Abstract
Rationale
Previous data suggest that food allergy may be more common in inner-city children; however, these studies have not collected data on both sensitization and clinical reactivity, or early life exposures.
Methods
Children in the URECA birth cohort were followed through age 5 years. Household exposures, diet, clinical history, and physical examinations were assessed yearly; specific-IgE to milk, egg, and peanut were measured at 1, 2, 3, and 5 years. Based on sensitization (IgE≥0.35 kU/L) and clinical history over the five-year period, children were classified as food allergic (FA), possibly allergic, sensitized but tolerant, or not allergic/not sensitized.
Results
516 children were included. Overall, 55.4% were sensitized (milk 46.7%, egg 31.0%, peanut 20.9%), while 9.9% were categorized as FA (peanut 6.0%, egg 4.3%, milk 2.7%, 2.5% >1 food). The remaining children were categorized as possibly allergic (17.0%), sensitized but tolerant (28.5%), and not sensitized (44.6%). Eighteen (3.5%) reported reactions to foods for which IgE was not measured. Food-specific IgE levels were similar in FA versus sensitized but tolerant children, except for egg, which was higher in FA at ages 1 and 2. FA was associated with recurrent wheeze, eczema, aeroallergen sensitization, male gender, breastfeeding, and lower endotoxin exposure in year 1, but not with race/ethnicity, income, tobacco exposure, maternal stress, or early introduction of solid foods.
Conclusions
Even given that this was designed to be a high-risk cohort, the cumulative incidence of food allergy is extremely high, especially considering the strict definition of food allergy that was applied and that only 3 common allergens were included.
Keywords: Food allergy, inner city, URECA cohort, specific IgE
Introduction
Food allergy is a common condition, affecting approximately 3–6% of the United States population,1 and this diagnosis is associated with a significant impairment in quality of life.2 The prevalence of this condition appears to be increasing in industrialized countries, with an estimated 18% increase in childhood food allergy between 1997 and 2007.3 Recent studies have also suggested that the prevalence of food allergy may vary by race/ethnicity and geographic location, possibly similar to the trends that have been consistently reported for asthma4 and other atopic conditions.5,6
Several studies have now shown that self-reported food allergy appears to be more prevalent in highly populated areas, after controlling for race/ethnicity and income,7 and among children of black race/ethnicity.8,9 These trends also persist when examining the estimated prevalence of food allergy based only on the measurement of food-specific IgE.10,11 All of these studies are limited, however, in that both self-report and sensitization substantially overestimate the prevalence of true food allergy.12,13 Furthermore, no studies have yet looked at exposures that are more common in urban environments, such as stress, changes in dietary habits, and allergens such as mouse and cockroach, which may promote the development of this condition. The aim of this study was to use clinical and serologic data from the Urban Environment and Childhood Asthma (URECA) study to better estimate the true prevalence of food allergy in inner-city children, and to evaluate the impact of urban environmental exposures on the development of food sensitization and food allergy.
Methods
Study Design
URECA is a prospective, observational, inner-city birth cohort designed to study the effects of specific urban exposures on the development of recurrent wheeze and asthma. Detailed information regarding the design, methods, and study population has been previously reported.14 Briefly, pregnant women in Baltimore, Boston, New York City, and St. Louis, were recruited between February 2005 and March 2007. Selection criteria included a mother or father with a history of allergic rhinitis, eczema, or asthma; gestational age ≥ 34 weeks; and collection of a suitable cord blood sample. Exclusion criteria included congenital anomalies, pulmonary or immunologic disorders, or maternal HIV infection. Overall, 1850 families were screened, 889 children were found to be eligible, and 560 were enrolled. An additional 49 children without a family history of atopy were also added to the study. Children who had food-specific IgEs measured at ≥1 timepoint were included in this study.
Maternal questionnaires regarding smoking, stress, and depression were collected prenatally, and cord blood and clinical information were collected at birth. Telephone surveys were performed every 3 months to assess the child’s respiratory and allergy symptoms, medications, tobacco exposure, and diet. Starting at one year, the children were seen annually, where physical exams and eczema assessments were performed, and blood samples were obtained. For this study, the children were followed through age 5.
Household dust samples were collected at 3 months and yearly thereafter and were analyzed for the common indoor allergens German cockroach (Bla g 1), dog (Can f 1), cat (Fel d 1), D. farinae (Der f 1), D. pteronyssinus (Der p 1), and mouse (Mus m 1) by two-site monoclonal antibody ELISA (Indoor Biotechnologies Inc., Charlottesville, VA). First year samples were also analyzed for endotoxin by the recombinant factor C assay15 and for ergosterol, a component of fungal cell membranes, by gas chromatography-mass spectroscopy.
Mononuclear cells from cord blood and samples obtained at ages 1 and 3 were incubated for 24 hours (PHA, LPS, poly-IC, CPG, peptidoglycan, respiratory syncytial virus, or medium alone) or 5 days (cockroach extract, D. pteronyssinus extract, tetanus toxoid, or medium alone). The supernatants were then collected and analyzed by multiplex assay (Beadlyte, Upstate Biotechnology, Lake Placid, NY) for the production of cytokines associated with both innate and adaptive immunity (see Table E1 in the Online Repository).
Food Allergy Data Collection and Definitions
At each annual visit, parents were asked specifically about the child’s ingestion of milk, egg, and peanut and if there was any concern for possible food allergy in a physician-administered food allergy questionnaire. If the study physician determined that the symptoms were consistent with food allergy, an allergy consult was recommended outside of the study protocol. In addition, allergen-specific IgE levels (ImmunoCap, Phadia, Uppsala, Sweden) were measured to milk, egg, and peanut at ages 1, 2, 3, and 5. An allergy consult was further recommended if food specific IgE levels exceeded the 95% positive predictive threshold and there was either ambiguity in the clinical or dietary history or a history of either atopic dermatitis or failure to thrive. As 95% predictive food-specific IgE cut-offs vary by age, we used previously validated values for pre-school aged children for milk (5 kU/L)16 and egg (2 kU/L)17 and the derived value for peanut from CoFAR (5 kU/L).18 Data on food allergy diagnosis and food avoidance recommendations were collected from all allergy consultations.
As oral food challenges were only performed as clinically indicated outside of this study, children were divided into four groups at each time point based on their food-specific IgE levels and clinical histories. Group 1 (Food Allergic) was defined as having a positive IgE (≥0.35 kU/L) to milk, egg, and / or peanut, documented dietary avoidance of foods to which they were sensitized, and clinical confirmation by any of the following: a) classified as food allergic to milk, egg, or peanut on allergy consultation; or b) parental documentation of a previous reaction to milk, egg, or peanut, confirmed as consistent with true food allergy by the site investigator. In addition, all children who met criteria for food allergy were individually reviewed by the authors to further ensure accurate categorization. Group 2 (Possibly Food Allergic) was defined as food sensitization with either documented dietary avoidance of the foods to which they were sensitized or unknown dietary consumption, but without a confirmed clinical history of food reaction. Group 3 (Sensitized but Tolerant) was defined as food sensitization but reported consumption of the culprit food without adverse reactions. Finally, Group 4 (Not Sensitized) was defined as all IgEs <0.35 kU/L.
Statistical Analysis
For the purpose of analyses, each child was placed in the highest food allergy category (with “Food Allergic” being highest) that he/she attained for milk, egg, or peanut at any time over the five years. The cumulative incidence of food allergy by age 5 was then calculated as a percentage of the total number of children included in the analysis (n=516). To compare baseline demographic and clinical characteristics among food allergy classifications, t-tests (continuous variables) and chi-squared tests (categorical variables) were performed. Differences in IgE and IgG4 between groups, as defined by yearly food allergy classifications, were tested by Wilcoxon Rank Sums two-sample tests. Comparisons across time points within each cumulative group were tested using a linear trend test. IgE and IgG4 data were positively skewed, and therefore values were log-transformed for all statistical analyses.
Several data reduction techniques were employed to reduce the 61-item cytokine panel to 12 composite factor scores, or linear combinations of the original, correlated, stimulant-specific cytokine responses (Table E1). The factors were identified separately for the innate and adaptive panels of stimulant-cytokine combinations and without including unstimulated responses. Clustering of responses first showed that there was little to no association across years. Hierarchical clustering with a distance matrix was done within each year, to determine which cytokine-stimulant responses were most similar to one another. These showed consistent results across the 3 years; when factors were calculated, all years were considered. Once the patterns of responses were determined from the hierarchical clusters, a separate independent factor analysis was performed. The factors obtained using factor analysis were similar to the ones obtained using hierarchical clustering, and confirmed that 6 factors accounted for the majority of the variance for each panel (66% for the innate panel and 84% for the adaptive panel). Odds ratios to determine associations between food allergy groups and cytokine factors were calculated using logistic regression, while controlling for site, sex, season of birth, and family history of atopy.
Associations between clinical outcomes, first year bedroom dust exposure, and food allergy classifications were examined using univariate and multivariable logistic regression, with adjustments for site, child’s sex, and family history of atopic disease. Statistical analyses were performed with R 3.0.2 (http://www.r-project.org) and SAS 9.2 (SAS Institute, Cary, NC).
Results
Study Population
Of the 609 children initially enrolled, 516 (85%) were included in this study. Individuals missing all food-specific IgE measurements were excluded (n=93), and comparison of this population to the remaining cohort revealed a difference only in gestational age (Table E2 in the Online Repository). Forty-four children from the non-atopic cohort were included in this study, and sensitivity analyses with removal of these children did not reveal any significant differences in our findings.
Overall, 284 children (55.4%) were sensitized (IgE≥0.35 kU/L) to milk, egg, or peanut (46.7%, 31.0%, 20.9%, respectively) at any age. Fifty-one children (9.9%) fulfilled our criteria for food allergy (milk 2.7%, egg 4.3%, peanut 6.0%) and were thus included in Group 1. While all 51 children had both documented sensitization and dietary avoidance, 14 children were classified as food allergic based on allergy consultation and 37 were classified based on parental report and investigator confirmation. The remaining children were classified into Group 2 (possibly food allergic, 17.0%), Group 3 (sensitized but tolerant, 28.5%), or Group 4 (not sensitized, 44.6%) (Table I). Thirteen children (2.5%) were allergic to more than one of these foods. An additional 9 children (1.7%) had reported reactions to other common food allergens (tree nuts, fish, shellfish, soy, and wheat), but were not included in Group 1 because IgE to these foods was not measured. Similarly, 9 children (1.7%) reported reactions to other less allergenic foods (e.g. chocolate, peaches) and were also not included in Group 1.
Table I.
Cumulative incidence of food allergy by age 5 in the URECA cohort (n=516)
| Allergic (Group 1) |
Possibly Allergic (Group 2) |
Sensitized & Tolerant (Group 3) |
Not Allergic (Group 4) |
|
|---|---|---|---|---|
| Milk | 14 (2.7) | 41 (7.9) | 186 (36.1) | 275 (53.3) |
| Egg | 22 (4.3) | 70 (13.5) | 68 (13.2) | 356 (69.0) |
| Peanut | 31 (6.0) | 45 (8.7) | 32 (6.2) | 408 (79.1) |
| Total | 51 (9.9) | 88 (17.0) | 147 (28.5) | 230 (44.6) |
Values reported as n (%)
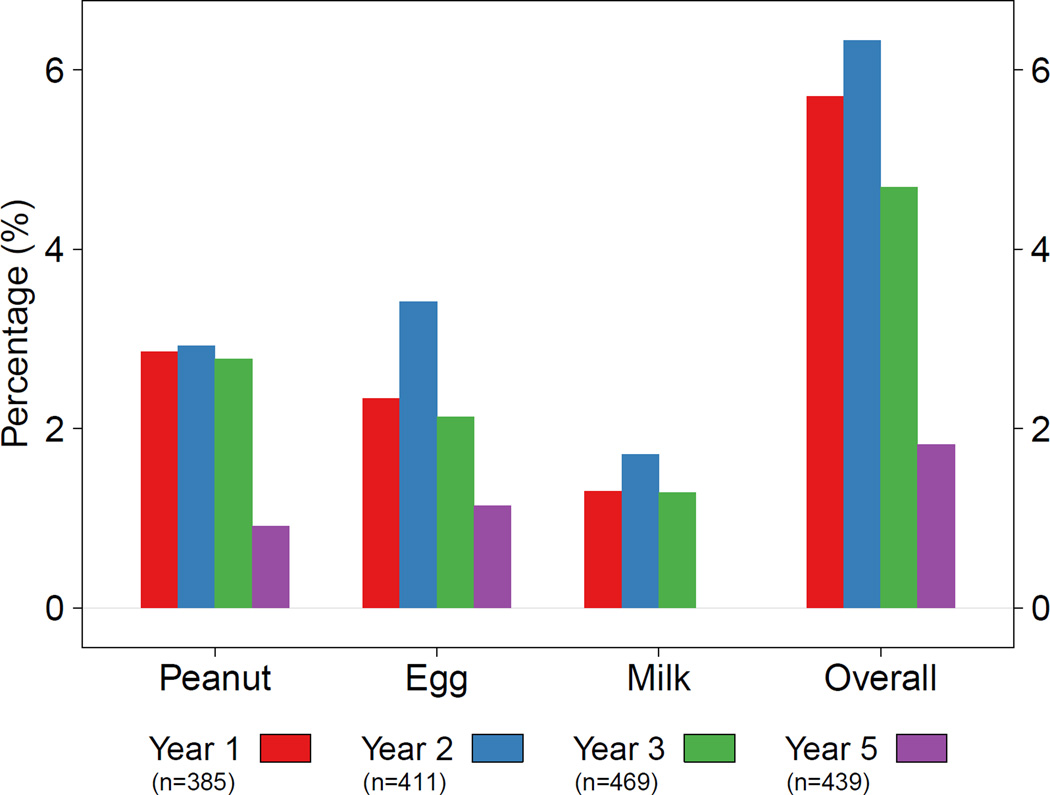
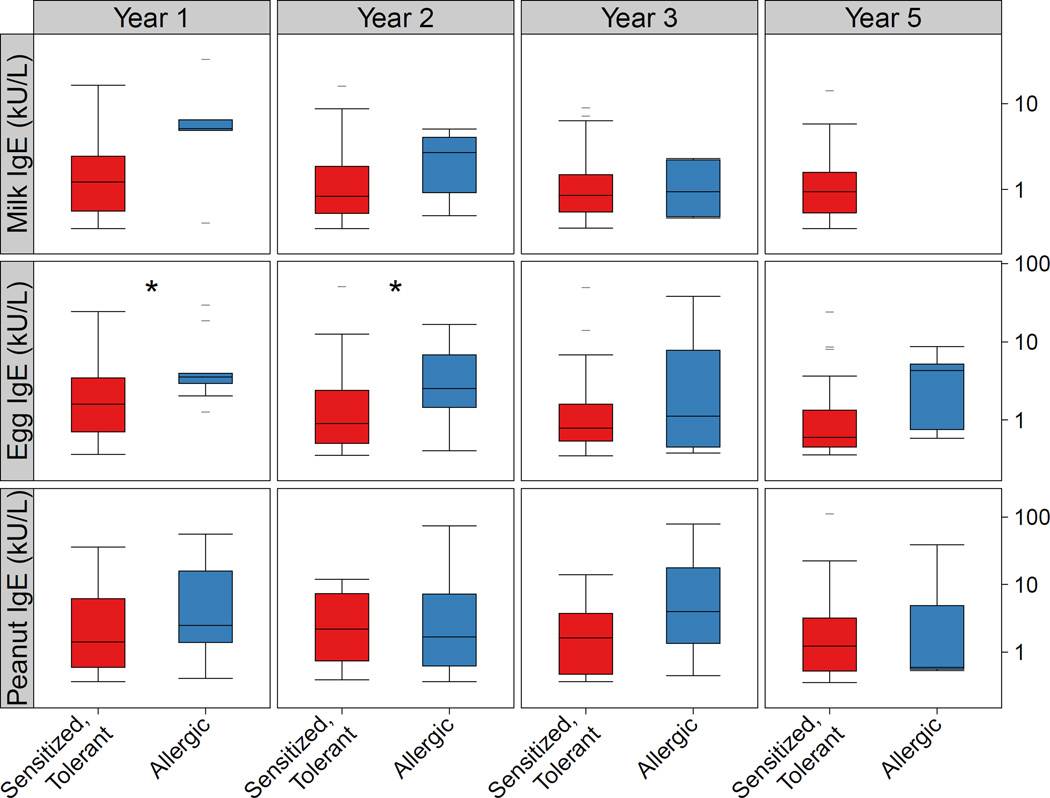
Over the five years of follow-up, when examining food allergy classifications cross-sectionally, milk, egg, and peanut allergy were all most prevalent at age 2 (milk 1.7%, egg 3.4%, peanut 2.9%, overall 6.3%, Figure 1). IgE levels in food allergic children, however, were highest for milk at age 1 (median 5.1 kU/L; IQR 4.9–6.5), peanut at age 3 (4.0 kU/L; IQR 1.3–17.8), and egg at age 5 (4.3 kU/L; IQR 0.8–5.2) (Figure 2). Food-specific IgE levels were similar in Group 1 versus Group 3 children at each age over the five years, except for egg, which was higher in those in Group 1 at ages 1 (median 3.5 kU/L versus 1.6; p=0.02) and 2 (2.5 kU/L versus 0.9; p=0.04). When examining the trend in IgE levels over time, food-specific IgE levels significantly decreased over time for milk (test for trend p < 0.001) and egg (p = 0.01) in those classified as food allergy but increased for sensitized but tolerant children for milk (p < 0.001) and peanut p < 0.001).
Figure 1.
The percentage of children classified as Food Allergic (Group 1) during each year of follow-up in the URECA cohort. Children were characterized as Food Allergic if they had serologic and clinical evidence of allergy to the food and documentation of food avoidance during that year.
Figure 2.
Differences in food-specific IgE levels between those classified as Group 3 (Sensitized but not Allergic, left panel) and Group 1 (Food Allergic, right panel) at ages 1, 2, 3, and 5. Significant differences (p < 0.05) were noted for egg at ages 1 and 2 and are depicted with an asterix (*).
Children in Group 1, when compared to those in Groups 3 and 4, were more likely to be male (p=0.01) and to be diagnosed with other allergic conditions (Table II and Table E3), including eczema prior to age 3, aeroallergen sensitization at ages 2, 3 and 5, and wheezing during years 3, 4 and 5.
Table II.
Demographic characteristics of food allergic and food sensitized children in the URECA cohort
| Allergic (n=51) |
Not Allergic† (n=377) |
p value | Sensitized (n = 286) |
Not Sensitized (n = 230) |
p value | |
|---|---|---|---|---|---|---|
| Male gender | 36 (70.6) | 192 (50.9) | 0.01 | 148 (51.7) | 120 (52.2) | 0.99 |
| Race/ethnicity | 0.56 | 0.16 | ||||
| Black | 41 (80.4) | 271 (71.9) | 207 (72.4) | 161 (70.0) | ||
| Hispanic | 6 (11.8) | 75 (19.9) | 57 (19.9) | 47 (20.4) | ||
| Other | 4 (7.8) | 31 (8.2) | 22 (7.7) | 22 (9.6) | ||
| Annual household income <$15,000 | 39 (76.5) | 255 (67.8) | 0.28 | 196 (68.5) | 160 (69.9) | 0.82 |
| Mother completed high school | 35 (68.8) | 211 (56.1) | 0.12 | 182 (63.9) | 123 (53.5) | 0.02 |
| Mother married | 6 (11.8) | 52 (13.8) | 0.85 | 45 (15.8) | 26 (11.3) | 0.18 |
| Mother’s age at delivery (yr)* | 24.2 ± 6.0 | 24.5 ± 6.0 | 0.73 | 24.5 ± 6.0 | 24.3 ± 5.9 | 0.84 |
| Type of delivery | 0.31 | 0.96 | ||||
| Vaginal | 39 (76.5) | 258 (68.4) | 196 (68.5) | 159 (69.1) | ||
| Cesarean section | 12 (23.5) | 119 (31.6) | 90 (31.5) | 71 (30.9) | ||
| Gestational age (weeks)* | 38.5 ± 1.7 | 38.8 ± 1.5 | 0.24 | 38.9 ± 1.5 | 38.7 ± 1.5 | 0.14 |
| Maternal asthma | 25 (49.0) | 165 (44.0) | 0.60 | 133 (46.8) | 101 (43.9) | 0.57 |
| Maternal stress¥ at age 1 | 4.9 ± 2.9 | 4.6 ± 2.8 | 0.56 | 4.5 ± 2.8 | 4.7 ± 2.6 | 0.40 |
| Maternal depression¥ at age 1 | 6.9 ± 6.6 | 6.5 ± 6.7 | 0.23 | 5.9 ± 6.4 | 6.7 ± 6.5 | 0.19 |
| Smokers in the home (#) | 0.63 | 0.72 | ||||
| 0 | 29 (56.9) | 186 (49.6) | 150 (52.8) | 116 (50.4) | ||
| 1–2 | 19 (37.3) | 168 (44.8) | 117 (41.2) | 102 (44.4) | ||
| 3+ | 3 (5.8) | 21 (5.6) | 17 (6.0) | 12 (5.2) | ||
| Breastfeeding‡ | ||||||
| Ever | 35 (68.8) | 193 (52.9) | 0.05 | 161 (58.3) | 121 (53.8) | 0.35 |
| At 3 months | 16 (32.7) | 76 (22.8) | 0.18 | 64 (25.1) | 48 (23.4) | 0.76 |
| Solid food intro (wk)* † | 13.9 ± 7.4 | 15.1 ± 8.9 | 0.27 | 14.8 ± 8.5 | 15.2 ± 8.6 | 0.63 |
| Vitamin D3 (ng/mL)* | ||||||
| Cord blood | 19.9 ± 9.4 | 20.1 ± 8.8 | 0.85 | 20.3 ± 9.6 | 20.3 ± 8.7 | 0.99 |
| At age 3∫ | 19.1 ± 8.4 | 20.1 ± 6.8 | 0.48 | 19.8 ± 6.9 | 20.1 ± 6.8 | 0.66 |
Values expressed as n (%) unless otherwise noted
Mean ± SD
Children who were classified as either Group 3 or Group 4; Group 2 is not included in this analysis
Data collected at age 3 months; N=501
N=413 from age 3 blood collections
P values determined by Pearson chi-squared test or t-test
Stress was measured by perceived stress scale, depression was measured by Edinburgh postnatal depression scale
Dietary and Environmental Exposures
Breastfeeding for any duration was found to be significantly associated with food allergy (p=0.05, Table II), while higher maternal education was associated with food sensitization (p=0.03) but not food allergy. Race/ethnicity, household income, type of delivery, maternal smoking during pregnancy, maternal stress and / or depression, timing of introduction of complementary foods, and Vitamin D levels were not found to be different between those with and without food sensitization or food allergy.
With regard to other exposures, neither food sensitization nor food allergy was associated with exposure to ergosterol or environmental tobacco smoke. Food allergy and sensitization were likewise not associated with cat, dog, mouse, cockroach, or dust allergen exposure in the bedroom during the first year of life (Supplementary Table E4). In contrast, higher levels of endotoxin in the bedroom during the first year of life were significantly protective for the development of overall food allergy (OR 0.4; 95% CI 0.2 – 0.8) and egg allergy (OR 0.2; 95% CI 0.1 – 0.6).
Immunologic Profiling
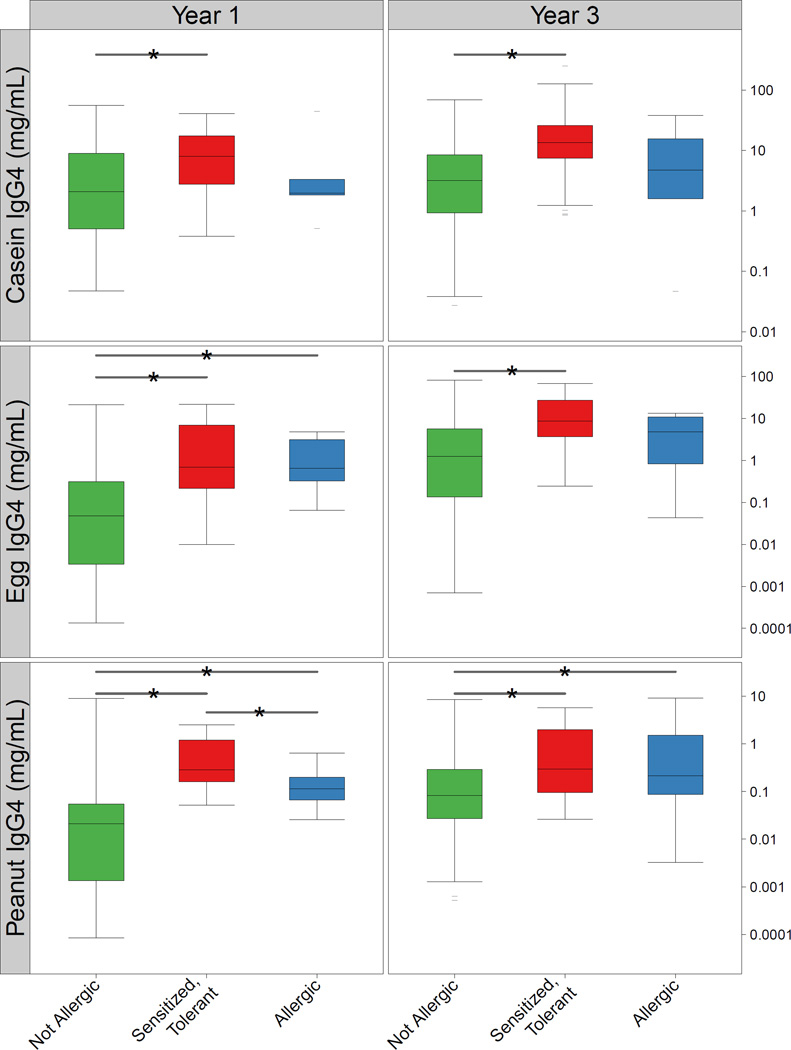
To examine immunological changes associated with the development of food allergy, food-specific IgG4 was measured at ages 1 and 3, and mononuclear cell (PBMC) cytokine profiles were analyzed at birth, and at ages 1 and 3. Looking year by year, children in Group 1 were found to have higher levels of IgG4 to peanut and egg at age 1 and peanut at age 3 than those in Group 4 (Figure 3). Similarly, those in Group 3 who were sensitized but tolerant to casein, egg, and peanut had significantly higher IgG4 levels to these foods at both ages 1 and 3 when compared to Group 4. Mean IgG4 values increased for all children between ages 1 and 3, and this trend was significant for all foods in Group 4 (p <0.01), casein and egg in Group 3 (p < 0.001), and solely egg for Group 1 (p < 0.001).
Figure 3.
Food-specific IgG4 levels among those in Group 4 (green), Group 3 (red), and Group 1 (blue) at ages 1 and 3. Significant differences between groups (P < 0.05) are noted with a line and asterix (*).
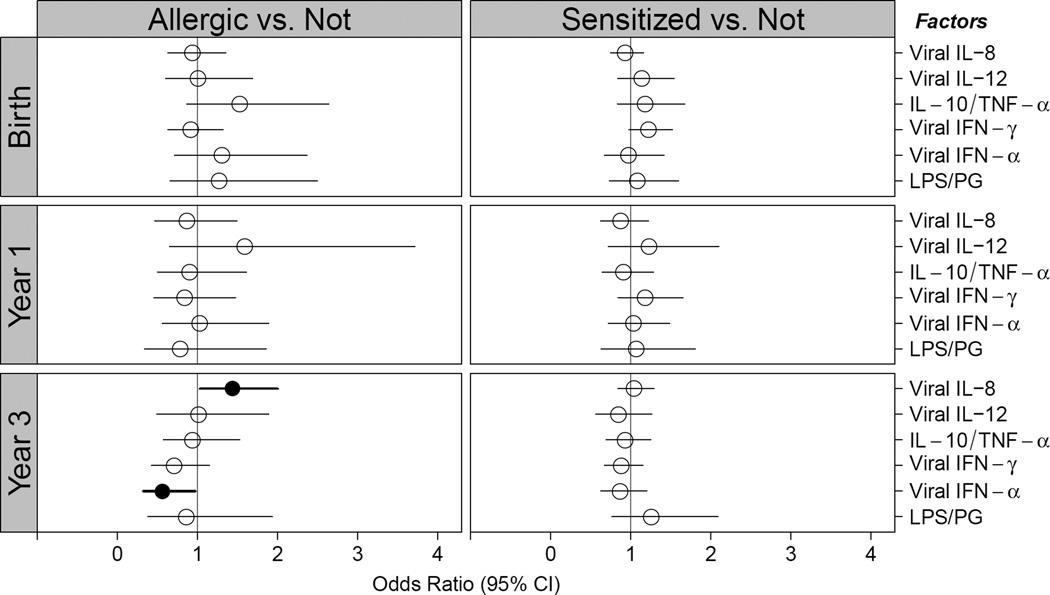
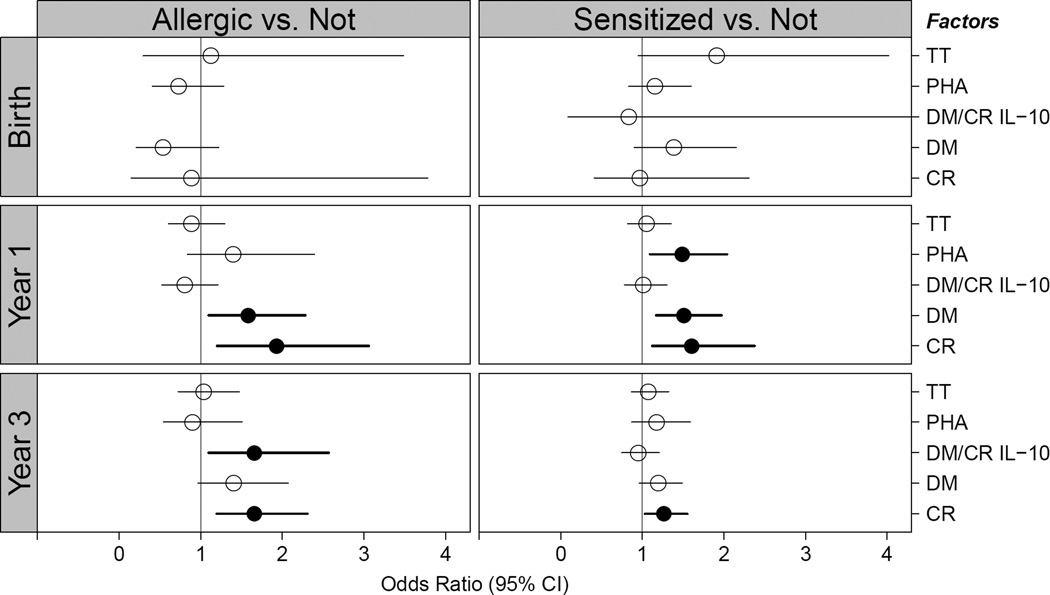
Children in Group 1 were further found to have altered innate and adaptive cytokine profiles as early as one year of age (Figure 4). These children were found to have increased IL-8 and decreased IFN-α production upon viral stimulation at age 3 compared to Group 4. Those with food allergy and food sensitization had increased PBMC expression of pro-Th2 cytokines (IL-4, IL-5, IL-13, and IFN-γ) upon dust mite and cockroach stimulation at 1 year of age, when compared to those without food allergy or sensitization, and this altered adaptive immune response persisted upon cockroach stimulation at age 3.
Figure 4.
Odds ratio for development of food allergy (left panel) or food sensitization (right panel) for summary variables of PBMC cytokine response. “Allergic” represents Group 1 (food allergic) versus Groups 3 (sensitized but tolerant) and 4 (not sensitized). “Sensitized” represents Groups 1, 2 (possibly food allergic), and 3 versus 4. Solid circles represent statistical significance in models adjusted for site, gender, season of birth, and family history of atopy. A. Innate panel. B. Adaptive panel. See Supplementary Table E1 for abbreviation definitions and list of stimulants and cytokines measured.
Discussion
In this observational, high-risk, inner-city birth cohort, we found that 9.9% of children had convincing clinical and serologic evidence of food allergy by age 5. While previous studies have suggested that food allergy may be more common among children living in urban environments,7 this study supports this finding by using a stringent definition of food allergy based on both clinical and serologic data. Furthermore, this is the first study to longitudinally examine the influence of urban environmental exposures on the development of both food allergy and food sensitization.
While it is possible that the true incidence is lower, it is far more likely that the cumulative incidence estimate of 9.9% is an underestimation of true food allergy in this population. Not only did we use a strict definition requiring both sensitization and clinical correlation, our cumulative incidence estimate was only based on milk, egg, and peanut, which have been shown to account for only about 80% of food allergy in young children.19,20 While we did not include them in our incidence estimate, it is likely that at least some of the additional 1.7% of children who reported reactions to fish, shellfish, tree nuts, wheat, or soy truly did have food allergy, not to mention those who reported reactions to less common food allergens. Finally, some of the children who were classified as “possibly allergic” because of lack of confirmatory clinical data could also be truly allergic and were not included in our estimate. In fact, if only serologic evidence of FA was required as in other studies,10 an additional 37 children (7.2%) would have been classified as food allergic using 95% positive predictive cut-offs.
This cumulative incidence estimate of 9.9% is higher than the recent prevalence estimate of 6.5% for self-reported food allergy in the general pediatric population from NHANES 2007–2010.21 However, differences in definitions used, time periods examined, and populations included make meaningful comparisons across food allergy studies very difficult. In a recent systematic review, the prevalence of food allergy was found to be greater than 1–2% but less than 10% of the population.22 While it has been shown that studies which rely on self-report overestimate the true prevalence of food allergy when compared to oral food challenges,12 performing oral food challenges in large-scale epidemiologic studies is often unfeasible. Thus, studies such as the URECA cohort that rely on robust clinical and serologic data to provide accurate estimates are extremely valuable.
In a recent study examining the effect of urbanization on food allergy, Gupta et al found that self-reported food allergy was more prevalent in urban (9.8%) versus rural (6.2%) locations.7 While the URECA estimate is similar to Gupta’s urban prevalence estimate, it is difficult to directly compare these studies, as our estimate is based on cumulative incidence over 5-years, was ascertained in a high-risk cohort, involves a population younger than 5 years, and only includes food allergy to milk, egg, and peanut, whereas Gupta reported a cross-sectional prevalence estimate based on a population younger than 18 years who had reactions to any food. In contrast, our estimate was significantly higher than the recently published prevalence estimate of 3.8% by Taylor-Black et al in a general pediatrics clinic in East Harlem, NY,23 although they did express concern that their population may be under-diagnosed or under-treated.
Consistent with previous studies, food allergy in our cohort was more common among males.18 Food allergy was further found to be more common among children who were breastfed, for which conflicting results have been shown in other studies on asthma and atopic dermatitis.24–27 Interestingly, other risk factors that are more commonly associated with inner-city environments, such as stress, black race/ethnicity, poverty, Vitamin D deficiency, and early introduction of complementary foods, were not found to be associated with food allergy. It is possible that this may be explained by the fact that the URECA population was relatively homogenous in terms of race/ethnicity and poverty level, and thus there was not substantial variation in these measures.
In contrast, there was a significant protective effect of endotoxin exposure for the development of egg and overall food allergy. This finding is similar to those of previous longitudinal studies for eczema28 and asthma,29 but to our knowledge, this is the first study to prospectively examine this relationship for the development of food allergy. Previous studies have demonstrated that endotoxin levels are lower in urban environments than in rural farming areas,30,31 and a recent study examining the indoor microbiome in the same URECA cohort found that exposure to specific allergens and bacteria was associated with reduced sensitization to inhalant allergens as well as recurrent wheeze (in press). Therefore, while it has been previously suggested that the high rates of allergy and asthma in the inner city contradict the hygiene hypothesis,32 both of these studies suggest that this is not actually the case.
Among children who were considered allergic to each food in a given year, milk-specific IgE levels peaked at age 1 and then trended down, whereas peanut and egg-specific IgE peaked at ages 3 and 5, respectively. Interestingly, with the exception of IgE to egg at ages 1 and 2, food-specific IgE levels were similar among those with food allergy and those who were asymptomatically sensitized, in whom levels actually increased over time for milk and peanut. This finding highlights the fact that the presence of food-specific IgE alone cannot be used to diagnose food allergy, either in the clinic or in epidemiologic studies.13,22 Our data further suggest that peanut allergy decreased at five years of age, a finding that would be inconsistent with the usual persistence of peanut allergy. We suspect that this finding is most likely an artifact related to missing data at age 5, since when the 31 children who were ever classified as peanut allergic were individually examined at age 5, 19 did not have sufficient information regarding ingestion to be accurately classified, although 5 (16.1%) did have serologic or clinical evidence suggesting they may have outgrown the allergy. We similarly suspect that children were not diagnosed with milk allergy at age 5 due to this missing dietary data.
Children with food allergy and those who were asymptomatically sensitized were found to have higher levels of food-specific IgG4 compared to children who were not sensitized. These findings are similar to previous studies demonstrating an association between peanut sensitization and increased peanut IgG4,33–35 although our findings are inconsistent with the notion that IgG4 is a marker of tolerance, as it may be in food immunotherapy.36,37 As previous authors have hypothesized,35 it is possible that this increased IgG4 level in sensitized children in this cohort indicates that the aberrant immune response in food allergic children may occur prior to IgE class-switching.
Children with food allergy were also found to have evidence of abnormal innate and adaptive immune responses as early as one year of age, with increased production of IL-4, IL-5, and IL-13, consistent with a Th2 phenotype. At age 3, they were further found to have increased IL-8 and decreased IFN-α production in response to viral infections. This finding is consistent with recent studies demonstrating that children with higher FcεR1 expression on plasmacytoid dendritic cells have decreased IFN-α production in response to human rhinovirus exposure.38
Our study is limited by the fact that the children did not undergo double-blind placebo controlled food challenges, the gold standard for food allergy diagnosis. However, the robust clinical, dietary, and serologic information available enabled us to make informed assessments regarding those likely to have true food allergy. We are further limited as a large proportion of children (n=88) were still classified as “possibly allergic.” While our power to detect potential risk factors for the development of food allergy may have been diminished by not including these children in our analyses, we felt that it was most important to focus on the subset with a firm diagnosis. Finally, as this is a prospective cohort study, sufficient data was only available for 85% of the original participants, which may have introduced selection bias into our study and possibly underestimated the true prevalence of food allergy at year 5 due to missing dietary data.
In conclusion, this is the first study to examine the cumulative incidence of food allergy in inner-city children through the use of both prospective clinical and serologic data. While the results may not be fully generalizable given that this was designed to be a high-risk cohort in a small number of U.S. cities, we found that despite a strict definition of food allergy and the inclusion of only three food allergens, the cumulative incidence of food allergy among inner-city children is substantially higher than recent estimates among the general US pediatric population. It is possible that previously lower estimates of food allergy in inner-city populations were a result of under-diagnosis and under-treatment, and future studies should be conducted to address this significant health disparity. While this study further identified potential environmental factors associated with the development of food allergy, additional study is clearly needed to further explore those factors that might be modifiable.
Supplementary Material
Clinical Implications.
When using a stringent definition, the cumulative incidence of food allergy in a high-risk inner city cohort was substantially higher than recent estimates among the general US pediatric population.
Acknowledgements
The Urban Environment and Childhood Asthma Study is a collaboration of the following institutions and investigators (principal investigators are indicated by an asterisk; protocol chair is indicated by double asterisks):
Johns Hopkins University, Baltimore, MD – R Wood*, E Matsui, H Lederman, F Witter, J Logan, S Leimenstoll, D Scott, L Daniels, L Miles, D Sellers, A Swift, K Smith; Boston University School of Medicine, Boston, MA – G O’Connor*, W Cruikshank, M Sandel, A Lee-Parritz, C Stamoulos, E Gjerasi, P Price-Johnson, B Caldwell, M Tuzova; Columbia University, New York, NY – M Kattan*, C Lamm, N Whitney, P Yaniv, C Sanabia, A Valones, R Rios, C Maher; Mount Sinai School of Medicine, New York, NY – H Sampson, M Mishoe; Washington University School of Medicine, St Louis, MO – G Bloomberg*, L Bacharier, Y Sadovsky, E Tesson, C Koerkenmeier, R Sharp, K Ray, I Bauer, A Freie, V Morgan; Statistical and Clinical Coordinating Center - Rho, Inc, Chapel Hill, NC – H Mitchell*, P Zook, C Visness, M Walter, R Bailey, S. Hicks, M Bader, W Taylor, R Budrevich; Scientific Coordination and Administrative Center – University of Wisconsin, Madison, WI – W Busse*, J Gern**, P Heinritz, C Sorkness, WM Lee, K Grindle, A Dresen, T Pappas; National Institute of Allergy and Infectious Diseases, Bethesda, MD – P Gergen, A Togias, E Smartt, K Thompson.
Funding
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract numbers NO1-AI-25496, NO1-AI-25482, HHSN272200900052C and HHSN272201000052I. Additional support was provided under grants RR00052, M01RR00533, 1UL1RR025771, M01RR00071, 1UL1RR024156, and 5UL1RR024992-02.
Abbreviations
- FA
Food Allergy/Food Allergic
- IQR
Inter-quartile Range
- LPS
Lipopolysaccharide
- NHANES
National Health and Nutrition Examination Survey
- PHA
Phytohemagglutinin
- Poly-IC
Polyinosinic-polycytidylic acid
- URECA
Urban Environment and Childhood Asthma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Emily C. McGowan, Johns Hopkins University School of Medicine, Department of Medicine, Division of Allergy and Clinical Immunology, Graduate Student, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (emcgowa4@jhmi.edu).
Gordon R. Bloomberg, Washington University School of Medicine, Division of Allergy, Immunology, and Pulmonary Medicine, St. Louis, MO (bloomberg@kids.wustl.edu).
Peter J. Gergen, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Bethesda, MD (pgergen@niaid.nih.gov).
Cynthia M. Visness, Rho, Inc., Chapel Hill, NC (cindy_visness@rhoworld.com).
Katy F. Jaffee, Rho, Inc., Chapel Hill, NC (katy_jaffee@rhoworld.com).
Megan Sandel, Boston University School of Medicine, Department of Medicine, Division of Pediatric Primary Care, Boston, MA (msandel@bu.edu).
George O’Connor, Boston University School of Medicine, Department of Medicine, Division of Pulmonary, Allergy, Sleep, and Critical Care Medicine, Boston, MA (goconnor@bu.edu).
Meyer Kattan, New York Presbyterian / Columbia University Medical Center, Department of Pediatrics, Division of Pediatric Pulmonology, New York, NY (mk2833@columbia.edu).
James Gern, University of Wisconsin School of Medicine, Department of Pediatrics, Division of Allergy and Immunology (gern@medicine.wisc.edu).
Robert A. Wood, Johns Hopkins University School of Medicine, Department of Pediatrics, Division of Allergy and Immunology, Baltimore, MD, (rwood@jhmi.edu).
References
- 1.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 2.Flokstra-de Blok BM, Dubois AE, Vlieg-Boerstra BJ, et al. Health-related quality of life of food allergic patients: comparison with the general population and other diseases. Allergy. 2010;65:238–244. doi: 10.1111/j.1398-9995.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Togias A, Fenton MJ, Gergen PJ, Rotrosen D, Fauci AS. Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases. J Allergy Clin Immunol. 2010;125:540–544. doi: 10.1016/j.jaci.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 5.Barnes M, Cullinan P, Athanasaki P, et al. Crete: does farming explain urban and rural differences in atopy? Clin Exp Allergy. 2001;31:1822–1828. doi: 10.1046/j.1365-2222.2001.01240.x. [DOI] [PubMed] [Google Scholar]
- 6.Von Ehrenstein OS, Von Mutius E, Illi S, Baumann L, Bohm O, von Kries R. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy. 2000;30:187–193. doi: 10.1046/j.1365-2222.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 7.Gupta RS, Springston EE, Smith B, Warrier MR, Pongracic J, Holl JL. Geographic variability of childhood food allergy in the United States. Clin Pediatr (Phila) 2012;51:856–861. doi: 10.1177/0009922812448526. [DOI] [PubMed] [Google Scholar]
- 8.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114:159–165. doi: 10.1016/j.jaci.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2009. Vital Health Stat. 2010;10:1–82. [PubMed] [Google Scholar]
- 10.Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126:798–806. e13. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar R, Tsai HJ, Hong X, et al. Race, ancestry, and development of food-allergen sensitization in early childhood. Pediatrics. 2011;128:e821–e829. doi: 10.1542/peds.2011-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods RK, Stoney RM, Raven J, Walters EH, Abramson M, Thien FC. Reported adverse food reactions overestimate true food allergy in the community. Eur J Clin Nutr. 2002;56:31–36. doi: 10.1038/sj.ejcn.1601306. [DOI] [PubMed] [Google Scholar]
- 13.Keet CA, Wood RA, Matsui EC. Limitations of reliance on specific IgE for epidemiologic surveillance of food allergy. J Allergy Clin Immunol. 2012;130:1207–1209. e10. doi: 10.1016/j.jaci.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gern JE, Visness CM, Gergen PJ, et al. The Urban Environment and Childhood Asthma (URECA) birth cohort study: design, methods, and study population. BMC Pulm Med. 2009;9:17. doi: 10.1186/1471-2466-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alwis KU, Milton DK. Recombinant factor C assay for measuring endotoxin in house dust: comparison with LAL, (1 --> 3)-beta-D-glucans. Am J Ind Med. 2006;49:296–300. doi: 10.1002/ajim.20264. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Ara C, Boyano-Martinez T, Diaz-Pena JM, Martin-Munoz F, Reche-Frutos M, Martin-Esteban M. Specific IgE levels in the diagnosis of immediate hypersensitivity to cows' milk protein in the infant. J Allergy Clin Immunol. 2001;107:185–190. doi: 10.1067/mai.2001.111592. [DOI] [PubMed] [Google Scholar]
- 17.Boyano-Martinez T, Garcia-Ara C, Diaz-Pena JM, Martin-Esteban M. Prediction of tolerance on the basis of quantification of egg white-specific IgE antibodies in children with egg allergy. J Allergy Clin Immunol. 2002;110:304–309. doi: 10.1067/mai.2002.126081. [DOI] [PubMed] [Google Scholar]
- 18.Sicherer SH, Wood RA, Stablein D, et al. Immunologic features of infants with milk or egg allergy enrolled in an observational study (Consortium of Food Allergy Research) of food allergy. J Allergy Clin Immunol. 2010;125:1077–1083. e8. doi: 10.1016/j.jaci.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osterballe M, Hansen TK, Mortz CG, Host A, Bindslev-Jensen C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatr Allergy Immunol. 2005;16:567–573. doi: 10.1111/j.1399-3038.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 20.Venter C, Pereira B, Voigt K, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63:354–359. doi: 10.1111/j.1398-9995.2007.01570.x. [DOI] [PubMed] [Google Scholar]
- 21.McGowan EC, Keet CA. Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007–2010. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chafen JJ, Newberry SJ, Riedl MA, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303:1848–1856. doi: 10.1001/jama.2010.582. [DOI] [PubMed] [Google Scholar]
- 23.Taylor-Black S, Wang J. The prevalence and characteristics of food allergy in urban minority children. Ann Allergy Asthma Immunol. 2012;109:431–437. doi: 10.1016/j.anai.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sears MR, Greene JM, Willan AR, et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet. 2002;360:901–907. doi: 10.1016/S0140-6736(02)11025-7. [DOI] [PubMed] [Google Scholar]
- 25.Bergmann RL, Diepgen TL, Kuss O, et al. Breastfeeding duration is a risk factor for atopic eczema. Clin Exp Allergy. 2002;32:205–209. doi: 10.1046/j.1365-2222.2002.01274.x. [DOI] [PubMed] [Google Scholar]
- 26.Kull I, Melen E, Alm J, et al. Breast-feeding in relation to asthma, lung function, and sensitization in young schoolchildren. J Allergy Clin Immunol. 2010;125:1013–1019. doi: 10.1016/j.jaci.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 27.Silvers KM, Frampton CM, Wickens K, et al. Breastfeeding protects against current asthma up to 6 years of age. J Pediatr. 2012;160:991–996. e1. doi: 10.1016/j.jpeds.2011.11.055. [DOI] [PubMed] [Google Scholar]
- 28.Perzanowski MS, Miller RL, Thorne PS, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol. 2006;117:1082–1089. doi: 10.1016/j.jaci.2005.12.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douwes J, van Strien R, Doekes G, et al. Does early indoor microbial exposure reduce the risk of asthma? The Prevention and Incidence of Asthma and Mite Allergy birth cohort study. Allergy Clin Immunol. 2006;117:1067–1073. doi: 10.1016/j.jaci.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Barnig C, Reboux G, Roussel S, et al. Indoor dust and air concentrations of endotoxin in urban and rural environments. Lett Appl Microbiol. 2013;56:161–167. doi: 10.1111/lam.12024. [DOI] [PubMed] [Google Scholar]
- 31.Lee A, Sangsupawanich P, Ma S, et al. Endotoxin levels in rural Thai and urban Singaporean homes. Int Arch Allergy Immunol. 2006;141:396–400. doi: 10.1159/000095467. [DOI] [PubMed] [Google Scholar]
- 32.Platts-Mills TA, Erwin E, Heymann P, Woodfolk J. Is the hygiene hypothesis still a viable explanation for the increased prevalence of asthma? Allergy. 2005;60(Suppl 79):25–31. doi: 10.1111/j.1398-9995.2005.00854.x. [DOI] [PubMed] [Google Scholar]
- 33.Sverremark-Ekstrom E, Hultgren EH, Borres MP, Nilsson C. Peanut sensitization during the first 5 yr of life is associated with elevated levels of peanut-specific IgG. Pediatr Allergy Immunol. 2012;23:224–229. doi: 10.1111/j.1399-3038.2012.01290.x. [DOI] [PubMed] [Google Scholar]
- 34.Tay SS, Clark AT, Deighton J, King Y, Ewan PW. Patterns of immunoglobulin G responses to egg and peanut allergens are distinct: ovalbumin-specific immunoglobulin responses are ubiquitous, but peanut-specific immunoglobulin responses are up-regulated in peanut allergy. Clin Exp Allergy. 2007;37:1512–1518. doi: 10.1111/j.1365-2222.2007.02802.x. [DOI] [PubMed] [Google Scholar]
- 35.Dreskin SC, Tripputi MT, Aubrey MT, et al. Peanut-allergic subjects and their peanut-tolerant siblings have large differences in peanut-specific IgG that are independent of HLA class II. Clin Immunol. 2010;137:366–373. doi: 10.1016/j.clim.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vickery BP, Lin J, Kulis M, et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J Allergy Clin Immunol. 2013;131:128–134. e1–e3. doi: 10.1016/j.jaci.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burks AW, Jones SM, Wood RA, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durrani SR, Montville DJ, Pratt AS, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130:489–495. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.