Summary
There is a growing consensus that the long term solution to the asthma epidemic lies in prevention and not in treatment of established disease. Atopic asthma arises from gene x environment interactions which most commonly occur during a relatively narrow window period in pre- and postnatal development. These interactions are incompletely understood, and hence the holy grail of primary prevention remains an elusive goal. We contend that a lack of understanding of the role of atopy in early life in the development of persistent asthma in children exists amongst primary care physicians, paediatricians and specialists. In this review we argue that early identification of high risk children is feasible based on currently available technology, and worthwhile in relation to potential benefits to the children so identified. Knowledge of an asthmatic child's atopic status in early life has practical clinical and prognostic implications, as well as forming the basis for future preventative strategies.
Introduction
There is a growing consensus that the long term solution to the asthma epidemic lies in prevention and not in the development of increasingly more sophisticated anti-inflammatory drugs for treatment of established disease 1. International research over the last 20 years has tracked the roots of the most common form of this disease, atopic asthma, to gene x environment interactions which most commonly occur during a relatively narrow window period in pre- and postnatal development1. These interactions are incompletely understood, and hence the holy grail of primary prevention remains an elusive goal. However the progress that has been achieved in this area is sufficient to justify a more structured approach to early identification of high risk children. Current treatment strategies do not alter the long term outlook for children with asthma despite controlling symptoms and improving quality of life2, implying that a different approach is necessary. We argue below that early identification of high risk children is feasible based on currently available technology, and worthwhile in relation to potential benefits to the children so identified.
Asthma related phenotypes in childhood: insights into the role of atopy from prospective birth cohort studies
A number of epidemiologically distinct wheezing phenotypes have been recognised in childhood 3, the most common being: transient infantile wheeze, where children have recurrent wheeze during the first two to three years of life but rarely afterwards; viral-associated wheeze, where children typically have episodic wheeze associated with respiratory viral infections and may not wheeze at other times; and atopic asthma, where children have wheeze associated with sensitization to aeroallergens and frequently have features of other atopic diseases such as dermatitis and rhino-conjunctivitis. While all of these wheezing phenotypes add to the burden of disease imposed on families and the community, asthma that persists throughout childhood and into adulthood imposes the greatest burden and, as such, is the major target for prevention programs. In this review, the term “persistent asthma” will be used to describe asthma that persists from early childhood into adult life and is not a description of symptoms as for staging asthma in clinical guidelines. The association between asthma and sensitization to aeroallergens continues through adolescence into the teen years 4-11 and in many cases into early adulthood 12, at least in the developed world 13.
Physiologically, the presence of wheeze implies expiratory flow limitation but the symptom does not give any clue about the underlying cause. Any insult that decreases airway calibre or alters airway wall compliance can potentially result in wheeze. Wheezing is more common in the first years of life in boys 4. One potential mechanism underlying this gender imbalance is that boys are thought to have relatively smaller airways than girls when related to lung size 14, however, direct proof of this is lacking. Wheeze with viral infections is common during childhood, especially during the first years of life. Viral lower respiratory infections (LRI) are likely to result in wheezing if they induce inflammation and oedema of the airway epithelium, resulting in a decrease in airway calibre. At least 20% of all children have one or more episodes of LRI with wheezing (wLRI) in the first year of life and up to 90% of these cases are associated with documented viral infections 15. Thirty percent of children enrolled in the Tucson Children's Respiratory Study had ≥1 wLRI in the first three years of life 3. Cohort studies in different parts of the world have shown that such wLRI are most commonly caused by rhinoviruses (RV), respiratory syncytial virus (RSV), parainfluenza viruses, and to a lesser extent, adenoviruses, human metapneumo virus and influenza virus 16-20.
The majority of children with viral-induced wheeze lose their symptoms in their early school years 21,22. These children generally have normal spirometry and are more commonly nonatopic 21. In contrast children who become sensitized to common aeroallergens are more likely to retain their symptoms and have lower lung function at school age 3,10,16,21. In a cohort of children enrolled between the age of 7 and 17 years and re-evaluated 6 years later, subjects with either persistent or new atopy to house dust mites (HDM) had lower lung function compared to those who remained unsensitized. The loss in lung function was greater in those with persistent as opposed to new onset of atopy 23. Furthermore, the loss of lung function reported in the first six years of life 3 continues in allergic asthmatics during adolescence 24. Longitudinal follow up of birth cohorts in Europe, USA and Australia shows that early sensitization 10,16,21,25,26, and more severe sensitization 27, are risk factors for persistence of asthma. Interestingly, while transient sensitization conveys increased risk of wheezing 10 the risk for persistence of symptoms is more pronounced if sensitization itself is also persistent10,21,28. The enhanced asthma risk associated with early sensitization may be related to the fact that this is associated with more intensely Th2-polarised long-term memory responses 29, and/or interactions with other risk factors during the early postnatal period (see below).
The role of the environment: a differential response to allergen and bacterial products
A recent prospective cohort study assessed postnatal development of lung function in high risk children raised in normal UK household environments versus those subject to a strict environmental controls to reduce exposure to indoor allergens and airborne irritants 30. While these measures did not improve sensitization rates (in fact the opposite result was seen), children living in the clean environment had better lung function at 3 years of age than children in the control group. Lung function was not different between the groups at 4 weeks of age, showing that this was a postnatal effect. These data raise the intriguing suggestion that the more “pro-inflammatory” conventional environment was associated with decreased lung growth. The potential significance of these findings is not known, however, studies (e.g. 31,32) have demonstrated that low lung function in early life is an independent risk factor for persistent asthma. The precise mechanisms underlying these effects of early environmental control are incompletely understood, but the clear association between indoor allergen levels and frequency of wheezing episodes 10,21,33 and development of new sensitizations, persistent bronchial responsiveness, and doctor-diagnosed asthma 34 in sensitised subjects suggests that successful reduction of allergen exposure leading to reduced allergy-mediated airway inflammation, may be one factor.
Atopic sensitization also increases the susceptibility of the airways to non-allergenic but nevertheless pro-inflammatory components of house dust such as microbial lipopolysaccharide (LPS) 35, and reduction of exposure to these in early life may also be a contributing factor. Exposure to LPS prior to sensitization is associated with reduction in risk for sensitization 36. Active exposure to immunogenic levels of aeroallergen seems to be a prerequisite for the development of normal immunological tolerance, which provides protection against de novo sensitization 37. This implies that an understanding of the significance of exposure to these agents to asthma pathogenesis in individual children can only be gained in light of knowledge of their current atopic status. Similar conclusions can be drawn for the role of viral infections in early life as risk factors for asthma (detailed discussion below).
Respiratory viral infections: direct and atopy-dependent roles in asthma development
The bulk of wheeze in the first few years of life is attributable to viral infection. Acute LRI, including bronchiolitis, resulting from RV and RSV, account for the majority of hospitalisations in children under 3 years of age 20,38. The mechanism(s) underlying the wheeze-promoting effects of these infections are only partially understood, but they are associated with the spread of infections to the lower airways and subsequent intensification, presumably resulting in inflammatory damage to airway tissues. Animal studies support a role for neurogenic inflammation resulting from RSV infection 39.
The contribution of individual inflammatory effector mechanisms to the host response to respiratory viruses is controversial, exemplified by the ongoing saga surrounding RSV. On the one hand, a prominent contribution from recruited neutrophils to the anti-RSV response in the airways of human infants has been reported 40,41, but the role of these cells in viral clearance is unknown 40. The presence of an underlying Th2 “signature” in the lung response to RSV in the form of local IgE production has also been reported 42. This Th2 signature has become a repeating theme in much of the literature on RSV infection in children and in murine models, particularly in relation to the apparent link between Th2-dependent eosinophil responses and infection severity. Reports to this effect include the presence of eosinophil secretory products in airway secretions of infants during acute infection 43-46, the presence of eosinophils in nasal secretions 46, and accompanying changes in the activation status of eosinophils in the blood 47,48. A consistent finding has been that circulating eosinophil numbers transiently drop during acute RSV infection 48-51, which has been widely interpreted as indicating active migration to the site of infection. The possibility that such recruitment may be a normal part of the host anti-viral defence response in this age group 50 is indirectly supported by recent findings from a murine RSV model demonstrating a direct role for eosinophils in accelerating viral clearance via a TLR-7-dependent pathway 52. In this context cytokine receptor expression on blood borne eosinophil precursors in infants differs markedly from the IL-5-dominant pattern characteristic of older age groups, and is dominated by receptors for the ubiquitous cytokines IL-3 and GM-CSF 53 which are “first line” cytokines produced early in most classes of cellular immune responses including those against pathogens. This may contribute to the increased prominence of eosinophils in inflammatory exudates in general in early life 53,54.
Severe viral bronchiolitis is more frequent in infants with elevated IgE 46,55 suggesting that predisposition to both states may involve common genetic or epigenetic mechanisms 1. These factors may act in concert with others involving innate mechanisms (such as type III IFN production) more specifically related to host anti-viral defense 56. Severe asthma exacerbations in older children are commonly associated with respiratory viral infections and the susceptible subgroup is again dominated by those sensitised to aeroallergens 20,57-60. These and other findings suggest that inflammatory pathways triggered by viral infections and inhalant allergy can function independently or in combination to drive asthma symptomatology in subjects with established disease 1,20.
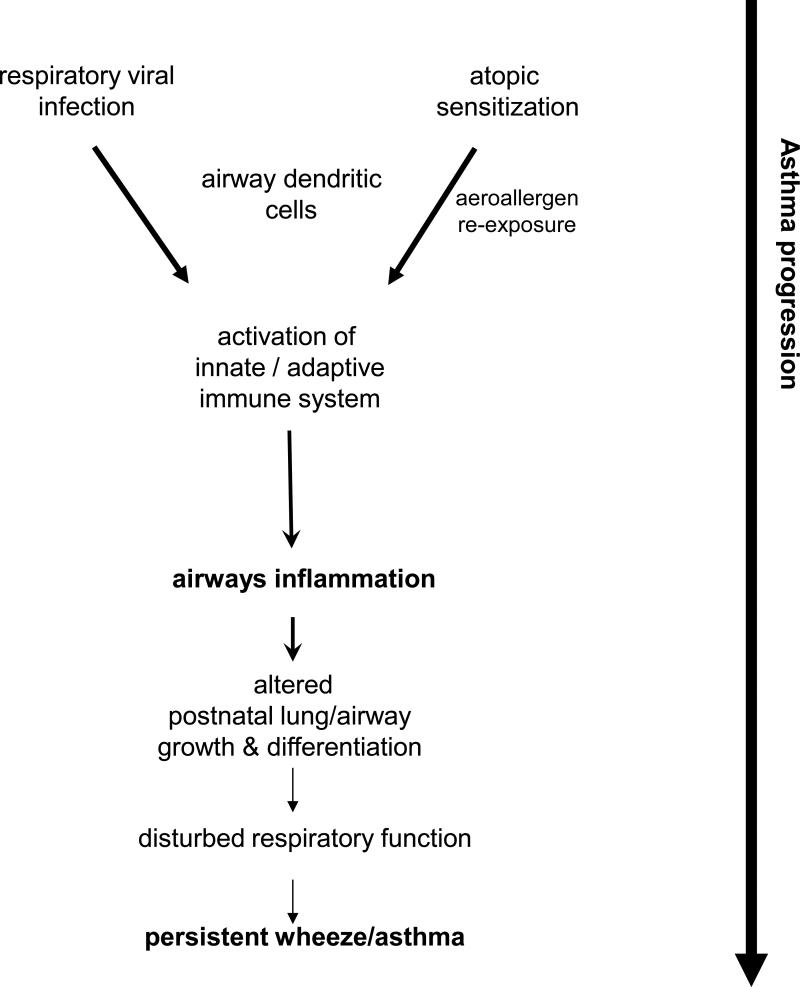
The results of a series of prospective cohort studies that have tracked large groups of children over varying periods indicate that the effects of these pathways are not restricted to asthma exacerbations but may also be central to disease aetiology. The bulk of the early studies in this area 5 focused primarily on the role of atopy in isolation whereas more recent prospective birth cohort studies have examined the role of atopy and viral infections in asthma aetiology in parallel 3,9,10,16,21,22,61. Collectively, these findings are consistent with a “two hit” model for asthma 1 in which airways inflammation triggered by viral infection or allergy during postnatal lung growth disrupts underlying tissue differentiation programs, leading to aberrations in respiratory function which “track” over prolonged periods into later life 1,62. While both allergy and respiratory infections during early life are independently associated with risk for subsequent development of asthma, the highest odds ratios for persistent asthma are observed in children who suffer both stresses 1,9(Fig. 1).
Figure 1.
Schematic representation of the interactions between early respiratory viral infections and atopic sensitization on the pathway to persistent wheeze/asthma. The link between activation of immune defence mechanisms to viral infections and activation of airway dendritic cells (DC) is highlighted.
The precise nature of the interactions between atopy and infections in this context are unclear. The finding in a high risk cohort that early RV/RSV infections impose the greatest risk on development of persistent asthma in children who are sensitised by two years suggests that the mechanism may involve cumulative airway damage accruing from the two pathways 16. However, other manifestations of the atopic phenotype, in particular excessively Th2-polarised cellular immune responses to the infecting virus in high risk infants 63, may also contribute. In this context it is of interest to note that developmental deficiencies in capacity to produce Th1 cytokines exemplified by interferon γ, which are a hallmark of infants at high risk of atopy 64, are also associated with risk for RSV 65,66 or RV infection in infancy 67. It is additionally noteworthy that respiratory viral infection markedly upregulates local innate immune functions in particular the activity of airway mucosal dendritic cell (DC) populations68,69 which control development and expression of adaptive cell-mediated immunity against the pathogen. These DC also provide the initial trigger for the CD4+ Th2-cell component of the asthma late phase response in atopics37, and via this route the viral infection may thus enhance the immunopathogenic potential of pre-existing inhalant allergy (see scheme in Fig 1) creating a heterogeneous inflammatory milieu which, as noted above, is associated with maximal risk of asthma development 9,70.
The role of atopy in asthma on a population basis has been clarified by a recent analysis of the Third National Health and Nutrition Examination Survey (NHANES III) which concluded that 56.3% of the cases of asthma in the USA were attributable to atopy 71. This report included data from people aged between 6 and 59 year old and found that the proportion of asthma attributable to atopy was higher in males (74.1% vs 43.2% in females), with further variations related to education and housing status. 71. Initial analyses of 1400 subjects from the 14yr follow-up of the community-based birth cohort from Perth (6yr data in 9) indicate that the proportion of current asthma attributable to atopy is 52% overall. However, in contrast to the NHANES III data, the atopy accounts for more asthma in females (65.6%) than in males (46.4%). It is intriguing to speculate that this pattern of gender difference may be specific to adolescence and thus not seen in populations with wider age ranges. It is also important to note that these data all come from developed Western countries and that the relationship between atopy and asthma may not be the same in populations from developing countries 13. However, even under conditions where non-atopic asthma is the most common phenotype, atopy is still associated with more severe asthma 13.
Predicting children at risk of persistent asthma: developing clinical indicators
A number of attempts have been made to produce models or clinical indicators of risk of persistent asthma at early ages 32,72,73. These models have used asthma risk factors identified in epidemiological studies, including: parental history of asthma and atopy; wheeze history; the presence of other atopic conditions such as eczema, rhino-conjunctivitis or food allergy; increased serum IgE antibodies; and in vitro cytokine production. In general, while children identified via these models do have a higher risk of developing persistent asthma, the negative predictive values are higher than the positive predictive values 32,72,73, indicating that they are better at excluding asthma than predicting it and are thus not suitable for identifying the high risk children likely to benefit from prevention strategies.
Longitudinal follow-up in a range of birth cohort studies have demonstrated that optimal utilisation of atopy indices for assessment of asthma risk can only be achieved in an appropriate clinical context. In particular, they need to be considered against a background of information including family history, age and gender which is collected under standardised conditions. This list is expanding with new candidate markers such as Body Mass Index 71, race-ethnicity 71, sleep quantity and quality 74,75 environmental pollution such as traffic noise and fumes 76, and will undoubtedly eventually include genetic markers 77.
Accumulating evidence suggests that quantitative measures of atopy 27,78-81 in particular cumulative titres of IgE specific for perennial inhalant allergens 82,83, provide more robust assessments of atopy associated risk than simple binary classifications such as sensitised/non sensitised. In a large birth cohort from Perth Australia, the severity of atopy, judged from both the number of positive SPT and the wheal size, was related to both the risk of current asthma and severity of asthma at 6 years of age 7. Linear trends were also seen between serum levels of eosinophil cationic protein, a biomarker of eosinophil activation, and SPT wheal size as well as to asthma severity 7. Associations between quantitative measures of atopy and an increased risk of asthma, severity of food allergy 27, and responsiveness to bronchial allergen challenge have been reported in other paediatric cohorts 79. These findings suggest that there is little justification for the continued use of atopy as a binary variable for assessing asthma risk.
Personal atopic history in early life appears to be one of the most important factors determining an individual's risk of persistent asthma. Recent data from longitudinal cohort studies address the inter-relationships between different clinical manifestations of atopy 27,84. The German Multicentre Allergy Study (MAS) reported a cumulative prevalence of atopic dermatitis (AD) in the first 2 years of life of 21.5% 84. When associated with allergic sensitization, AD was a significant predictor of asthma at school age; this risk was not seen with AD in the absence of sensitization. However, in many of the children in this cohort wheeze was seen before or at the onset of AD rather than as a progression of atopic disease. The finding that AD per se does not constitute a risk for asthma, but only when associated with allergic sensitization was supported by the findings a cohort of high risk Australian children 27. The latter study also demonstrated a significant risk for asthma [odds ratio (OR) 3.52, 95%CI 1.88-6.59] at early school age in children with AD and sensitization to food allergens in the first 2 years of life. The risk was greater with large wheal size predictive of clinical food allergy [OR 4.61, 2.34-9.09]. They also found no increased risk of asthma with AD in the absence of allergic sensitization. These data, and others from high risk cohorts 85, demonstrate that infantile eczema and genuine AD (i.e. that associated with allergic sensitization) are not synonymous, and that non-atopic eczema does not convey a risk for asthma. Thus, objective measurement of atopic status including quantification of markers of sensitization is required to quantify asthma risk. Sensitization to hen's egg appears to convey the greatest risk 27,84. These conclusions come from both high-risk 27,85 and community-based cohorts 84 suggesting that they are applicable to the general population.
In addition to atopy and respiratory infections, a range of environmental factors contribute to risk for asthma development through pre- or postnatal exposures, including: ETS 86-88, type of bedding 89,90, indoor and outdoor air pollutants 91-94, psychosocial factors 95, and bioaerosols containing microbial breakdown products36 and/or allergens 37. The effects of these exposures may be modified by genetic 96,97 and epigenetic 98,99 factors. In vitro models suggest that environmental conditions that compromise the barrier function of the airway epithelium could enhance replication of respiratory viruses 100,101 and, consequently, respiratory morbidity. We must, however, acknowledge that the role of some environmental exposures in inducing asthma is not clear. In particular, exposure to pets or farm animals is protective in many but not all studies and controversy still exists regarding whether prolonged breast feeding increases the risk of persistent asthma in all children or only in those with atopic mothers.
At present it may not be possible to accurately define a “high risk” child with absolute certainty. Current research points to some indicators, including: family history of asthma and allergies, especially a maternal history; early and more severe sensitisation to some food antigens, especially hen's egg, and to aeroallergens; early viral infection associated with wheeze and adverse environmental exposures. However, further comprehensive studies are needed to develop good clinical indicators of high risk.
What would early identification of high risk children achieve?
A significant proportion of children who develop persistent disease are not being detected until their disease has already consolidated, thus denying them any potential benefits of early intervention. But are these potential benefits real or imagined? One argument in support of reality is the “Hawthorne effect” which is seen in the control arm of intervention studies and in observational studies, notably the improved disease outcomes (relative to the community at large) which accrue from nothing other than the provision of “current best practise” care to at-risk subjects 102. We argue that the ability to identify at-risk children with a high degree of certainty would potentially allow a number of important interventions to be undertaken, including: early institution of appropriate therapy in children destined to suffer persistent asthma which can control asthma symptoms and reduce the burden imposed on the child and the family 2 and the community 103; provision of appropriate advice to parents of wheezing children, including advice of allergen avoidance in sensitized children104; and potentially institution of specific allergen immunotherapy in children most likely to benefit 6,8. In addition, routine advice that should be given by all primary care physicians and paediatricians to avoid exposing young children to environmental contaminants, such cigarette smoke, pollutants and noxious chemical, could be further strengthened for parents of high risk children. However, ensuring that high risk children are actively engaged with clinical follow-up may also provide an important benefit.
Intensive research is in progress internationally on a broad range of more aggressive early intervention strategies (discussed in 62), including: immunotherapy; immunoprophylaxis; and prevention of wLRI with immunomodulatory approaches and/or antiviral approaches. Testing these strategies requires identification of those children most likely to benefit. It is clear that at present our capacity to accurately define the high risk state is limited and is an issue requiring further research. Nevertheless, the approach discussed above, which includes as a cornerstone the early identification and quantification of specific allergic sensitization, together with accurate assessment of severity and stability of sensitization, represents a logical step in this direction.
In conclusion, objective assessment of atopy by quantitating allergen-specific IgE levels in serum to common food allergens (e.g. hens egg and cows milk) and local aeroallergens (e.g. house dust mite, alternaria, etc) by two years of age in conjunction with the presence of other atopic manifestations (e.g. AD, or clinical food allergy) can help identify which wheezing children are at high risk of developing persistent asthma. The ability to identify high risk children may provide individual benefit by ensuring they are actively engaged with clinical monitoring and providing them with current “best practice” management. This will also facilitate appropriate selection of subjects for clinical trials designed to evaluate new prevention strategies.
Search strategy and selection criteria
The OVID search engine was used to find articles in the Medline database using key words related to childhood asthma, allergic sensitization, longitudinal cohort studies and asthma risk factors. This general search was supplemented by specific searches for articles addressing particular points of relevance to the review. As this was not a systematic review conducted using Cochrane guidelines, no “selection” or “rejection” criteria were used.
Footnotes
Author's contributions:
The idea for this review came from Pat Holt and Peter Sly and they wrote the first draft. They then invited a group of peers to join with them and assimilated their comments into the final version. Authors were invited based on their contribution to the literature in this area, especially in relation to major prospective birth cohort studies.
Conflict of Interest statement
No commercial entity was involved in the development or production of this article. The undersigned authors declare that they have no commercial relationship with any entity that could benefit from publication of this article and have no actual or perceived conflict.
References
- 1.Holt PG, Upham JW, Sly PD. Contemporaneous maturation of immunological and respiratory functions during early childhood: Implications for development of asthma prevention strategies. J Allergy Clin Immunol. 2005;116:16–24. doi: 10.1016/j.jaci.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma.[see comment]. New England Journal of Medicine. 2006;354(19):1985–97. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 3.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates.[see comment]. New England Journal of Medicine. 1995;332(3):133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 4.Anderson HR, Pottier AC, Strachan DP. Asthma from birth to age 23: incidence and relation to prior and concurrent atopic disease. Thorax. 1992;47(7):537–42. doi: 10.1136/thx.47.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burrows B, Martinez F, Halonen M, Barbee R, Cline M. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62(8):943–8. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 7.Joseph-Bowen J, de Klerk N, Holt PG, Sly PD. Relationship of asthma, atopy and bronchial responsiveness to serum eosinophil cationic proteins in early childhood. J Allergy & Clinical Immunology. 2004;114(5):1040–1045. doi: 10.1016/j.jaci.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 8.Niggemann B, Jacobsen L, Dreborg S, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006;61(7):855–9. doi: 10.1111/j.1398-9995.2006.01068.x. [DOI] [PubMed] [Google Scholar]
- 9.Oddy WH, de Klerk NH, Sly PD, Holt PG. The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. European Respiratory Journal. 2002;19(5):899–905. doi: 10.1183/09031936.02.00103602. [DOI] [PubMed] [Google Scholar]
- 10.Rhodes HL, Thomas P, Sporik R, Holgate ST, Cogswell JJ. A birth cohort study of subjects at risk of atopy: twenty-two-year follow-up of wheeze and atopic status. American Journal of Respiratory & Critical Care Medicine. 2002;165(2):176–80. doi: 10.1164/ajrccm.165.2.2104032. [DOI] [PubMed] [Google Scholar]
- 11.Xuan W, Marks GB, Toelle BG, et al. Risk factors for onset and remission of atopy, wheeze, and airway hyperresponsiveness. Thorax. 2002;57(2):104–109. doi: 10.1136/thorax.57.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sears MR, Greene JM, Willan AR, et al. A Longitudinal, Population-Based, Cohort Study of Childhood Asthma Followed to Adulthood. N Engl J Med. 2003;349(15):1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 13.Pereira MU, Sly PD, Pitrez PM, et al. Non-atopic asthma is associated with helminth infections and bronchiolitis in poor children. Eur Respir J. 2007;29:1154–1160. doi: 10.1183/09031936.00127606. [DOI] [PubMed] [Google Scholar]
- 14.Tepper RS, Morgan WJ, Cota K, Wright A, Taussig LM. Physiologic growth and development of the lung during the first year of life. American Review of Respiratory Disease. 1986;134(3):513–9. doi: 10.1164/arrd.1986.134.3.513.[erratum appears in Am Rev Respir Dis 1987;136(3):800]
- 15.Papadopoulos NG, Xepapadaki P, Mallia P, et al. Mechanisms of virus-induced asthma exacerbations: state-of-the-art. A GA2LEN and InterAirways document. Allergy. 2007;62(5):457–470. doi: 10.1111/j.1398-9995.2007.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusel MMH, de Klerk NH, Kebadze T, et al. Early-life respiratory viral infections, atopic sensitization and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee W-M, Kiesner C, Pappas T, et al. A Diverse Group of Previously Unrecognized Human Rhinoviruses Are Common Causes of Respiratory Illnesses in Infants. PLoS ONE. 2007;2(10):e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemanske RF, Jr., Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing.[see comment]. Journal of Allergy & Clinical Immunology. 2005;116(3):571–7. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Montalbano MM, Lemanske RF., Jr. Infections and asthma in children. Current Opinion in Pediatrics. 2002;14(3):334–7. doi: 10.1097/00008480-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Singh AM, Moore PE, Gern JE, Lemanske RF, Jr., Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. American Journal of Respiratory & Critical Care Medicine. 2007;175(2):108–19. doi: 10.1164/rccm.200603-435PP. [DOI] [PubMed] [Google Scholar]
- 21.Illi S, von Mutius E, Lau S, et al. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9537):763–70. doi: 10.1016/S0140-6736(06)69286-6.[erratum appears in Lancet. 2006 Sep 30;368(9542):1154].
- 22.Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years.[see comment]. Lancet. 1999;354(9178):541–5. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 23.Ulrik CS, Backer V. Markers of Impaired Growth of Pulmonary Function in Children and Adolescents. Am. J. Respir. Crit. Care Med. 1999;160(1):40–44. doi: 10.1164/ajrccm.160.1.9806059. [DOI] [PubMed] [Google Scholar]
- 24.Strunk RC, Weiss ST, Yates KP, Tonascia J, Zeiger RS, Szefler SJ. Mild to moderate asthma affects lung growth in children and adolescents. Journal of Allergy and Clinical Immunology. 2006;118(5):1040–1047. doi: 10.1016/j.jaci.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 25.Peat JK, Salome CM, Woolcock AJ. Longitudinal changes in atopy during a 4-year period: Relation to bronchial hyperresponsiveness and respiratory symptoms in a population sample of Australian schoolchildren. J Allergy Clin Immunol. 1990;85:65–74. doi: 10.1016/0091-6749(90)90223-q. [DOI] [PubMed] [Google Scholar]
- 26.Sherrill D, Stein R, Kurzius-Spencer M, Martinez F. Early senstization to allergens and development of respiratory symptoms. Clin Exp Allergy. 1999;29:905–11. doi: 10.1046/j.1365-2222.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- 27.Lowe AJ, Hosking CS, Bennett CM, et al. Skin prick test can identify eczematous infants at risk of asthma and allergic rhinitis. Clinical & Experimental Allergy. 2007;37(11):1624–31. doi: 10.1111/j.1365-2222.2007.02822.x. [DOI] [PubMed] [Google Scholar]
- 28.Kulig M, Bergmann R, Tacke U, Wahn U, Guggenmoos-Holzmann I. Long-lasting sensitization to food during the first two years precedes allergic airway disease. The MAS Study Group, Germany. Pediatric Allergy & Immunology. 1998;9(2):61–7. doi: 10.1111/j.1399-3038.1998.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 29.Turner SW, Heaton T, Rowe J, et al. Early-onset atopy is associated with enhanced lymphocyte cytokine responses in 11-year-old children. Clinical & Experimental Allergy. 2007;37(3):371–80. doi: 10.1111/j.1365-2222.2007.02668.x. [DOI] [PubMed] [Google Scholar]
- 30.Woodcock A, Lowe LA, Murray CS, et al. Early life environmental control: effect on symptoms, sensitization, and lung function at age 3 years. American Journal of Respiratory & Critical Care Medicine. 2004;170(4):433–9. doi: 10.1164/rccm.200401-083OC. [DOI] [PubMed] [Google Scholar]
- 31.Haland G, Lodrop Carlsen K, Sandvik L, Devulapalli C, Carlsen K-H. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–1689. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- 32.Devulapalli CS, Carlsen KCL, Haland G, et al. Severity of obstructive airways disease by age 2 years predicts asthma at 10 years of age. Thorax. 2008;63(1):8–13. doi: 10.1136/thx.2006.060616. [DOI] [PubMed] [Google Scholar]
- 33.Lane J, Siebers R, Pene G, Howden-Chapman P, Crane J. Tokelau: a unique low allergen environment at sea level. Clinical & Experimental Allergy. 2005;35(4):479–482. doi: 10.1111/j.1365-2222.2005.02202.x. [DOI] [PubMed] [Google Scholar]
- 34.Matheson MC, Abramson MJ, Dharmage SC, et al. Changes in indoor allergen and fungal levels predict changes in asthma activity among young adults. Clinical & Experimental Allergy. 2005;35(7):907–913. doi: 10.1111/j.1365-2222.2005.02272.x. [DOI] [PubMed] [Google Scholar]
- 35.Michel O, Kips J, Duchateau J, et al. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med. 1996;154:1641–6. doi: 10.1164/ajrccm.154.6.8970348. [DOI] [PubMed] [Google Scholar]
- 36.Gereda JE, Leung DYM, Thatayatikom A, et al. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet. 2000;355:1680–83. doi: 10.1016/s0140-6736(00)02239-x. [DOI] [PubMed] [Google Scholar]
- 37.Holt PG, Thomas WR. Sensitization to airborne environmental allergens: unresolved issues. Nature Immunol. 2005;6:957–960. doi: 10.1038/ni1005-957. [DOI] [PubMed] [Google Scholar]
- 38.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 39.Piedimonte G. Neural Mechanisms of Respiratory Syncytial Virus-induced Inflammation and Prevention of Respiratory Syncytial Virus Sequelae. Am. J. Respir. Crit. Care Med. 2001;163(3):18S–21. doi: 10.1164/ajrccm.163.supplement_1.2011113. [DOI] [PubMed] [Google Scholar]
- 40.Jones A, Qui JM, Bataki E, et al. Neutrophil survival is prolonged in the airways of healthy infants and infants with RSV bronchiolitis.[see comment]. European Respiratory Journal. 2002;20(3):651–7. doi: 10.1183/09031936.02.00278902. [DOI] [PubMed] [Google Scholar]
- 41.Smith PK, Wang SZ, Dowling KD, Forsyth KD. Leucocyte populations in respiratory syncytial virus-induced bronchiolitis. Journal of Paediatrics and Child Health. 2001;37(2):146–151. doi: 10.1046/j.1440-1754.2001.00618.x. [DOI] [PubMed] [Google Scholar]
- 42.Everard ML, Fox G, Walls AF, et al. Tryptase and IgE concentrations in the respiratory tract of infants with acute bronchiolitis. Archives of Disease in Childhood. 1995;72(1):64–9. doi: 10.1136/adc.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dimova-Yaneva D, Russell D, Main M, Brooker RJ, Helms PJ. Eosinophil activation and cysteinyl leukotriene production in infants with respiratory syncytial virus bronchiolitis. Clinical & Experimental Allergy. 2004;34(4):555–8. doi: 10.1111/j.1365-2222.2004.1918.x. [DOI] [PubMed] [Google Scholar]
- 44.Harrison AM, Bonville CA, Rosenberg HF, Domachowske JB. Respiratory syncytical virus-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. American Journal of Respiratory & Critical Care Medicine. 1999;159(6):1918–24. doi: 10.1164/ajrccm.159.6.9805083. [DOI] [PubMed] [Google Scholar]
- 45.Kristjansson S, Bjarnarson SP, Wennergren G, et al. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. Journal of Allergy & Clinical Immunology. 2005;116(4):805–11. doi: 10.1016/j.jaci.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Rakes GP, Arruda E, Ingram JM, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. American Journal of Respiratory & Critical Care Medicine. 1999;159(3):785–90. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 47.Lindemans CA, Kimpen JLL, Luijk B, et al. Systemic eosinophil response induced by respiratory syncytial virus. Clinical & Experimental Immunology. 2006;144(3):409–17. doi: 10.1111/j.1365-2249.2006.03084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oymar K, Elsayed S, Bjerknes R. Serum eosinophil cationic protein and interleukin-5 in children with bronchial asthma and acute bronchiolitis. Pediatric Allergy & Immunology. 1996;7(4):180–6. doi: 10.1111/j.1399-3038.1996.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 49.Kawasaki Y, Hosoya M, Kanno H, Suzuki H. Serum regulated upon activation, normal T cell expressed and presumably secreted concentrations and eosinophils in respiratory syncytial virus infection. Pediatrics International. 2006;48(3):257–60. doi: 10.1111/j.1442-200X.2006.02199.x. [DOI] [PubMed] [Google Scholar]
- 50.Martinez FD, Stern DA, Wright AL, Taussig LM, Halonen M. Differential immune responses to acute lower respiratory illness in early life and subsequent development of persistent wheezing and asthma. J Allergy Clin Immunol. 1998;102:915–20. doi: 10.1016/s0091-6749(98)70328-8. [DOI] [PubMed] [Google Scholar]
- 51.Nagayama Y, Tsubaki T, Sawada K, Taguchi K, Nakayama S, Toba T. Age and sex as factors of response to RSV infections among those with previous history of wheezing. Pediatric Allergy & Immunology. 2006;17(5):376–81. doi: 10.1111/j.1399-3038.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 52.Phipps S, Lam CE, Mahalingam S, et al. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110(5):1578–86. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 53.Fernandes R, Kusel M, Cyr M, et al. Cord blood hemopoietic progenitor profiles predict fever and wheeze during acute respiratory illness in infancy. Pediatric Allergy & Immunology. 2008;19:239–247. doi: 10.1111/j.1399-3038.2007.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holt PG. Postnatal maturation of immune competence during infancy and early childhood. Pediatr Allergy Immunol. 1995;6:59–70. doi: 10.1111/j.1399-3038.1995.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 55.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Björkstén B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- 56.Contoli M, Message SD, Laza-Stanca V, et al. Role of deficient type III interferon-lambda production in astham exacerbations. Nature Medicine. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 57.Johnston SL, Pattemore PK, Sanderson G, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–60. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 58.Murray CS, Poletti G, Kebadze T, et al. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children.[see comment]. Thorax. 2006;61(5):376–82. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parry DE, Busse WW, Sukow KA, Dick CR, Swenson C, Gern JE. Rhinovirus-induced PBMC responses and outcome of experimental infection in allergic subjects. Journal of Allergy & Clinical Immunology. 2000;105(4):692–8. doi: 10.1067/mai.2000.104785. [DOI] [PubMed] [Google Scholar]
- 60.Xatzipsalti M, Kyrana S, Tsolia M, et al. Rhinovirus viremia in children with respiratory infections.[see comment]. American Journal of Respiratory & Critical Care Medicine. 2005;172(8):1037–40. doi: 10.1164/rccm.200502-315OC. [DOI] [PubMed] [Google Scholar]
- 61.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 62.Holt Prevention of allergic respiratory disease in infants: current aspects and future perspectives. Current opinion in allergy and clinical immunology. 2007:547–555. doi: 10.1097/ACI.0b013e3282f14a17. [DOI] [PubMed] [Google Scholar]
- 63.Holt PG, Sly PD. Interactions between RSV infection, asthma, and atopy: unraveling the complexities. J Exp Med. 2002;196:1271–1275. doi: 10.1084/jem.20021572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holt PG, Clough JB, Holt BJ, et al. Genetic 'risk' for atopy is associated with delayed postnatal maturation of T-cell competence. Clin Exp Allergy. 1992;22:1093–9. doi: 10.1111/j.1365-2222.1992.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 65.Copenhaver CC, Gern JE, Li Z, et al. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004;170:175–180. doi: 10.1164/rccm.200312-1647OC. [DOI] [PubMed] [Google Scholar]
- 66.Rowe J, Macaubas C, Monger T, et al. Heterogeneity in diphtheria-tetanus-acellular pertussis vaccine-specific cellular immunity during infancy: relationship to variations in the kinetics of postnatal maturation of systemic Th1 function. J Infect Dis. 2001;184:80–8. doi: 10.1086/320996. [DOI] [PubMed] [Google Scholar]
- 67.Gern JE, Brooks GD, Meyer P, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. Journal of Allergy & Clinical Immunology. 2006;117(1):72–8. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 68.McWilliam A, Marsh A, Holt P. Inflammatory infiltration of the upper airway epithelium during Sendai virus infection: invovlement of epithelial dendritic cells. J Virol. 1997;71:226–236. doi: 10.1128/jvi.71.1.226-236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McWilliam A, Napoli S, Marsh A, et al. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996;184:2429–2432. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holt PG, Strickland DH, M.E. W, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nature Rev Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 71.Arbes SJ, Jr., Gergen PJ, Vaughn B, Zeldin DC. Asthma cases attributable to atopy: results from the Third National Health and Nutrition Examination Survey.[see comment]. Journal of Allergy & Clinical Immunology. 2007;120(5):1139–45. doi: 10.1016/j.jaci.2007.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. American Journal of Respiratory & Critical Care Medicine. 2000;162(4 Pt 1):1403–6. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 73.Clough JB, Keeping KA, Edwards LC, Freeman WM, Warner JA, Warner JO. Can we predict which wheezy infants will continue to wheeze? American Journal of Respiratory & Critical Care Medicine. 1999;160(5 Pt 1):1473–80. doi: 10.1164/ajrccm.160.5.9807019. [DOI] [PubMed] [Google Scholar]
- 74.Smaldone A, Honig JC, Byrne MW. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation's children. Pediatrics. 2007;119(Suppl 1):S29–37. doi: 10.1542/peds.2006-2089F. [DOI] [PubMed] [Google Scholar]
- 75.Stein MA, Mendelsohn J, Obermeyer WH, Amromin J, Benca R. Sleep and behavior problems in school-aged children. Pediatrics. 2001;107(4):E60. doi: 10.1542/peds.107.4.e60. [DOI] [PubMed] [Google Scholar]
- 76.Ising H, Lange-Asschenfeldt H, Moriske HJ, Born J, Eilts M. Low frequency noise and stress: bronchitis and cortisol in children exposed chronically to traffic noise and exhaust fumes. Noise Health. 2004;6(23):21–28. [PubMed] [Google Scholar]
- 77.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nature Rev Immunol. 2008;8:169–182. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 78.Carroll WD, Lenney W, Child F, et al. Asthma severity and atopy: how clear is the relationship? Archives of Disease in Childhood. 2006;91(5):405–9. doi: 10.1136/adc.2005.088278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Douglas TA, Kusel M, Pascoe EM, Loh RKS, Holt PG, Sly PD. Predictors of response to bronchial allergen challenge in 5- to 6-year-old atopic children. Allergy. 2007;62(4):401–407. doi: 10.1111/j.1398-9995.2007.01329.x. [DOI] [PubMed] [Google Scholar]
- 80.Guilbert TW, Morgan WJ, Zeiger RS, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma.[see comment]. Journal of Allergy & Clinical Immunology. 2004;114(6):1282–7. doi: 10.1016/j.jaci.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 81.Mrazek DA, Klinnert M, Mrazek PJ, et al. Prediction of early-onset asthma in genetically at-risk children. Pediatric Pulmonology. 1999;27(2):85–94. doi: 10.1002/(sici)1099-0496(199902)27:2<85::aid-ppul4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 82.Marinho S, Simpson A, Soderstrom L, Woodcock A, Ahlstedt S, Custovic A. Quantification of atopy and the probability of rhinitis in preschool children: a population-based birth cohort study. Allergy. 2007;62(12):1379–86. doi: 10.1111/j.1398-9995.2007.01502.x. [DOI] [PubMed] [Google Scholar]
- 83.Simpson A, Soderstrom L, Ahlstedt S, Murray CS, Woodcock A, Custovic A. IgE antibody quantification and the probability of wheeze in preschool children. Journal of Allergy & Clinical Immunology. 2005;116(4):744–9. doi: 10.1016/j.jaci.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 84.Illi S, von Mutius E, Lau S, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. Journal of Allergy & Clinical Immunology. 2004;113(5):925–31. doi: 10.1016/j.jaci.2004.01.778. [DOI] [PubMed] [Google Scholar]
- 85.Kusel MM, Holt PG, de Klerk N, Sly PD. Support for 2 variants of eczema. J Allergy Clin Immunol. 2006;116:1067–1072. doi: 10.1016/j.jaci.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 86.Macaubas C, de Klerk NH, Holt BJ, et al. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet. 2003;362:1192–1197. doi: 10.1016/s0140-6736(03)14542-4. [DOI] [PubMed] [Google Scholar]
- 87.Stick SM, Burton PR, Gurrin L, Sly PD, LeSouef PN. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet. 1996;348(9034):1060–4. doi: 10.1016/s0140-6736(96)04446-7. [DOI] [PubMed] [Google Scholar]
- 88.Vork KL, Broadwin RL, Blaisdell RJ. Developing asthma in childhood from exposure to secondhand tobacco smoke: insights from a meta-regression. Environ Health Perspect. 2007;115(10):1394–400. doi: 10.1289/ehp.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ponsonby A, Dwyer T, Kemp A, Cochrane J, Couper D, Carmichael A. Synthetic bedding and wheeze in childhood. Epidemiol. 2003;14:37–44. doi: 10.1097/00001648-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 90.Trevillian L, Ponsonby A, Dwyer T, et al. A prospective association between cocoon use in infancy and childhood asthma. Pediatr Perinatal Epidemiol. 2004;18:281–289. doi: 10.1111/j.1365-3016.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 91.Björkstén B. Risk factors in early childhood for the development of atopic diseases. Allergy. 1994;49:400–7. doi: 10.1111/j.1398-9995.1994.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 92.Brauer M, Hoek G, Smit HA, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. 2007;29(5):879–888. doi: 10.1183/09031936.00083406. [DOI] [PubMed] [Google Scholar]
- 93.Brauer M, Hoek G, Van Vliet P, et al. Air Pollution from Traffic and the Development of Respiratory Infections and Asthmatic and Allergic Symptoms in Children. Am. J. Respir. Crit. Care Med. 2002;166(8):1092–1098. doi: 10.1164/rccm.200108-007OC. [DOI] [PubMed] [Google Scholar]
- 94.Schwartz DA, Freedman JH, Linney EA. Environmental genomics: a key to understanding biology, pathophysiology and disease. Hum Mol Genet. 2004;13(Spec No 2):R217–24. doi: 10.1093/hmg/ddh228. [DOI] [PubMed] [Google Scholar]
- 95.Klinnert MD, Nelson HS, Price MR, Adinoff AD, Leung DY, Mrazek DA. Onset and persistence of childhood asthma: predictors from infancy. Pediatrics. 2001;108(4):E69. doi: 10.1542/peds.108.4.e69. [DOI] [PubMed] [Google Scholar]
- 96.Cole Johnson C, Ownby DR, Havstad SL, Peterson EL. Family history, dust mite exposure in early childhood, and risk for pediatric atopy and asthma. Journal of Allergy & Clinical Immunology. 2004;114(1):105–10. doi: 10.1016/j.jaci.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 97.Simpson A, John SL, Jury F, et al. Endotoxin exposure, CD14, and allergic disease: an interaction between genes and the environment.[see comment]. American Journal of Respiratory & Critical Care Medicine. 2006;174(4):386–92. doi: 10.1164/rccm.200509-1380OC. [DOI] [PubMed] [Google Scholar]
- 98.Adcock IM, Tsaprouni L, Bhavsar P, Ito K. Epigenetic regulation of airway inflammation. Curr Opin Immunol. 2007;19(6):694–700. doi: 10.1016/j.coi.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 99.Yu-Fen L, Langholz B, Salam MT, Gilliland FD. Maternal and grandmatrenal smoking patterns are associated with early childhood asthma. Chest. 2005;127:1232–1241. doi: 10.1378/chest.127.4.1232. [DOI] [PubMed] [Google Scholar]
- 100.Jakiela B, Brockman-Schneider R, Amineva S, Lee W-M, Gern JE. Basal Cells of Differentiated Bronchial Epithelium Are More Susceptible to Rhinovirus Infection. Am. J. Respir. Cell Mol. Biol. 2007:2007–0050OC. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lopez-Souza N, Dolganov G, Dubin R, et al. Resistance of differentiated human airway epithelium to infection by rhinovirus. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L373–381. doi: 10.1152/ajplung.00300.2003. [DOI] [PubMed] [Google Scholar]
- 102.Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. Journal of Clinical Epidemiology. 2001;54:217–224. doi: 10.1016/s0895-4356(00)00305-x. [DOI] [PubMed] [Google Scholar]
- 103.Silverstein MD, Mair JE, Katusic SK, Wollan PC, O'Connell EJ, Yunginger JW. School attendance and school performance: a population-based study of children with asthma. Journal of Pediatrics. 2001;139(2):278–83. doi: 10.1067/mpd.2001.115573. [DOI] [PubMed] [Google Scholar]
- 104.Morgan WJ, Crane E, Gruchalla R, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]