialysis. Ther Apher Dial 2012; 16: 87–90 [DOI] [PubMed] [Google Scholar]
- 34.Akabayashi A, Kodama S. Lessons from Japan's March 2011 earthquake regarding dialysis patients. Ther Apher Dial 2011; 15: 334. [DOI] [PubMed] [Google Scholar]
- 35.Perkins R, Simon J, Jayakumar A, et al. Renal replacement therapy in support of Operation Iraqi Freedom: a tri-service perspective. Military Med 2008; 173: 1115–1121 [DOI] [PubMed] [Google Scholar]
- 36.Chung KK, Perkins RM, Oliver JD., 3rd Renal replacement therapy in support of combat operations. Crit Care Med 2008; 36(7 Suppl): S365–S369 [DOI] [PubMed] [Google Scholar]
- 37.Vanholder R, van der TA, De SM, et al. Earthquakes and crush syndrome casualties: lessons learned from the Kashmir disaster. Kidney Int 2007; 71: 17–23 [DOI] [PubMed] [Google Scholar]
- 38.Sever MS, Erek E, Vanholder R, et al. Lessons learned from the Marmara disaster: time period under the rubble. Crit Care Med 2002; 30: 2443–2449 [DOI] [PubMed] [Google Scholar]
- 39.Sever MS, Erek E, Vanholder R, et al. Effect of gender on various parameters of crush syndrome victims of the Marmara earthquake. J Nephrol 2004; 17: 399–404 [PubMed] [Google Scholar]
- 40.Sever MS, Erek E, Vanholder R, et al. Lessons learned from the catastrophic Marmara earthquake: factors influencing the final outcome of renal victims. Clin Nephrol 2004; 61: 413–421 [DOI] [PubMed] [Google Scholar]
- 41.Sever MS, Vanholder R. Recommendations for the management of crush victims in mass disasters. Nephrol Dial Transplant 2012; 27(Suppl 1): i1–67 [DOI] [PubMed] [Google Scholar]
- 42.Sever MS, Vanholder R. Management of crush victims in mass disasters: highlights from recently published recommendations. Clin J Am Soc Nephrol 2013; 8: 328–335 [DOI] [PubMed] [Google Scholar]
- 43.Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int 1996; 49: 314–326 [DOI] [PubMed] [Google Scholar]
- 44.Sever MS, Vanholder R. Management of crush syndrome casualties after disasters. Rambam Maimonides Med J 2011; 2: e0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Better OS, Stein JH. Early management of shock and prophylaxis of acute renal failure in traumatic rhabdomyolysis. N Engl J Med 1990; 322: 825–829 [DOI] [PubMed] [Google Scholar]
- 46.Sever MS. Rhabdomyolysis. Acta Clin Belg Supplementum 2007; 2: 375–379 [DOI] [PubMed] [Google Scholar]
- 47.Better OS, Abassi Z, Rubinstein I, et al. The mechanism of muscle injury in the crush syndrome: ischemic versus pressure-stretch myopathy. Miner Electrolyte Metab 1990; 16: 181–184 [PubMed] [Google Scholar]
- 48.Tanaka H, Oda J, Iwai A, et al. Morbidity and mortality of hospitalized patients after the 1995 Hanshin-Awaji earthquake. Am J Emerg Med 1999; 17: 186–191 [DOI] [PubMed] [Google Scholar]
- 49.Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med 1988; 148: 1553–1557 [PubMed] [Google Scholar]
- 50.Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore) 1982; 61: 141–152 [DOI] [PubMed] [Google Scholar]
- 51.Ukai T. The Great Hanshin-Awaji Earthquake and the problems with emergency medical care. Ren Fail 1997; 19: 633–645 [DOI] [PubMed] [Google Scholar]
- 52.Hwang SJ, Shu KH, Lain JD, et al. Renal replacement therapy at the time of the Taiwan Chi-Chi earthquake. Nephrol Dial Transplant 2001; 16(Suppl 5): 78–82 [DOI] [PubMed] [Google Scholar]
- 53.Sever MS, Vanholder R, Lameire N. Management of crush-related injuries after disasters. N Engl J Med 2006; 354: 1052–1063 [DOI] [PubMed] [Google Scholar]
- 54.Scharman EJ, Troutman WG. Prevention of kidney injury following rhabdomyolysis: a systematic review. Ann Pharmacother 2013; 47: 90–105 [DOI] [PubMed] [Google Scholar]
- 55.Iraj N, Saeed S, Mostafa H, et al. Prophylactic fluid therapy in crushed victims of Bam earthquake. Am J Emerg Med 2011; 29: 738–742 [DOI] [PubMed] [Google Scholar]
- 56.Merin O, Ash N, Levy G, et al. The Israeli field hospital in Haiti--ethical dilemmas in early disaster response. N Engl J Med 2010; 362: e38. [DOI] [PubMed] [Google Scholar]
- 57.Merin O, Miskin IN, Lin G, et al. Triage in mass-casualty events: the Haitian experience. Prehosp Dis Med 2011; 26: 386–390 [DOI] [PubMed] [Google Scholar]
- 58.Flint P, Allen CF. Pelvic fracture complicated by bilateral ureteral obstruction: case report. J Trauma 1994; 36: 285–287 [DOI] [PubMed] [Google Scholar]
- 59.Clive DM, Stoff JS. Renal syndromes associated with nonsteroidal antiinflammatory drugs. N Engl J Med 1984; 310: 563–572 [DOI] [PubMed] [Google Scholar]
- 60.Bennett WM, Henrich WL, Stoff JS. The renal effects of nonsteroidal anti-inflammatory drugs: summary and recommendations. Am J Kidney Dis 1996; 28(1 Suppl 1): S56–S62 [DOI] [PubMed] [Google Scholar]
- 61.Schetz M, Dasta J, Goldstein S, et al. Drug-induced acute kidney injury. Curr Opin Crit Care 2005; 11: 555–565 [DOI] [PubMed] [Google Scholar]
- 62.Sever MS. The Crush Syndrome (and Lessons Learned From the Marmara Earthquake). Basel: S. Karger AG, 2005 [Google Scholar]
- 63.Morris JA, Jr, Mucha P, Jr, Ross SE, et al. Acute posttraumatic renal failure: a multicenter perspective. J Trauma 1991; 31: 1584–1590 [DOI] [PubMed] [Google Scholar]
- 64.Richards NT, Tattersall J, McCann M, et al. Dialysis for acute renal failure due to crush injuries after the Armenian earthquake. BMJ 1989; 298: 443–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kazancioglu R, Cagatay A, Calangu S, et al. The characteristics of infections in crush syndrome. Clin Microbiol Infect 2002; 8: 202–206 [DOI] [PubMed] [Google Scholar]
- 66.Keven K, Ates K, Sever MS, et al. Infectious complications after mass disasters: the Marmara earthquake experience. Scand J Infect Dis 2003; 35: 110–113 [DOI] [PubMed] [Google Scholar]
- 67.Saito K, Kim JI, Maekawa K, et al. The great Hanshin-Awaji earthquake aggravates blood pressure control in treated hypertensive patients. Am J Hypertens 1997; 10: 217–221 [DOI] [PubMed] [Google Scholar]
- 68.Minami J, Kawano Y, Ishimitsu T, et al. Effect of the Hanshin-Awaji earthquake on home blood pressure in patients with essential hypertension. Am. J. Hypertens. 1997; 10: 222–225 [DOI] [PubMed] [Google Scholar]
- 69.Kario K, Matsuo T, Ishida T, et al. “White coat” hypertension and the Hanshin-Awaji earthquake. Lancet 1995; 345: 1365. [DOI] [PubMed] [Google Scholar]
- 70.Lloyd CE, Dyer PH, Lancashire RJ, et al. Association between stress and glycemic control in adults with type 1 (insulin-dependent) diabetes. Diabetes Care 1999; 22: 1278–1283 [DOI] [PubMed] [Google Scholar]
- 71.Baba S, Taniguchi H, Nambu S, et al. The Great Hanshin earthquake. Lancet 1996; 347: 307–309 [DOI] [PubMed] [Google Scholar]
- 72.Sengul A, Ozer E, Salman S, et al. Lessons learnt from influences of the Marmara earthquake on glycemic control and quality of life in people with type 1 diabetes. Endocr J 2004; 51: 407–414 [DOI] [PubMed] [Google Scholar]
- 73.Trichopoulos D, Katsouyanni K, Zavitsanos X, et al. Psychological stress and fatal heart attack: the Athens (1981) earthquake natural experiment. Lancet 1983; 1: 441–444 [DOI] [PubMed] [Google Scholar]
- 74.Inui A, Kitaoka H, Majima M, et al. Effect of the Kobe earthquake on stress and glycemic control in patients with diabetes mellitus. Arch Intern Med 1998; 158: 274–278 [DOI] [PubMed] [Google Scholar]
- 75.Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med 1996; 334: 413–419 [DOI] [PubMed] [Google Scholar]
- 76.Vanholder R, Sever MS, De SM, et al. Intervention of the Renal Disaster Relief Task Force in the 1999 Marmara, Turkey earthquake. Kidney Int. 2001; 59: 783–791 [DOI] [PubMed] [Google Scholar]
- 77.Vanholder R, Van Biesen W, Hoste E, et al. The role of the Renal Disaster Relief Task Force in the prevention and treatment of Crush syndrome in mass disasters. Acta Clin Belg Supplementum 2007; 2: 405–407 [DOI] [PubMed] [Google Scholar]
- 78.Sakai R. The Japanese experience during the Kobe Earthquake: management of continuous ambulatory peritoneal dialysis patients in a disaster. Ren Fail 1997; 19: 693–699 [DOI] [PubMed] [Google Scholar]
- 79.Sever MS, Erek E, Vanholder R, et al. Features of chronic hemodialysis practice after the Marmara earthquake. J Am Soc Nephrol 2004; 15: 1071–1076 [DOI] [PubMed] [Google Scholar]
- 80.China S. Management of continuous ambulatory peritoneal dialysis patients in a disaster: the Japanese experience during the Kobe Earthquake. Ren Fail 1997; 19: 687–692 [DOI] [PubMed] [Google Scholar]
- 81.Ozener C, Ozdemir D, Bihorac A. The impact of the earthquake in northwestern Turkey on the continuous ambulatory peritoneal dialysis patients who were living in the earthquake zone. Adv Perit Dial 2000; 16: 182–185 [PubMed] [Google Scholar]
- 82.Haynes BE, Freeman C, Rubin JL, et al. Medical response to catastrophic events: California's planning and the Loma Prieta earthquake. Ann Emerg Med 1992; 21: 368–374 [DOI] [PubMed] [Google Scholar]
- 83.Fukagawa M. Nephrology in earthquakes: sharing experiences and information. Clin J Am Soc Nephrol 2007; 2: 803–808 [DOI] [PubMed] [Google Scholar]
- 84.Kenney RJ. Emergency preparedness concepts for dialysis facilities: reawakened after Hurricane Katrina. Clin J Am Soc Nephrol 2007; 2: 809–813 [DOI] [PubMed] [Google Scholar]
- 85.Sever MS, Lameire N, Vanholder R. Renal disaster relief: from theory to practice. Nephrol Dial Transplant 2009; 24: 1730–1735 [DOI] [PubMed] [Google Scholar]
- 86.Noji EK, Armenian HK, Oganessian A. Issues of rescue and medical care following the 1988 Armenian earthquake. Int J Epidemiol 1993; 22: 1070–1076 [DOI] [PubMed] [Google Scholar]
- 87.Noji EK. Public health issues in disasters. Crit Care Med 2005; 33(1 Suppl): S29–S33 [DOI] [PubMed] [Google Scholar]
- 88.Mahoney LE, Reutershan TP. Catastrophic disasters and the design of disaster medical care systems. Ann Emerg Med 1987; 16: 1085–1091 [DOI] [PubMed] [Google Scholar]
- 89.Seaman J. Disaster epidemiology: or why most international disaster relief is ineffective. Injury 1990; 21: 5–8 [DOI] [PubMed] [Google Scholar]
- 90.Waeckerle JF. Disaster planning and response. N Engl J Med 1991; 324: 815–821 [DOI] [PubMed] [Google Scholar]
- 91.Portilla D, Shaffer RN, Okusa MD, et al. Lessons from Haiti on disaster relief. Clin J Am Soc Nephrol 2010; 5: 2122–2129 [DOI] [PubMed] [Google Scholar]