Abstract
Myocardial aging is characterized by LV fibrosis leading to diastolic and systolic dysfunction. Studies have established the potent anti-fibrotic and anti-proliferative properties of C-type natriuretic peptide (CNP), however the relationship between circulating CNP, LV fibrosis and associated changes in LV function with natural aging are undefined. Accordingly, we characterized the relationship of plasma CNP with LV fibrosis and function in 2, 11 and 20 month old male Fischer rats. Further in vitro, we establish the anti-proliferative actions of CNP and the participation of the clearance receptor using adult human cardiac fibroblasts. Here we establish for the first time that a progressive decline in circulating CNP characterizes natural aging and is strongly associated with a reciprocal increase in LV fibrosis which precedes impairment of diastolic and systolic function. Additionally we demonstrate in cultured adult human cardiac fibroblasts that the direct anti-proliferative actions of high dose CNP may involve a non-cGMP pathway via the clearance receptor. Taken together these studies provide new insights into myocardial aging and the relationship to the anti-fibrotic and anti-proliferative peptide CNP.
Keywords: aging, heart, c-type natriuretic peptide, fibrosis, natriuretic peptides
INTRODUCTION
It is well established that myocardial aging is characterized by left ventricular (LV) fibrosis due to the progressive reduction in cardiomyocyte number, the increase in cardiac fibroblast (CF) proliferation and LV collagen deposition leading to ventricular dysfunction.1, 2 To date, advances have been made in experimental and clinical studies in identifying humoral and hemodynamic mechanisms which contribute to age-related fibrosis in the heart. Such mechanisms include activation of cytokines such as transforming growth factor beta (TGF-β) and loss of nitric oxide (NO) together with LV pressure overload secondary to the rise in arterial pressure.3–5
C-type natriuretic peptide (CNP) is an anti-fibrotic and anti-proliferative peptide which shares these key biological actions with the cardiac natriuretic peptides, atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP).6 CNP is mainly an endothelial cell derived peptide,7, 8 which also has been detected in various other tissues including the heart and kidneys9–11 and circulates at low concentrations. Because of low circulating concentrations, CNP is thought to be mainly an autocrine/paracrine factor. Yet recently, CNP production has been identified in the human heart, liver, and brain with clearance in the lung and kidney, suggesting a role as a circulating hormone.11 The relevance of CNP to fibrosis is compelling as CNP possesses potent anti-fibrotic properties12 through 3′,5′-cyclic guanosine monophosphate (cGMP).6, 13 Specifically in one key in vitro study, CNP possessed more potent anti-fibrotic and anti-proliferative properties in young rat cardiac fibroblasts (CFs) as compared to ANP and BNP.12 Further, these investigators demonstrated in vivo, that 14 days of continuous infusion of CNP in young rats with acute myocardial infarction (AMI) significantly attenuated post-AMI cardiac fibrosis.14 These two studies of CNP and its potential role as an inhibitor of cardiac fibrosis are complemented by reports that CNP possesses anti-fibrotic and extracellular matrix (ECM) regulatory actions in the lung, liver and kidney.15–17 While Soeki et al.14 and Horio et al.12 demonstrated that CNP suppression of collagen production is linked to cGMP and the natriuretic peptide B receptor (NPR-B) in the young, evidence suggests that the natriuretic peptides may possess anti-proliferative properties in part via activation of the non-cGMP natriuretic peptide clearance receptor (NPR-C), particularly in adults.18, 19
The current study tested the hypothesis that aging is characterized by a progressive decline in circulating CNP, which would be followed by a reciprocal increase in LV fibrosis as compared to BNP. To address this hypothesis, we characterized circulating CNP in a Fischer rat model of aging, which is equivalent to human aging from adolescence to the 6th decade of life.20 We also determined LV structure, including LV fibrosis and function and defined the correlation of LV fibrosis to circulating CNP and BNP. Lastly in vitro using adult human CFs, we tested the hypothesis that CNP’s anti-proliferative action involves NPR-C.
METHODS
Animals
Studies were performed in 2, 11 and 20 month old male Fischer rats (Harlan Laboratories, Inc., Madison, WI; n=10 per age group, unless otherwise specified). These age groups are equivalent to human aging from adolescence to the 6th decade of life.20 The experimental study was performed in accordance with the Animal Welfare Act and with approval of the Mayo Clinic Institutional Animal Care and Use Committee.
Standard and Two-Dimensional Speckle-Derived Strain Echocardiography (2DSE)
Standard transthoracic echocardiography was performed on anesthetized (1.5% isoflurane in oxygen) rats using the Vivid 7 ultrasound system (GE Medical Systems, Milwaukee, WI) and a 10S transducer (11.5 MHz) with ECG monitoring. M-mode images and gray scale 2D parasternal short axis images (300–350 frames/sec) at the mid-papillary level were recorded for off-line analysis using EchoPAC software (EchoPAC PC BTO 9.0.0, GE Healthcare, Milwaukee, WI). LV end-diastolic and end-systolic internal diameters and wall thicknesses were measured from M-mode images permitting calculation of LV ejection fraction (EF) based on the cubed method, LV fractional shortening (FS) and relative wall thickness (RWT). All parameters represent the average of 3 beats.
2DSE parasternal short axis images at the mid-papillary level were acquired with a frame rate ranging from 60 (full apical views) and 160 (narrow sector views) frames/sec as previously described.21 Three consecutive cardiac cycles were recorded as 2-D cine loops and the acquired raw data were saved for off-line analysis using EchoPAC software. Circumferential systolic peak values were determined for strain (sS) and strain rate (sSR). Circumferential early diastolic peak values were determined for myocardial strain rate (dSR-E).
Blood Pressure and Plasma Collection
Rats were anesthetized with isoflurane and PE-50 tubing was placed into the carotid artery for blood pressure (BP) monitoring and blood sampling. After BP acquisition using CardioSOFT Pro software (Sonometrics Corporation, London, Ontario), blood was collected from the carotid artery and placed in EDTA tubes on ice.7, 22 Blood was immediately centrifuged at 2,500 rpm at 4°C for 10 minutes and the plasma was stored in polystyrene tubes at −80°C for future radioimmunoassay.
LV Tissue Harvest
Hearts were removed for total cardiac and LV weights. The LV was dissected and quickly frozen in liquid nitrogen. A cross-section of the LV was preserved in 10% formalin for histological analysis and smaller cube sections were preserved in 2.5% glutaraldehyde for electron microscopy (EM) analysis.
Plasma CNP and BNP Analysis
As previously described, plasma CNP7 was determined by a non-equilibrium radioimmunoassay kit (Phoenix Pharmaceutical, Mountain View, CA), using an antibody to human CNP which is fully cross-reactive to rat CNP. One ml of plasma was extracted using C-18 Bond Elut cartridges. After washing cartridges with 4ml 100% methanol and 4ml water, plasma was applied and the cartridges were washed. Eluates were concentrated on a Savant speed vacuum concentrator, and pellets were re-suspended in 300 ul assay buffer. 100ul of standards and samples are incubated with 100 ul anti-human CNP at 4°C. After 18 hours 100ul (10,000 counts) of I125-labeled CNP was added and incubated at 4°C for 18 hours. Then a second antibody was added to all samples to separate the free and bound fractions and the samples were centrifuged, free fraction was aspirated and bound fraction was counted on a gamma counter. A standard curve was generated and used to calculate the concentrations of the unknown samples and reported in pg/ml. The range of the standard curve was 0.5–128 pg, with a lower limit of detection of 0.5 pg. Inter- and intra-assay variability was 11% and 5.2% respectively. Recovery was 72±6%. Cross-reactivity was <1% with ANP, BNP, endothelin, and adrenomedullin and 97% with CNP-53. Plasma BNP23 was also determined by non-equilibrium radioimmunoassay kits from Phoenix Pharmaceutical, using an antibody to rat BNP. All rat plasma samples were evaluated in one assay.
Histological and Electron Microscopy Analysis
For histology, fixed LV tissues were dehydrated, embedded in paraffin and sectioned at thickness of 4 μm. Collagen and extent of fibrosis was performed using picrosirius red staining (n=7 per age group). An Axioplan II KS 400 microscope (Carl Zeiss, Inc., Germany) was used to capture at least 4 randomly selected images from each slide using a 20x objective and KS 400 software was utilized to determined fibrotic area as a percentage of total tissue area. For EM, LV tissue fixed in 2.5% glutaraldehyde was dehydrated and embedded in a resin mould. Ultra-thin sections were cut according to the EM core facility procedures. LV collagen fibers were visualized at 8000x using a JEM-1400 transmission electron microscope.
Proliferation Assay
Seventy to 80% confluent passage 1 to 4 human adult CFs were serum starved for 24 hours and stimulated to proliferate using 10−8 M cardiotrophin-1 (CT-1) with or without 10−6 M BNP or CNP. For receptor antagonism studies, cells were treated with 10−6 M CNP with and without the non-selective NPR-A/B receptor antagonist, HS-142-1 (10−6 M) or the selective NPR-C antagonist, cANF(4–23) (des[Gln18, Ser19, Gly20, Leu21, Gly22] (ANP 4–23)-NH2 (10−6 M). Untreated CFs were processed as controls. Colormetric bromodeoxyuridine (BrdU) cell proliferation enzyme-linked immunoadsorbent assay (Roche, Indianapolis, Indiana) was performed. CFs were labelled with BrdU for 2 h in a CO2 37°C incubator. Antibromodeoxyuridine was added and allowed to react for 90 min at room temperature. The anti-BrdU was removed and the CFs were washed 3 times with a washing solution. Colormetric substrate solution was added, and color was allowed to develop for 30 min. Absorbance at 370 nm was measured on a SpectraMax spectrophotometer (Molecular Devices, Sunnyvale, California).
Statistical Analysis
Results are expressed as mean ± SEM. Comparisons within groups were made by 1-way analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison test. A general linear regression model was used to test the correlation between LV interstitial fibrosis and plasma natriuretic peptides. GraphPad Prism software (GraphPad Software, La Jolla, CA) was used for the above calculations. Statistical significance was accepted as P<0.05.
RESULTS
Cardiac Structure and Function with Aging
Cardiac structure and function and mean arterial pressure (MAP) are reported in Table 1. There was a significant increase in body, heart and LV weight, end-diastolic internal chamber diameter, wall thicknesses and RWT at 11 months, which were sustained at 20 months. When heart weights (HW) and LV weights (LVW) were normalized to body weight (BW), there was a significant reduction in both HW:BW and LVW:BW ratio at 11 months, which was sustained at 20 months. Notably, LV EF and FS were significantly reduced at 20 months. At 20 months there was a significant increase in MAP and there was no change in heart rate among the groups. Furthermore, 2DSE confirmed a trend for LV functional impairment as demonstrated by reductions in circumferential sSR (Figure 1B) and dSR-E (Figure 1C) at 11 months. More importantly, significant impairment of systolic and diastolic function was demonstrated by reductions in circumferential sS (Figures 1A), sSR (Figure 1B) and dSR-E (Figure 1C) at 20 months compared to the younger age groups.
Table 1.
Standard Cardiovascular Characteristics
| Parameter | 2 months | 11 months | 20 months |
|---|---|---|---|
| Body Weight (g) | 211 ± 2 | 465 ± 5 * | 445 ± 7 *† |
| Heart Weight (mg) | 654 ± 7 | 1149 ± 23 * | 1089 ± 29 * |
| HW:BW (mg/g) | 3.11 ± 0.03 | 2.47 ± 0.03 * | 2.44 ± 0.04 * |
| LV Weight (mg) | 472 ± 6 | 829 ± 15 * | 801 ± 23 * |
| LVW:BW (mg/g) | 2.24 ± 0.3 | 1.78 ± 0.02 * | 1.80 ± 0.03 * |
| HR (bpm) | 326 ± 13 | 319 ± 6 | 322 ± 11 |
| IVSd (mm) | 1.18 ± 0.02 | 1.73 ± 0.03 * | 1.65 ± 0.06 * |
| LVIDd (mm) | 6.71 ± 0.10 | 7.62 ± 0.06 * | 7.44 ± 0.08 * |
| LVPWd (mm) | 1.21 ± 0.03 | 1.69 ± 0.2 * | 1.66 ± 0.06 * |
| EF (%) | 88 ± 1 | 92 ± 1 * | 80 ± 1 *† |
| FS (%) | 51 ± 1 | 58 ± 2 * | 42 ± 1 *† |
| RWT | 0.36 ± 0.01 | 0.45 ± 0.01 * | 0.45 ± 0.02 * |
| MAP (mmHg) | 91 ± 1 | 91 ± 2 | 102 ± 4 *† |
Values are mean ± SEM. n=10 for all age groups.
P<0.05 versus 2 months (1-way ANOVA),
P<0.05 versus 11 months (1-way ANOVA).
HW = heart weight; BW = body weight; LV = left ventricle; LVW = left ventricle weight; HR = heart rate; IVSd = Interventricular septum end-diastole; LVIDd = left ventricular internal diameter end-diastole; LVPWd = left ventricular posterior wall end-diastole; EF = ejection fraction; FS = fractional shortening; RWT = relative wall thickness; MAP = mean arterial pressure.
Figure 1.
Assessment of the global average for sS circ. (A), sSR circ. (B) and dSR-E circ. (C) by 2DSE between 2,11 and 20 month old rats. Values are mean ± SEM. P<0.05. 2DSE = two-dimensional speckle-derived strain echocardiography; Circ. = circumferential; sS = systolic strain; sSR = systolic strain rate; dSR-E = early diastolic strain rate
LV Interstitial Fibrosis
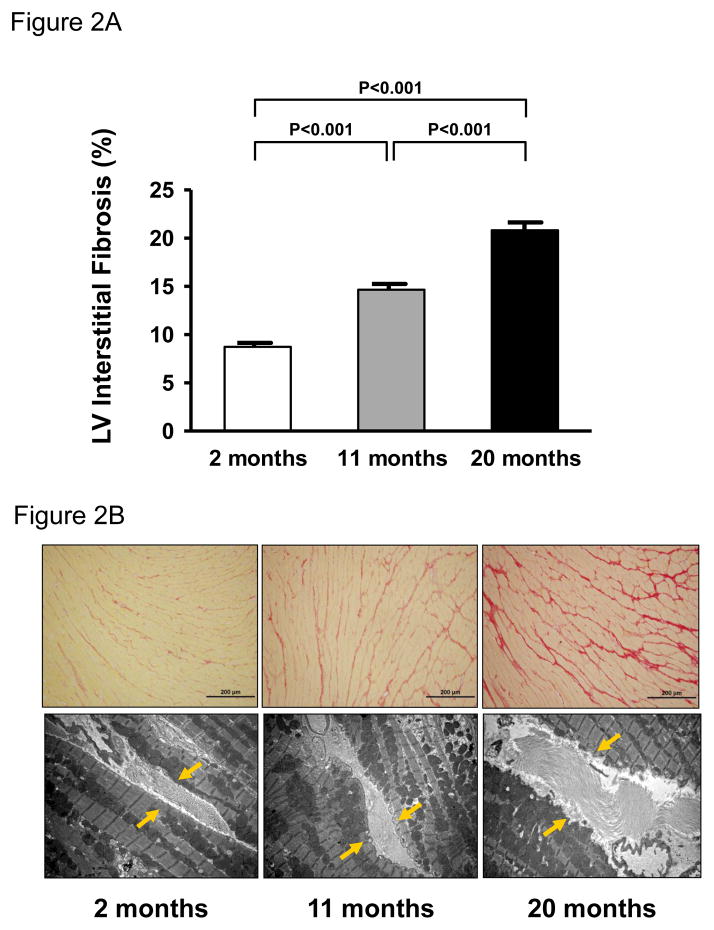
Figure 2 illustrates the quantification of picrosirius red staining (Figure 2A) and a representative image of LV interstitial fibrosis using picrosirius red staining (Figure 2B-upper panels) and electron microscopy (Figure 2B – lower panels) for each age group. Specifically, there was a significant and progressive increase in intensity of collagen staining (Figure 2A & 2B – upper panels) and abundance in collagen fibers (Figure 2B – lower panels, yellow arrows) between the age groups.
Figure 2.
Quantification of picrosirius red staining (A) and representative histology images at 20x objective magnification (B – upper panels) and electron micrographs at 8000x magnification (B – lower panels, yellow arrows) of LV interstitial fibrosis from 2,11 and 20 month old rats. Values are mean ± SEM. P<0.05.
Plasma CNP and BNP and the Relationship to LV Fibrosis
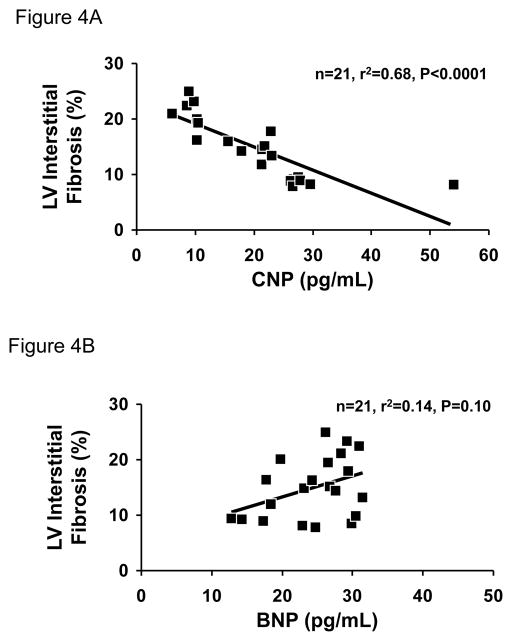
Figure 3 illustrates plasma CNP and BNP with aging. Importantly, there was a significant progressive decrease in plasma CNP (Figure 3A) from 2 to 11 to 20 months of age (2 month mean ± SE: 31.4 ± 3.6 pg/ml; 11 month mean ± SE: 20.6 ± 0.8 pg/ml; 20 month mean ± SE: 9.0 ± 0.4 pg/ml; P<0.0001). Whereas plasma BNP (Figure 3B) demonstrated a modest trend for an increase at 11 and 20 months (2 month mean ± SE: 21.0 ± 2.1 pg/ml; 11 month mean ± SE: 25.1 ± 1.3 pg/ml; 20 month mean ± SE: 26.5 ± 1.6 pg/ml; P=0.08). Further, Figure 4A illustrates a strong negative correlation between LV interstitial fibrosis and plasma CNP (n=21; r2=0.68; P<0.0001). In contrast, plasma BNP did not correlate with LV interstitial fibrosis (Figure 4B: n=21; r2=0.14; P=0.10).
Figure 3.
Plasma concentrations CNP (A) and BNP (B) with aging by radioimmunoassay. Values are mean ± SEM. P<0.05.
Figure 4.
Scatter plots showing the relationship between LV interstitial fibrosis and plasma CNP (A) and BNP (B).
Anti-Proliferative Effects of CNP on Adult Human Cardiac Fibroblasts
Figure 5A illustrates the ability of CNP to significantly suppress cell proliferation in adult human CFs induced by CT-1 as assessed by BrdU uptake as a measure of DNA synthesis and cellular proliferation. Further, we observed the anti-proliferative response to CNP was significantly blocked by the NPR-C antagonist cANF(4–23) as illustrated in Figure 5B, with only a trend to suppress inhibition with blockade of NPR-B with HS-1-141.
Figure 5.
In vitro proliferation analysis of adult human CFs in response to 10−8 M CT-1 stimulation with or without 10−6 M BNP or CNP was determined by BrdU incorporation (A) and in response to 10−6 M CNP with and without the NPR-A/NPR-B antagonist, HS-142-1 and NPR-C antagonist, cANF(4–23) was determined by BrdU incorporation (B). Values are the mean of three samples per treatment from four experiments. Passages 1–4 were used. P<0.05.
DISCUSSION
This is the first study to define the relationship between circulating CNP and myocardial fibrosis during natural aging using a Fischer rat model of aging. Specifically, we report that circulating CNP progressively declines with aging and there is a highly significant inverse relationship between decreasing circulating CNP and increasing LV fibrosis. We also demonstrate that the anti-proliferative action of CNP on adult human CFs involves NPR-C.
The first evidence of the biological importance of CNP was reported by Chusho et al.,24 in which targeted disruption of the CNP gene in mice resulted in severe dwarfism due to impaired endochondral ossification. More recently evidence has also shown that CNP has biological actions which go beyond skeletal remodeling and targets cardiac ECM regulation. Specifically, humans in their sixth decade of life with aortic stenosis, are reported to have reduced CNP production in their aortic valves compared to young disease free adults.25 Importantly, in vitro studies have demonstrated that CNP possesses more robust anti-fibrotic and anti-proliferative properties in young rat CFs as compared to ANP and BNP.12 Further, other investigations demonstrated that a two week infusion of CNP or a CNP-based peptide suppressed post-AMI cardiac fibrosis.14, 26 Together, these studies support an important cardio-protective role of CNP in inhibiting cardiac fibrosis and ECM remodelling.
In contrast to BNP, plasma CNP concentrations in the adult are low. Therefore CNP has been thought to primarily be an autocrine/paracrine factor. However, recent studies suggest a possible endocrine role for CNP in adults, by demonstrating modest but significant increases in circulating CNP in HF and myocardial ischemia.11, 27 In our study using a rat model of aging, we observed that aging is characterized by a progressive decline in circulating CNP. Our studies thus are in part consistent with the elegant human studies of Prickett et al.,22 which reported that plasma NT-proCNP and CNP were high in children when skeletal growth and development are occurring and progressively declined with aging. Our findings of a decline in circulating CNP was in contrast to circulating BNP, which modestly increased with aging, as supported by previous studies.28 Further and consistent with our hypothesis, circulating CNP significantly and inversely correlated with LV interstitial fibrosis. While the current study supports the conclusion that circulating CNP declines with age in association with myocardial aging and fibrosis, review of the clinical literature is less clear in both health and disease. While the work of Prickett et al.22 supports our observations, it is clear that especially in the presence of disease, circulating CNP may be elevated in adults including in the aged. Totsune et al.29 reported plasma CNP to be increased in adults with chronic kidney disease, however a correlation to age was not investigated. Moreover in the presence of HF, Del Ry et al. reported in two key papers,27, 30 the elevation of plasma CNP with some association with aging, in which age was associated with higher CNP levels in adult HF subjects. Such an observation would be consistent with the report of Palmer et al. 11 in the presence of myocardial ischemia. Meanwhile, Gulberg et al.31 reported a decrease in plasma CNP in adults with liver cirrhosis and normal renal function as compared to controls, however no correlation to age was noted. Based upon these previous studies, a careful prospective study in humans from infancy to old age, especially without concomitant disease is clearly warranted.
Aging is increasingly recognized as an endocrine deficiency state involving many biologically active molecules. Such deficiencies have been linked to senescent endothelial cell production of NO,32 impaired vitamin D synthesis in aging skin33 and reduced growth hormone production.34 Here we demonstrate the progressive decline in circulating CNP with aging in an experimental model of aging. Although the mechanism of the reduction in CNP with aging was not a goal of the current study, further studies in both animal models of aging and in humans should address three key mechanisms which include 1) diminished production or release of CNP from endothelial cells due to a reduction in endothelial cell number; 2) decreasing production of CNP in aging bone to due cessation of skeletal growth, and 3) an increase in CNP degradation by neutral endopeptidase.
It is well known that aging is associated with LV fibrosis and altered function. However as demonstrated here, the natural history of global LV functional impairment was not completely progressive compared to LV fibrosis. Interestingly, at 11 months we observed a significant reduction in circulating CNP together with a significant increase in LV fibrosis which preceded any significant alterations in LV function. Structurally, LV weight plateaued at 11 months as did LV chamber diameter and wall thickness. Importantly, LV systolic function, specifically LV EF, circumferential sS and sSR were significantly reduced only at the more advanced age of 20 months which is equivalent to the 6th decade of human life. In addition, impairment of LV diastolic function at 20 months was present as circumferential dSR-E was also significantly reduced. The mechanisms of this late reduction in LV function are likely multi-factorial. First, the reduction in function may be related to increased LV fibrosis but to a higher level that exceeded 11 months of age. This reduction in LV function would be secondary to accumulation of replacement fibrosis in order to preserve structural myocardial integrity due to necrotic loss of cardiomyocytes35 and/or related to changes in collagen turnover due to an imbalance in procollagen biosynthesis, postsynthetic procollagen processing and collagen degradation.36, 37 In addition, the modest but significant increase in MAP at 20 months could also potentially contribute to increased LV fibrosis and impaired function. Nonetheless, this progressive increase of LV fibrosis, which is most likely to be high tensile strength type I collagen fibers,38 may ultimately impact diastolic and systolic function, particularly at the more advanced age of 20 months. Therefore as age related increases in myocardial fibrosis is a complex process involving multiple pathways and humoral factors in addition to CNP, further studies are warranted to clarify these mechanisms.
To date, only two previous in vitro studies have reported the actions of CNP on CF proliferation and production of collagen. These studies demonstrate the ability of CNP to activate cGMP in young rat CFs, which may be linked to binding of CNP to NPR-B.12, 14 Others report that CNP may have biological effects through the activation of the non-cGMP linked receptor NPR-C, 39, 40 which originally was thought to function only as a clearance receptor. In our in vitro studies, pharmacological levels of CNP possessed potent anti-proliferative actions on adult human CFs. Further, the anti-proliferative effect of CNP was attenuated by the NPR-C antagonist cANF(4–23), suggesting involvement of NPR-C. Such a concept of inhibiting proliferation of adult CFs by a natriuretic peptide via the activation of NPR-C is consistent with the report of Huntley et al.,19 which demonstrated that the anti-proliferative actions of BNP on adult human CFs was also attenuated by the NPR-C antagonist cANF(4–23). However the anti-proliferative actions of CNP involving NPR-C must be viewed with caution as high doses of CNP were used, without defining a dose response relationship. Interestingly, Kaneki and co-workers41 demonstrated that the signalling pathway for CNP regulation of bone formation in osteoblasts switches from the NPR-B/cGMP/protein kinase G pathway to the NPR-C/Gi protein/phosphatidylinositol-specific phospholiase C pathway with aging, strengthening the concept of a functional role for NPR-C in adults. Indeed, a physiological role for NPR-C continues to grow with studies suggesting that NPR-C can hyperpolarize vascular smooth muscle cells, activate a non-selective cation current in CFs, diminish endothelial cell permeability and exert anti-proliferative actions on human CFs.19, 39, 40, 42
STUDY LIMITATIONS
Our study was designed to define for the first time the evolution of endogenous circulating CNP, a potent anti-fibrotic peptide, and its relationship to alterations in myocardial structure and function in an experimental model of aging. Additional studies are warranted in humans from childhood into advanced age, to establish if the current findings are relevant to human aging. Moreover, the dose response relationship of CNP and the anti-proliferative effects on CFs needs to be defined in CFs in which NPR-C has been knocked down or in CFs harvested from mice in which NPR-C has been genetically deleted to strengthen the current findings. Further studies are also needed in experimental aging to determine if physiological and/or pharmacological replacement therapy with CNP can delay myocardial aging and LV fibrosis advancing a role for CNP as a fibro-inhibiting therapeutic. Finally, it is important to keep in mind that LV fibrosis with aging is complex involving many humoral and mechanical mechanisms in addition to CNP.
PERSPECTIVES
Aging, in the absence of disease, is a complex phenomenon which may be associated with changes in LV structure including pronounced fibrosis and a subsequent decrease in LV function, increasing the risk of cardiovascular morbidity. This study demonstrates that a progressive decline in circulating CNP characterizes natural aging and is strongly associated with a reciprocal increase in LV fibrosis which precedes subsequent reductions in diastolic and systolic function. We also demonstrate using adult human CFs, that the anti-proliferative actions of high dose CNP may involve a non-cGMP pathway via NPR-C. Thus, a relative decline in CNP bioavailability could be a contributor to age related LV fibrosis. Further, these findings underscore the potential of a CNP-based therapy for myocardial aging as well as pathophysiological conditions where excessive LV fibrosis affects LV function.
Acknowledgments
The authors acknowledge the outstanding contributions of Denise M. Heublein and Sharon M. Sandberg.
SOURCES OF FUNDING
This study was supported by grants RO1 HL36634, R01 HL83231 and PO1 HL76611 from the National Institute of Health and the Mayo Foundation.
Footnotes
CONFLICT(S) OF INTEREST/DISCLOSURE(S)
None.
References
- 1.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 2.Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993;27:341–348. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- 3.Gardin JM, Arnold A, Gottdiener JS, Wong ND, Fried LP, Klopfenstein HS, O’Leary DH, Tracy R, Kronmal R. Left ventricular mass in the elderly. The Cardiovascular Health Study. Hypertension. 1997;29:1095–1103. doi: 10.1161/01.hyp.29.5.1095. [DOI] [PubMed] [Google Scholar]
- 4.Katusic ZS. Mechanisms of endothelial dysfunction induced by aging: role of arginase I. Circ Res. 2007;101:640–641. doi: 10.1161/CIRCRESAHA.107.162701. [DOI] [PubMed] [Google Scholar]
- 5.Luo S, Shaw WM, Ashraf J, Murphy CT. TGF-beta Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. PLoS Genet. 2009;5:1–15. doi: 10.1371/journal.pgen.1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Souza SP, Davis M, Baxter GF. Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther. 2004;101:113–129. doi: 10.1016/j.pharmthera.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Stingo AJ, Clavell AL, Heublein DM, Wei CM, Pittelkow MR, Burnett JC., Jr Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am J Physiol. 1992;263:H1318–1321. doi: 10.1152/ajpheart.1992.263.4.H1318. [DOI] [PubMed] [Google Scholar]
- 8.Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of “vascular natriuretic peptide system”. J Clin Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei CM, Heublein DM, Perrella MA, Lerman A, Rodeheffer RJ, McGregor CG, Edwards WD, Schaff HV, Burnett JC., Jr Natriuretic peptide system in human heart failure. Circulation. 1993;88:1004–1009. doi: 10.1161/01.cir.88.3.1004. [DOI] [PubMed] [Google Scholar]
- 10.Mattingly MT, Brandt RR, Heublein DM, Wei CM, Nir A, Burnett JC., Jr Presence of C-type natriuretic peptide in human kidney and urine. Kidney Int. 1994;46:744–747. doi: 10.1038/ki.1994.329. [DOI] [PubMed] [Google Scholar]
- 11.Palmer SC, Prickett TC, Espiner EA, Yandle TG, Richards AM. Regional release and clearance of C-type natriuretic peptides in the human circulation and relation to cardiac function. Hypertension. 2009;54:612–618. doi: 10.1161/HYPERTENSIONAHA.109.135608. [DOI] [PubMed] [Google Scholar]
- 12.Horio T, Tokudome T, Maki T, Yoshihara F, Suga S, Nishikimi T, Kojima M, Kawano Y, Kangawa K. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology. 2003;144:2279–2284. doi: 10.1210/en.2003-0128. [DOI] [PubMed] [Google Scholar]
- 13.Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H, Goeddel DV. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP) Science. 1991;252:120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- 14.Soeki T, Kishimoto I, Okumura H, Tokudome T, Horio T, Mori K, Kangawa K. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol. 2005;45:608–616. doi: 10.1016/j.jacc.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 15.Murakami S, Nagaya N, Itoh T, Fujii T, Iwase T, Hamada K, Kimura H, Kangawa K. C-type natriuretic peptide attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1172–1177. doi: 10.1152/ajplung.00087.2004. [DOI] [PubMed] [Google Scholar]
- 16.Tao J, Mallat A, Gallois C, Belmadani S, Mery PF, Nhieu JT, Pavoine C, Lotersztajn S. Biological effects of C-type natriuretic peptide in human myofibroblastic hepatic stellate cells. J Biol Chem. 1999;274:23761–23769. doi: 10.1074/jbc.274.34.23761. [DOI] [PubMed] [Google Scholar]
- 17.Canaan-Kuhl S, Ostendorf T, Zander K, Koch KM, Floege J. C-type natriuretic peptide inhibits mesangial cell proliferation and matrix accumulation in vivo. Kidney Int. 1998;53:1143–1151. doi: 10.1046/j.1523-1755.1998.00895.x. [DOI] [PubMed] [Google Scholar]
- 18.Cahill PA, Hassid A. ANF-C-receptor-mediated inhibition of aortic smooth muscle cell proliferation and thymidine kinase activity. Am J Physiol. 1994;266:R194–203. doi: 10.1152/ajpregu.1994.266.1.R194. [DOI] [PubMed] [Google Scholar]
- 19.Huntley BK, Sandberg SM, Noser JA, Cataliotti A, Redfield MM, Matsuda Y, Burnett JC., Jr BNP-induced activation of cGMP in human cardiac fibroblasts: interactions with fibronectin and natriuretic peptide receptors. J Cell Physiol. 2006;209(3):943–949. doi: 10.1002/jcp.20793. [DOI] [PubMed] [Google Scholar]
- 20.Ruth EB. Metamorphosis of the pubic symphysis: I. The white rat (Mus norvegicus albinus) Anat Rec. 1935;64:1–7. [Google Scholar]
- 21.Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–1029. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Prickett TC, Lynn AM, Barrell GK, Darlow BA, Cameron VA, Espiner EA, Richards AM, Yandle TG. Amino-terminal proCNP: a putative marker of cartilage activity in postnatal growth. Pediatr Res. 2005;58:334–340. doi: 10.1203/01.PDR.0000169964.66260.4B. [DOI] [PubMed] [Google Scholar]
- 23.Sudoh T, Maekawa K, Kojima M, Minamino N, Kangawa K, Matsuo H. Cloning and sequence analysis of cDNA encoding a precursor for human brain natriuretic peptide. Biochem Biophys Res Commun. 1989;159:1427–1434. doi: 10.1016/0006-291x(89)92269-9. [DOI] [PubMed] [Google Scholar]
- 24.Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, Itoh H, Tanaka K, Saito Y, Katsuki M, Nakao K. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98:4016–4021. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peltonen TO, Taskinen P, Soini Y, Rysa J, Ronkainen J, Ohtonen P, Satta J, Juvonen T, Ruskoaho H, Leskinen H. Distinct downregulation of C-type natriuretic peptide system in human aortic valve stenosis. Circulation. 2007;116:1283–1289. doi: 10.1161/CIRCULATIONAHA.106.685743. [DOI] [PubMed] [Google Scholar]
- 26.Martin FL, Sangaralingham SJ, McKie PM, Huntley BK, Harders GE, Chen HH, Burnett JC., Jr Prevention of cardiorenal fibrosis and suppression of proteinuria and aldosterone activation following experimental myocardial infarction with the novel natriuretic peptide CD-NP. J Card Fail. 2009;15:S3. (Abstract) [Google Scholar]
- 27.Del Ry S, Cabiati M, Lionetti V, Emdin M, Recchia FA, Giannessi D. Expression of C-type natriuretic peptide and of its receptor NPR-B in normal and failing heart. Peptides. 2008;29:2208–2215. doi: 10.1016/j.peptides.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 29.Totsune K, Takahashi K, Murakami O, Satoh F, Sone M, Mouri T. Elevated plasma C-type natriuretic peptide concentrations in patients with chronic renal failure. Clin Sci (Lond) 1994;87:319–322. doi: 10.1042/cs0870319. [DOI] [PubMed] [Google Scholar]
- 30.Del Ry S, Giannessi D, Maltinti M, Prontera C, Iervasi A, Colotti C, Emdin M, L’Abbate A, Neglia D. Increased levels of C-type natriuretic peptide in patients with idiopathic left ventricular dysfunction. Peptides. 2007;28:1068–1073. doi: 10.1016/j.peptides.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Gulberg V, Moller S, Henriksen JH, Gerbes AL. Increased renal production of C-type natriuretic peptide (CNP) in patients with cirrhosis and functional renal failure. Gut. 2000;47:852–857. doi: 10.1136/gut.47.6.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrera MD, Mingorance C, Rodriguez-Rodriguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res Rev. 2010;9:142–152. doi: 10.1016/j.arr.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, Knol DL, Lips P. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 34.Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest. 1981;67:1361–1369. doi: 10.1172/JCI110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber KT, Pick R, Jalil JE, Janicki JS, Carroll EP. Patterns of myocardial fibrosis. J Mol Cell Cardiol. 1989;21:121–131. doi: 10.1016/0022-2828(89)90778-5. [DOI] [PubMed] [Google Scholar]
- 36.Mays PK, McAnulty RJ, Campa JS, Laurent GJ. Age-related changes in collagen synthesis and degradation in rat tissues. Importance of degradation of newly synthesized collagen in regulating collagen production. Biochem J. 1991;276:307–313. doi: 10.1042/bj2760307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Bonnema DD, Zile MR. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in post-synthetic procollagen processing. Am J Physiol Heart Circ Physiol. 2010;298:H614–622. doi: 10.1152/ajpheart.00474.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45:203–212. doi: 10.1016/0047-6374(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 39.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci U S A. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose RA, Hatano N, Ohya S, Imaizumi Y, Giles WR. C-type natriuretic peptide activates a non-selective cation current in acutely isolated rat cardiac fibroblasts via natriuretic peptide C receptor-mediated signalling. J Physiol. 2007;580:255–274. doi: 10.1113/jphysiol.2006.120832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaneki H, Kurokawa M, Ide H. The receptor attributable to C-type natriuretic peptide-induced differentiation of osteoblasts is switched from type B- to type C-natriuretic peptide receptor with aging. J Cell Biochem. 2008;103:753–764. doi: 10.1002/jcb.21448. [DOI] [PubMed] [Google Scholar]
- 42.Schreier B, Borner S, Volker K, Gambaryan S, Schafer SC, Kuhlencordt P, Gassner B, Kuhn M. The heart communicates with the endothelium through the guanylyl cyclase-A receptor: acute handling of intravascular volume in response to volume expansion. Endocrinology. 2008;149:4193–4199. doi: 10.1210/en.2008-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]