Abstract
Sequence-specific RNA-binding proteins (RBPs) bind to pre-mRNA to control alternative splicing, but it is not yet possible to read the ‘splicing code’ that dictates splicing regulation on the basis of genome sequence. Each alternative splicing event is controlled by multiple RBPs, the combined action of which creates a distribution of alternatively spliced products in a given cell type. As each cell type expresses a distinct array of RBPs, the interpretation of regulatory information on a given RNA target is exceedingly dependent on the cell type. RBPs also control each other’s functions at many levels, including by mutual modulation of their binding activities on specific regulatory RNA elements. In this Review, we describe some of the emerging rules that govern the highly context-dependent and combinatorial nature of alternative splicing regulation.
The split arrangement of protein-coding information in DNA and its reconstitution by RNA splicing after transcription remain one of the most intriguing features of eukaryotic genome structure and evolution. The seemingly inefficient strategy of keeping introns in a gene only to transcribe and remove them using a large, complex macromolecular machine (that is, the spliceosome) must have provided evolutionary advantages1,2, particularly during the rise of metazoan organisms. Such advantages may have included protection from mutation and transposons, expanded and flexible genomic coding capacity, protein diversity and opportunities for regulation3. Large-scale surveys of alternative splicing reveal its pervasive impact on the transcriptomes of Drosophila melanogaster4 and other metazoans5,6. In present-day mammalian genomes, RNA splicing has evolved to serve complex regulatory functions that are coupled with other gene expression steps7–9.
The splicing reactions are carried out in the spliceosome — a dynamic RNA–protein complex with a highly regulated functional cycle found in all eukaryotes10. This sophisticated RNA–protein enzyme has remarkable plasticity in substrate recognition and can incorporate different parts of a pre-mRNA into a mature mRNA under the influence of a large number of regulatory proteins, many of which have the ability to directly bind to the pre-mRNA. Through alternative splicing, pre-mRNA transcripts can be processed to produce mRNA isoforms with different stability or coding potential, thus allowing quantitative control of protein production and synthesis of related proteins with qualitatively distinct functions11.
The large number of splicing regulatory proteins that recognize distinct RNA sequences across the transcriptome suggests the existence of a ‘splicing code’ (REF. 12), which could be used to predict splicing responses in different cell types under different experimental conditions. However, the depths of the complexity of this code have yet to be understood and measured. Deciphering the splicing code will require a comprehensive list of splicing regulatory RNA-binding proteins (RBPs) and their cis-acting binding sites. A major challenge is that the same genomic sequences are recognized differently by a given RBP in different cell types, partly depending on which other RBPs are expressed in that cell type (see below). Many proteins may function in complex with other proteins, thus influencing the binding specificity of one another. Complicating the problem, the same short degenerate RNA sequence motif can be recognized by multiple different proteins or protein isoforms. Furthermore, there is no simple division of positive and negative splicing regulators, as this behaviour frequently depends on the location of the binding site relative to the regulated exon. Attempts to predict splicing regulation from sequence features and tissue splicing data through computer learning approaches13,14 have so far been limited by the fact that tissues are composed of multiple cell types with superimposed splicing responses, as well as by the inability to assign a unique protein to a unique RNA sequence motif.
As a complete understanding of alternative splicing and its regulation will be necessary for comprehending its biological impact on developmental and disease processes15–18, our goal in this Review is to outline the emerging regulatory rules with attention to their limitations. Future experimentation will be necessary both for understanding the mechanisms of splicing regulation and for identifying the genomic location at which each regulator functions in different cell types.
Defining the regulatory landscape
When considering splicing regulation, it is helpful to distinguish between two classes of splicing events: first, constitutive splicing events that are recognized efficiently by the spliceosome and that are spliced the same way in each pre-mRNA from a given gene; second, alternative splicing events, in which recognition and joining of a 5′ and 3′ splice site pair are in competition with at least one other 5′ or 3′ splice site (FIG. 1a). Accordingly, cis-acting RNA elements involved in splicing reaction and regulation may also be divided into two classes. The first comprises the pre-mRNA reactive sites known as splicing signals, including the 5′ splice site, the branchpoint and the 3′ splice site, each of which is strictly required by the spliceosome for substrate recognition and catalysis10, whereas the second class comprises other RNA elements — collectively known as splicing regulatory elements (SREs) — which are often target sites for trans-acting factors.
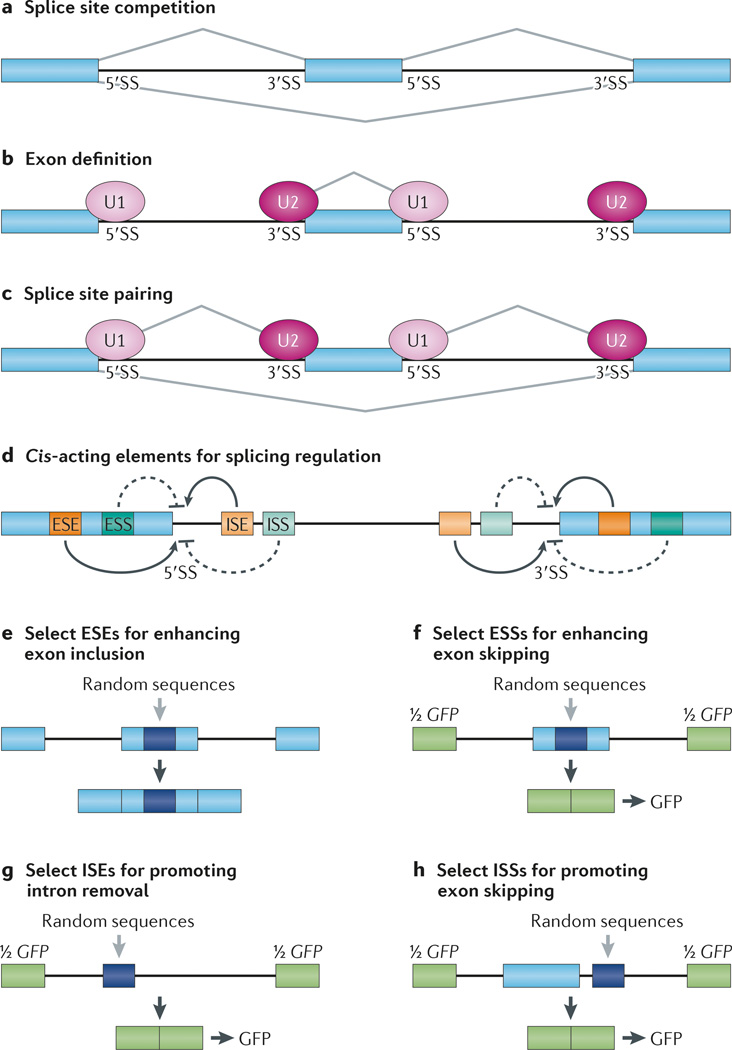
Figure 1. Regulation of alternative splicing by cis-acting enhancers and silencers.
a | A pre-mRNA substrate has two 5′ splice sites (5′SSs) and two 3′SSs that are engaged in competition with each other for assembly of splicing complexes, which causes the alternative exon in the middle to be either included or skipped in the final mRNA products. In a given cell type, the ratio of included and skipped isoforms varies, and such variation is regulated by a large array of splicing regulators. b | Most exons in mammals, including both constitutive and alternative ones, are recognized by U1 small nuclear ribonucleoprotein (snRNP) binding at the 5′SS and U2 snRNP binding at the 3′SS that enhance one another. This exon definition process determines the selection of functional splice sites in the genome, which is subjected to modulation by various RNA-binding splicing regulators. c | Upon initial splice site selection, functional splice sites are paired to allow subsequent steps of spliceosome assembly to occur. Such a pairing process can also be regulated to generate alternatively spliced mRNA isoforms. d | Cis-acting RNA elements that positively or negatively influence splice site selection are shown. Depending on their locations and functions, they are referred to as exonic splicing enhancers (ESEs), exonic splicing silencers (ESSs), intronic splicing enhancers (ISEs) and intronic splicing silencers (ISSs). e | A selection of ESEs from a random sequence library are inserted into an alternative exon in a splicing reporter. In this setting, the middle exon is weakly included. From a random pool of sequences that have been individually inserted in the alternative exon, specific ESEs can be isolated, amplified and reselected from the mRNA product pool generated by in vitro splicing or from transfected cells that contain the alternative exon. f–h | Selection of ESSs (part f), ISEs (part g) and ISSs (part h) are shown. In each case, a sequence from a random sequence library is inserted into different locations in a splicing reporter that splits the GFP gene (green) into two halves. Upon transfection into an experimental cell line, GFP-positive cells are selected, and the pool of inserted sequences are then amplified by PCR and sequenced to identify sequence motifs that can modulate splicing.
The context and positional relationship of these signals are paramount to their function. For example, a weak 3′ splice site is more efficiently recognized when there is a strong functional 5′ splice site within 100–150 nucleotides downstream through a phenomenon called exon definition19, whereby splice sites influence each other’s recognition by the U1 small nuclear ribonucleoprotein (snRNP) at the 5′ splice site and the U2 snRNP at the 3′ splice site20 (FIG. 1b). Exon definition for genes with complex architecture is followed by splice site pairing, which is another key regulatory step in alternative splicing (FIG. 1c). Importantly, the accessibility of any sequence-based RNA signal will depend on the folding landscape of the sequence environment in which it is embedded21. This has been exemplified by long-distance RNA–RNA interactions that facilitate pairing of a common exon to different alternative exons22. Recent fine-mapping of RNA secondary structure in yeast, plant and mammalian genomes23–25 provides rich information for associating local RNA structure with function of specific RBP-binding sites in an effort to refine the prediction of functional sites from genomic context.
In principle, regulatory elements may act from any location in a pre-mRNA, but most studies focus on 200–300 nucleotides adjacent to observed splice sites, which seem to have the most identifiable sequence features26. However, a recent study showed that remote regulatory sequences are just as important as those proximal to a functional splice site27, which suggests a major limitation associated with current experimental and computational approaches in defining functional RNA elements in mammalian genomes. In fact, many long introns in mammals may carry more regulatory information than we currently appreciate. It is now more feasible to dissect these distant regulatory regions by genome engineering using CRISPR–Cas (clustered regularly interspaced short palindromic repeat– CRISPR-associated) technology28,29. Mutations of SREs in the proximal intron are known to contribute to many human diseases, which indicates that more such mutations might be found in distal regions. This highlights a major limitation of exome sequencing efforts, which will not detect crucial disease-associated mutations that may be found by complete sequencing of patient genomes. Below, we focus on the class of SREs that act by capturing specific RBPs to achieve splicing regulation.
Dissecting SREs on model genes
Two approaches have traditionally been used to identify and dissect cis-acting SREs, which can be divided into exonic splicing enhancers (ESEs), exonic splicing silencers (ESSs), intronic splicing enhancers (ISEs) and intronic splicing silencers (ISSs) (FIG. 1d). As extensively reviewed previously30, serial deletion constructs can be analysed to expose positive and negative SREs or to characterize putative SREs in a heterozygous exon. Mutational analyses of model alternative splicing substrates can be informative for single cases but suffer from limited generalizability owing to undefined contributions of sequence context. Furthermore, it is often unclear how results from mini-gene constructs relate to sequence elements and functions in the endogenous gene in its natural genomic context.
The second approach is in vitro selection of RNAs that bind to a splicing regulatory protein of interest to identify sequences that resemble those found in natural pre-mRNAs. These studies often use stringent in vitro binding conditions that result in overspecification of a sequence that does not represent the more diverse set of natural sites that function in vivo. A new method that selects sequences with high rates of RNA binding in a kinetic capture experiment has revealed sequence specificity that is not found using thermodynamic end-point binding studies31. Kinetic sequence preferences are beyond the reach of widely used methods, and yet many of these have important regulatory roles. Even so, the general limitation with single model reporters is that most regulatory contexts arise from multiple overlapping sets of RNA elements and that one reporter only samples the activity of the regulator under study in only one such context. More inclusive global approaches that are now widely used in the field (see below) have the potential to offset and deconvolute some of these limitations in defining the regulatory landscape in a genome.
In silico capture of regulatory RNA elements
Computational approaches have been used to filter out some regulatory sequence features. One study attempted to identify sequence motifs representing elements that promote exon inclusion (that is, ESEs) by postulating that they would be needed to compensate for exons with weak matches to the consensus sequences at the 5′ and 3′ splice sites. A set of motifs were enriched in exons with weak splice sites32, some of which were further linked to specific features in the splice sites33. Related studies compared internal non-coding exons to pseudoexons and 5′ untranslated regions (UTRs) of intronless genes34,35; alternatively, sequence conservation at wobble positions can be taken into consideration to minimize the confounding effect of protein-coding information36. Except in cases of alternative 5′ or 3′ splice site usage in which an intronic regulatory sequence may overlap with an extension of an exon, the superposition of splicing regulatory information and protein-coding information does not interfere with identification of ISEs and ISSs.
Motif collections deduced by different computational methods are not identical, which suggests that not all features associated with regulatory RNA sequences are represented by any one approach. In addition, many computationally identified sequences cannot be easily matched to a known RBP. The same motifs are also sometimes found to function as either an enhancer or a silencer in different locations in the same exon36. The poor reliability of any single prediction, together with the challenge in experimentally connecting a motif to a particular regulator, limits the usefulness of computational approaches for dissecting biological functions.
Reporter-based sequence library screens
Diverse sequence populations can be screened for function as SREs using reporters that have been engineered to accept randomized oligonucleotides at positions within or near an alternative exon. For example, to identify ESEs, each sequence from a library can be inserted in the internal alternative exon of a three exon splicing reporter to select specific sequences that can enhance the inclusion of the alternative exon in an in vitro splicing reaction37 or in transfected cells38 (FIG. 1e). Common motifs can then be deduced from these sequences and further characterized as ESEs, which are largely purine-rich, consistent with their functions as SR protein-binding sites. However, there are other non-purine-rich sequences, which suggests the existence of other types of ESEs. Again, these results are constrained by the context of both the chosen reporter and the cell type in which the selection is carried out, as well as by the uncertainty in pinpointing the specific trans-acting regulatory RBPs involved.
More elaborate strategies have used GFP-based vectors in transfected HEK293T cells, in which a short randomized sequence from a library was inserted into an efficiently included internal exon that prevents GFP expression, and sorting of GFP-positive cells allowed recovery of ESSs39 (FIG. 1f). More recently, this approach was adapted to screen for ISEs or ISSs by inserting a random sequence from a library into a weak intron or a location downstream of an internal exon that splits the GFP gene40,41 (FIG. 1g,h). These approaches yielded a large collection of sequences that function as putative SREs, many of which resemble binding sites for some known splicing regulators. A major caveat to these approaches is that the motifs might function only in the specific contexts (reporter gene and cell type) in which the selection was carried out. This was underscored in a recent screen in which library sequences were inserted into two different positions in a three exon reporter with different outcomes42. Thus, although these screens provide valuable information on SREs, they offer little insight into the problem of context.
With each new SRE sequence there is the question of which RBP binds to it. However, affinity purification of RBPs using SREs shows that a one to one relationship between an SRE and an RBP is not always the case40,41. As association under native conditions used in these studies does not distinguish between direct and indirect interactions, some of the identified proteins may interact indirectly with RNA through protein–protein interactions. In any case, these possibilities create ample mechanistic scenarios to positively or negatively modulate splice site selection in the cell.
High-throughput approaches
It is crucial to answer the following questions: how many proteins encoded in mammalian genomes have the capacity to bind to RNA, and how many of them are involved in splicing control? Recent proteomic studies indicate that up to 1,000 proteins can be directly crosslinked to mRNA in HeLa cells or mouse embryonic stem cells43,44. Interestingly, many of these proteins do not contain known RNA-binding domains that are similar to those in the known repertoire of RBPs45. As the first step to characterize their roles in splicing regulation or RNA metabolism in general, we need to know the RNA-binding site sequences for known RBPs. A high-throughput methodology was recently applied to this question46. In this study, individual purified RBPs (although in most cases only known RNA-binding domains were expressed instead of whole proteins) were incubated with a pool of RNA oligonucleotides. After affinity selection, enriched sequences were identified by microarray, and the consensus motifs were deduced. This information enables inference of potentially functional RNA elements in the genome and lends itself to experimental tests. This approach can be coupled with system-wide RNA interference (RNAi) and splicing profiling to deduce a full complement of splicing regulators in a genome47,48.
As RBPs exert their function partly by binding to pre-mRNA, the knowledge of where and when they bind could help to define the context in which splicing regulation takes place. Transcriptome-wide identification of binding sites can be sampled for a given RBP using the crosslinking and immunoprecipitation (CLIP) method, which detects direct RNA–protein interactions because ultraviolet (UV) radiation induced crosslinking requires the RNA and the protein to be within ~1 Å of each other49,50. After UV crosslinking in live cells or extracts, immunoprecipitation and denaturing gel electrophoresis can be used to purify covalently linked protein–RNA adducts. RNA is then recovered, sequenced and mapped back to the genome. Since the initial development of the CLIP technology, several refinements have been made to increase the crosslinking efficiency (such as photoactivatable ribonucleoside-enhanced CLIP (PAR-CLIP))51 or the mapping precision (such as individual-nucleotide-resolution CLIP (iCLIP))52. Although each method has intrinsic caveats, such as variable crosslinking efficiencies and recovery biases at different RNA sites, the CLIP approach greatly enables qualitative identification of specific binding sites and deduction of consensus binding sequences, and it also allows specific binding events (or patterns) to be linked to function (TABLE 1). This approach is particularly powerful if the derived information is cross-analysed with high-throughput analyses of binding specificity by individual purified RBPs46,53. Owing to its power to identify cis-acting regulatory RNA elements in mammalian genomes, multiple regulatory principles have emerged from the use of the CLIP technology.
Table 1.
Genomic landscape of splicing regulators as revealed by CLIP experiments
| Splicing factors | Binding profile and functions | Motifs* | Refs |
|---|---|---|---|
| Splicing regulators | |||
| CUGBP1 | Binds to intronic regions and antagonizes MBNL1 | UGUU | 166 |
| MBNL1 and MBNL2 | Bind to intronic regions with positional effects | YGCY | 83,167 |
| NOVA | Binds to both exons and introns with positional effects | YCAY | 79 |
| PTB and nPTB | Bind to intronic regions to mediate splicing repression | CU rich | 68,123 |
| RBFOX1 and RBFOX2 | Bind to intronic regions with positional effects | GCAUG | 82,168 |
| TIA-1 and TIAL1 | Bind to intronic regions with positional effects | U rich | 169 |
| QKI | Binds to intronic regions with positional effects | UAA | 51 |
| ESRPs | Bind to intronic regions with positional effects | GU rich | 87 |
| RBM4 | Binds to intronic regions with positional effects | CGG or CTAACG | 40,170 |
| RBM5 | Binds to intronic regions with positional effects | UCAUC or UGUAA | 118 |
| RBM6 and RBM10 | Bind to intronic regions with positional effects | CUCUGAA | 118 |
| ELAV1 | Binds to intronic and 3′ untranslated regions | U-rich or AU rich | 171–173 |
| 3′splice site factors | |||
| U2AF65 | Defines functional 3′ splice sites in most introns | Pyrimidine rich | 139 |
| SF1 | Binds to branchpoint sequences and other intronic regions | ACUNAC | 174 |
| SR proteins | |||
| SRSF1 | Enhances splicing on ESEs in a context-sensitive manner | GGAGGA | 58,61 |
| SRSF2 | Enhances splicing on ESEs in a context-sensitive manner | SSNG | 59 |
| SRSF3 | Has a slight preference for exonic over intronic regions | Pyrimidine rich | 58 |
| SRSF4 | Has a slight preference for exonic over Intronic regions | Purine rich | 58 |
| hnRNPs | |||
| hnRNP A1 | Binds to intronic regions to repress splicing on ESSs | GNNAGN | 78 |
| hnRNP A2 and hnRNP B1 | Bind to intronic regions to repress splicing on ESSs | GGUAGU | 78 |
| hnRNP C | Binds to intronic regions and competes with U2AF65 at Alu sequences | Pyrimidine rich | 52 |
| hnRNP F | Binds to intronic regions to enhance or repress splicing | GU rich | 78 |
| hnRNP H1 | Binds to intronic regions to enhance or repress splicing | G run | 78 |
| hnRNP L | Binds to intronic regions to enhance or repress splicing | CA rich | 175,176 |
| hnRNP M | Binds to intronic regions to enhance or repress splicing | GGUGG | 78 |
| hnRNP U | Binds to intronic regions to regulate U2 snRNP maturation | GU rich | 78,92 |
CLIP, crosslinking and immunoprecipitation; CUGBP1; CUG-binding protein 1; ELAV1, Elav-like protein 1 (also known as HuR); ESE, exonic splicing enhancer; ESRP, epithelial splicing regulatory protein; ESS; exonic splicing silencer; hnRNP, heterogeneous nuclear ribonucleoprotein; MBNL1, muscleblind-like protein 1; nPTB, neural polypyrimidine tract-binding protein (PTB); QKI, protein quaking; RBFOX1, RNA-binding protein fox 1 homologue 1; RBM4, RNA-binding protein 4; SF1, splicing factor 1; snRNP, small nuclear ribonucleoprotein; SRSF1, serine/arginine-rich splicing factor 1; TIA 1, nucleolysin TIA 1 isoform p40; TIAL1, nucleolysin TIAR; U2AF65, U2 auxiliary factor 65 kDa subunit.
S represents C or G; R represents G or A; Y represents C or U; and N is any nucleotide.
Trans-acting splicing regulators
Our understanding of splicing regulation has benefitted tremendously from biochemical studies on in vitro splicing systems. Classic trans-acting splicing regulators identified are SR proteins and heterogeneous nuclear ribonucleoproteins (hnRNPs)54. The SR protein family can be further divided into core members and RS domain-containing polypeptides. SR proteins are widely viewed as positive splicing regulators that promote exon inclusion both by binding to exons and by recruiting U1 snRNP to the 5′ splice site and U2 auxiliary factor (U2AF) to the 3′ splice site through protein–protein interactions in early steps of spliceosome assembly55–57 (FIG. 2a,b). In general, SR proteins have a binding preference for purine-rich exonic sequences, and this observation is now largely confirmed by global mapping of protein–RNA interactions58–61.
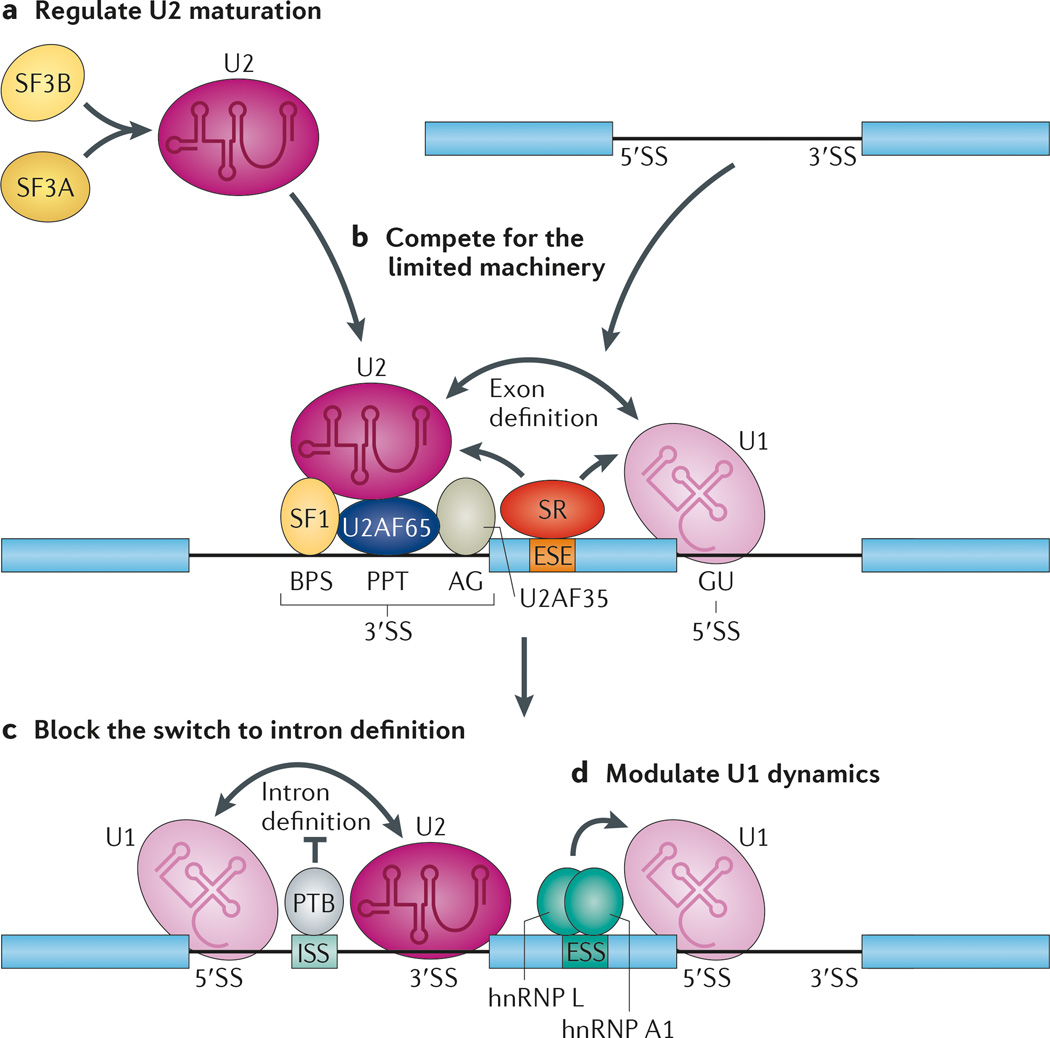
Figure 2. Regulated splicing through controlling the assembly of the core splicing machinery.
All splicing regulators must exert their activities, either directly or indirectly, through the core splicing machinery. a | Modulation of small nuclear ribonucleoprotein (snRNP) assembly can change the pool of active splicing components in the cell, thereby influencing alternative splice sites that are particularly sensitive to the levels of spliceosome components. b | The available functional splice sites in different pre-mRNAs — including the branchpoint sequence (BPS), polypyrimidine tract (PPT) and AG sequence in the 3′ splice site (3′SS), and the GU sequence in the 5′SS — are all competing for the splicing machinery. As a result, strong splice sites may titrate core components of the splicing machinery away from weak splice sites, thus indirectly influencing global splicing patterns in the cell. c | After initial recognition of splicing signals, the functional sites have to be efficiently paired for next steps of spliceosome assembly to take place, which also represents key steps for regulation. The binding of polypyrimidine-tract binding protein (PTB) to an intronic splicing silencer (ISS) inhibits intron definition. d | The U1 snRNP is about tenfold higher in abundance than other splicing snRNPs in mammalian cells. This may allow efficient recognition of most 5′SSs in both constitutive and alternative exons, as well as protection of inappropriate polyadenylation sites. However, the U1 snRNP has to be released later in the spliceosome cycle, which is essential for the formation of the catalytic core in the spliceosome. Interference of U1 release by heterogeneous nuclear ribonucleoprotein L (hnRNP L) and hnRNP A1 can thus also inhibit the selection of a regulated splice site, which acts as a repressive complex. ESE, exonic splicing enhancer; ESS, exonic splicing silencer; SF3A, splicing factor 3A; U2AF65, U2 auxiliary factor 65 kDa subunit.
The best-characterized hnRNPs involved in splicing control are negative regulators hnRNP A/B and polypyrimidine tract-binding protein (PTB; also known as hnRNP I). hnRNP A/B seems to antagonize the function of SR proteins62,63. The mechanism by which this is accomplished is unclear, as high-affinity hnRNP A/B-binding sites do not often overlap with SR protein-binding sites on exons. A potential mechanism involves cooperative binding of hnRNP oligomers that spreads along a transcript to prevent the binding of SR proteins over an extended region of RNA64,65. PTB has a binding preference for polypyrimidine sequences66, mostly in intronic regions, and this finding has also been confirmed by genome-wide mapping67,68. The binding specificity of PTB is similar but not identical to that of U2AF65, which promotes U2 snRNP binding to the intron, suggesting that PTB may interfere with functional recognition of the 3′ splice site at least for certain genes. PTB prevents onward spliceosome assembly after initial exon definition69,70 (FIG. 2c), and a more recent study indicates that PTB might additionally bind to U1 snRNA to cause an extended interaction between U1 and the 5′ splice site71. Extended U1–5′ splice site interaction has also been attributed to the splicing repressor activities of hnRNP L and hnRNP A1 (REF. 72). Although this interaction is subtle and non-rate limiting in most cases, under other conditions this extended base-pairing may be kinetically manifested to shift net splice site choice markedly73 (FIG. 2d). These findings underscore the fact that there is no substitute for biochemistry to understand the mechanism of splicing regulation and that much remains to be done to illuminate the mechanisms of action of SRE-recognizing RBPs. A wide range of functional screens and differential expression profiling strategies have successfully identified tissue-specific trans-acting splicing regulators and have demonstrated the roles of epithelial splicing regulatory proteins (ESRPs)74, hnRNP LL in the control of T cell signalling75,76 and nSR100 in the development of the nervous system77.
Although the simple ‘division of labour’ between SR proteins as positive splicing factors and hnRNPs as negative ones is generally useful, it does not hold true in every case. Splicing profiling experiments have now shown roughly equal numbers of induced exon inclusion and skipping events in cells treated with small interfering RNA (siRNA) against SR proteins59 or hnRNPs67,68,78. This is also true for several well-studied tissue-specific splicing regulators, such as NOVA79,80, RBFOX81,82, muscleblind-like proteins (MBNLs)83,84, protein quaking (QKI)85 and ESRPs86,87. Although some of the documented events may be indirectly affected by depletion of a splicing factor, biochemical characterization, motif enrichment analyses and CLIP all reveal binding sites that are indicative of direct action. Despite these advances, it is clear that we are still far from deciphering all the regulatory rules and mechanisms for both known and new splicing regulators.
Binding and context: rules
Numerous splicing factors have now been characterized, and they seem to share sets of behaviours. Below, we summarize these behaviours in the form of rules. The general message is that all regulatory activities of RBPs are coordinated with the function of the core splicing machinery to first define functional splice sites and then to modulate selective pairing of differentially defined splice sites.
Core machinery threshold for splice site recognition
A common feature of alternative splice sites is their divergence from the consensus derived from ‘strong’ constitutive splice sites, which has been considered to render them ‘weaker’ than typical constitutive splice sites. Consistent with an important regulatory role preserved during evolution, weak alternative splice sites tend to be more conserved than constitutive splice sites88. This suggests that the sequence context sets idiosyncratic constraints for the core splicing machinery to recognize alternative splice sites, which would then be influenced by regulatory factors. This implies that the level or functionality of the core splicing machinery also contributes to splicing control (FIG. 3a), and this possibility may explain why mutations in the core splicing machinery lead to highly specific phenotypes and diseases. Accordingly, depletion of some core components of the spliceosome in RNAi-treated cells shows selective effects on certain alternative splicing events48,89, which could reflect the fact that some RBPs interact with specific core components of the spliceosome to achieve regulation. For example, during 5′ splice site selection, the RBP nucleolysin TIA 1 isoform p40 (TIA 1) functions through the U1 snRNP protein U1 C90, and the RBP P-element somatic inhibitor (PSI) exerts its effect through U1 70K91. In cassette exons where the proximal splice sites are regulated, this rule predicts that a compromised core splicing machinery would induce exon skipping to a large extent, which seems to be the case in a study that broadly examined the splicing responses upon reducing the level of a specific U2 snRNP component92.
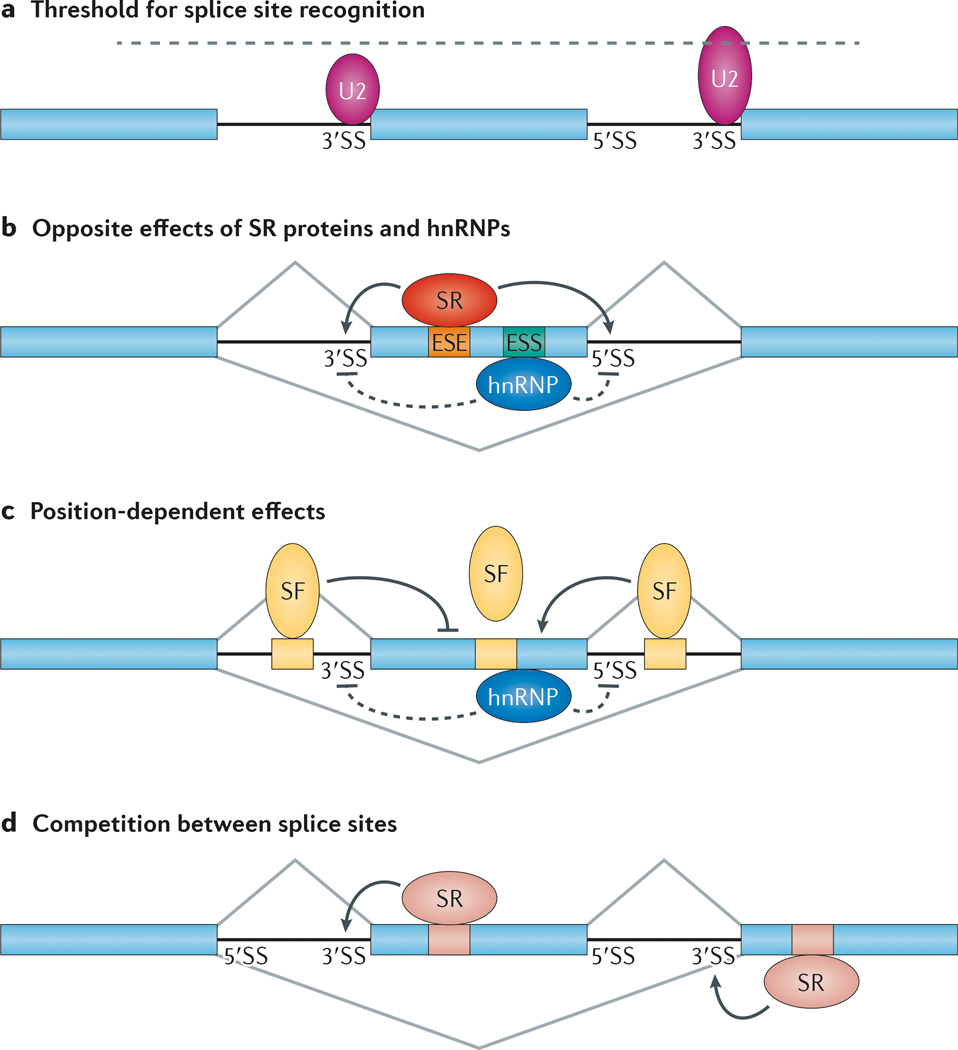
Figure 3. Rules for context-dependent and position-sensitive regulation of alternative splicing.
a | In general, the alternative exon (in the middle) is associated with weak splice site signals, which render partial selection by core components of the splicing machinery. A small U2 small nuclear ribonucleoprotein (snRNP) is illustrated to show its inefficient interaction with the alternative splice site. The flanking sites, which are strongly recognized by the splicing machinery, are engaging in competition with the alternative splice sites. A large U2 snRNP is illustrated to show its strong interaction with the flanking 3′ splice site (3′SS). According to the threshold rule, a partially compromised splicing machinery would selectively affect the alternative splice sites relative to the competing sites, thus leading to induced exon skipping. b | Exon-bound SR proteins and heterogeneous nuclear ribonucleoproteins (hnRNPs) have opposite effects on the recognition of the adjacent splice sites by the core splicing machinery. c | For many sequence-specific splicing factors (SFs) — for example, NOVA — their binding on exons generally inhibits the selection of the alternative exon. They are inhibitory when they are bound to an upstream intronic region but enhance the selection of the alternative exon when bound to a downstream intronic region. The mechanism for the polarity effect remains to be fully understood. d | Enhanced recognition of the alternative splice site induces exon inclusion, whereas increased recognition of the flanking competing splice site causes exon skipping. ESE, exonic splicing enhancer; ESS, exonic splicing silencer.
The differential response of splicing substrates to depletion of core components of the splicing machinery suggests that competition between pre-mRNAs for the available splicing machinery may influence splicing across the whole transcriptome. Consistent with this, extensive changes in pre-mRNA pools in budding yeast that reduce demand for the splicing machinery increase splicing efficiency for poorly spliced transcripts93. The phenomenon whereby changes in the activity of core splicing components alter the entire balance between competing splicing events may underlie numerous specific diseases, such as retinitis pigmentosa94, spinal muscular atrophy95 and myelodysplasia96.
Opposite effects from exon versus intron binding
In general, hnRNPs inhibit splicing when binding to exons but can have either positive or negative effects on splicing when bound to intronic sites. This is best illustrated with hnRNP L and hnRNP H/F. On several model substrates, hnRNP L represses or enhances splicing by binding to CA rich motifs in exons97,98 or introns99,100, respectively. hnRNP H binds to intronic G rich elements to promote the selection of nearby 5′ splice sites101,102 but represses splicing when bound to exons103–105. In many cases, hnRNP H seems to function together with the related hnRNP F105–108.
This rule has been generalized by tethering several well-studied SR proteins and hnRNPs to either side of a regulated 5′ splice site in a three exon reporter109 (FIG. 3b). An SR protein tethered to an intronic region upstream of the functional 5′ splice site interferes with exon definition, which mimics the activity of natural decoys or pseudoexons in regulated splicing14,110,111. By contrast, an hnRNP tethered to an exon represses productive splicing in a similar manner to PTB69,70, and the mechanism involved formation of non-productive complexes that prevent subsequent splice site pairing109. However, there are exceptions to this general rule because positioning of an SR protein-binding site in two different regions in an alternative exon elicited opposite splicing responses36; similarly, the inhibitory effect of exon-bound hnRNP L could be switched to a positive one when an adjacent sequence was mutated112. These observations suggest that the positional effect is also subject to modulation by sequence context that has yet to be defined.
Intron binding position and polarity
The positional effect of splicing regulators in different intronic locations was first illustrated with NOVA79. In this study, NOVA activated exon inclusion when binding downstream of the alternative exon but repressed its inclusion when binding upstream of the exon. Subsequently, these polarity effects were observed with multiple splicing regulators based on CLIP–seq maps and motif enrichment analyses that link their binding events to functional responses (FIG. 3c; TABLE 1).
The molecular mechanism for this phenomenon remains to be fully understood, and this represents a key area for future experimentation. A recent study using tethered RBFOX1 showed that the carboxy-terminal region of this protein was sufficient to promote exon inclusion when tethered downstream of the alternative exon, but both its RNA-binding domain and the carboxy terminal region seemed to be essential for repression when tethered upstream of the alternative exon, which indicated distinct requirements for the activating and repressing functions of RBFOX1 in splicing regulation113. Although several other proteins — including hnRNP H, a newly identified splicing regulator TFG and the mRNA export factor RALY — have all been found to bind to RBFOX1, none of these factors showed selectivity in RBFOX1 mediated exon inclusion or skipping in different intronic locations. Thus, both the mechanism and the auxiliary proteins that might be involved to account for the position–polarity effect remain elusive. In this regard, it is interesting to note that a dominant-negative RBFOX protein was found to interfere with exon activation but not repression, which indicates that repression might be mediated by other factors at or near some RBFOX-binding sites114. This may also provide an explanation for the negative regulation of apparent MBNL-regulated splicing before the induction of MBNL expression in embryonic stem cells115. It is also important to emphasize that this positional rule needs to be further generalized because some reporter-selected SREs seem to activate inclusion both when they are placed in the upstream intron and when they are in the downstream intron40.
Effect of binding on distal competing sites
Splice site selection is almost never an isolated event that solely depends on the intrinsic properties of the splice site; more often, it occurs in cases in which two or more potential splice sites are engaged in competition. Using an alternative cassette exon as an example (FIG. 3d), the 5′ splice site of the upstream exon competes for the 3′ splice sites of both the internal exon and the downstream exon; similarly, the 3′ splice site of the downstream exon competes for the 5′ splice sites of both the upstream exon and the internal exon. In principle, inclusion of the internal exon could be enhanced by strengthening the recognition of either its 5′ splice site or its 3′ splice site, or by weakening the recognition of either the 5′ splice site on the upstream exon or the 3′ splice site of the downstream exon. By tethering an SR protein to the internal exon or to a flanking competing exon of model genes, the predicted opposite effects were achieved. The presence of the SR protein on the internal exon promoted its inclusion, but the opposite result (that is, repression of the internal exon) was obtained when the SR protein was tethered to either of the adjacent exons116,117.
Similarly, when a repressive PTB-binding site was placed near the 5′ splice site of the upstream exon or the 3′ splice site of the downstream exon, the inclusion of the internal exon was enhanced in a PTB-dependent manner68. This is consistent with the positive effect of PTB when tethered in an intronic location in a related study67. Interestingly, opposite to the effect of PTB, RNA-binding protein 6 (RBM6) seems to promote exon skipping by enhancing the selection of the competing flanking splice site118. Therefore, an intronic splicing repressor or activator may be selectively directed to one of the competing sites to induce exon inclusion or skipping. This rule may apply to several other hnRNPs and could account for the roughly equal numbers of induced exon inclusion and skipping events in response to RNAi-mediated depletion of traditionally classified positive and negative splicing regulators in the cell59,78.
In concluding this section, it is important to emphasize that regulatory RBPs must function in conjunction with specific components of the core splicing machinery to modulate splice site selection and pairing (FIG. 2). Therefore, regulated splicing is a highly orchestrated biochemical process in mammalian cells.
Strategies to play on the rules
It has become increasingly evident that splicing regulators themselves are also subjected to regulation. Below, we highlight the emerging mechanisms that underlie combinatorial splicing controls, which have previously been highlighted119. More recent studies further emphasize that the consequences observed in any loss of function study are likely to reflect not only the loss of function of the factor under investigation but also the effect of perturbing its cooperating factors, thus providing an opportunity for gain of function conditions for other splicing regulators. These mechanisms are likely to provide an explanation for the composite and complex phenotypes observed in splicing-factor-knockout animals80,120–123.
Cooperativity between splicing regulators
An earlier study provided evidence for cooperativity between SUP 12 and the worm homologue of RBFOX1 in the regulation of tissue-specific splicing in Caenorhabditis elegans124. A systematic study of co occurring regulatory motifs in mammals also identified a global relationship between NOVA and RBFOX family proteins125. One of the best-characterized cases is perhaps the cooperativity between two T STAR family members in C. elegans, in which ASD 2 and SUP 12 act together on the unc 60 gene to regulate its splicing in muscle126. These two related splicing factors each bind to a separate motif with low affinity, but one binding event enhances the other, thereby creating a synergy between the two factors. This proves to be required for muscle-specific processing of their target genes. Another recent study documented extensive overlap between the splicing regulatory networks of PTB and QKI in muscle cells85, which indicates the broader regulatory potential of such interactions. These findings illustrate synergistic interactions both between the same family of RBPs and between different families of RBPs in regulating their target genes (FIG. 4a), and our knowledge of such partnerships is almost certain to be expanded in the future.
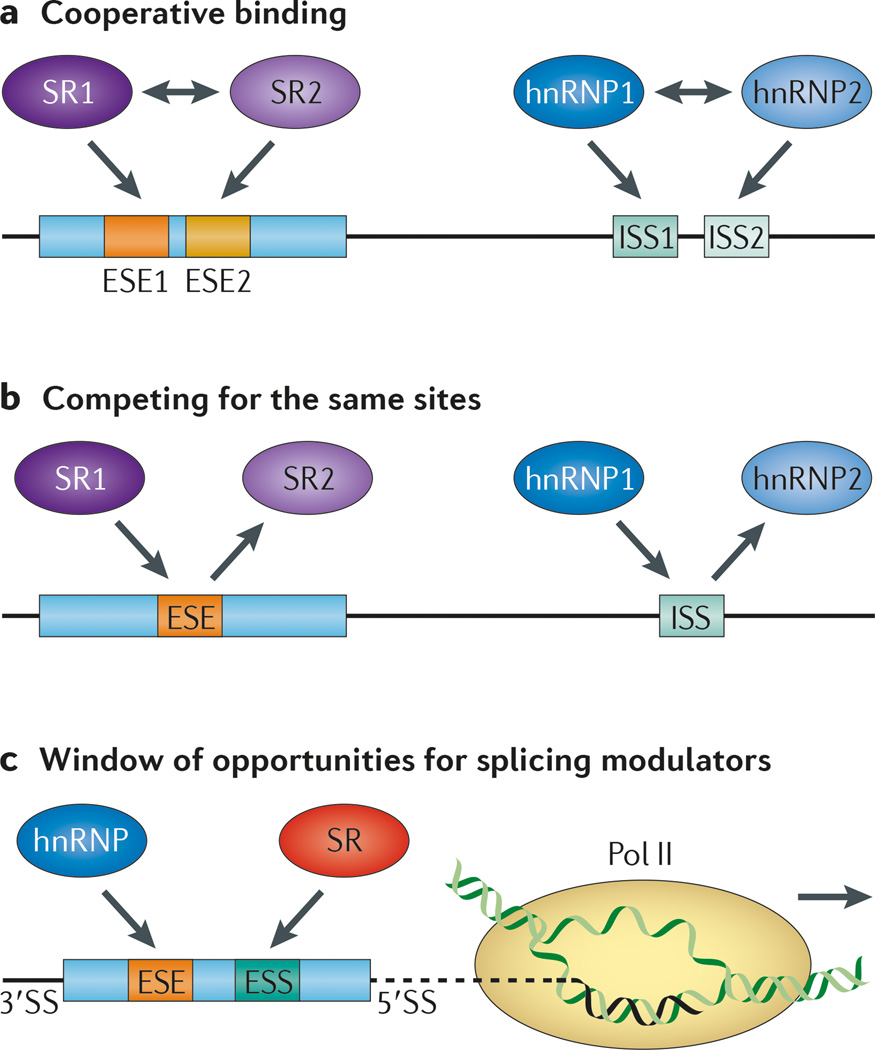
Figure 4. Cooperation and competition of splicing factors in regulated splicing.
a | Distinct splicing regulators may bind to adjacent splicing regulatory elements (SREs) in a cooperative manner, which may be mediated, at least partly, by their protein–protein interactions (indicated by two-headed arrows). b | The same cis-acting SREs, regardless of whether they are located in exons or introns, may be recognized by related RNA-binding splicing regulators. This results in competition between related splicing regulators. c | An increasing amount of evidence suggests that the speed of transcription elongation may influence splice site selection, which may create window of opportunities for both positive and negative splicing regulators to recognize their binding sites co transcriptionally, thus modulating alternative splicing during these integrated events in gene expression. 3′SS, 3′ splice site; ESE, exonic splicing enhancer; ESS, exonic splicing silencer; hnRNP, heterogeneous nuclear ribonucleoprotein; ISS, intronic splicing silencer; Pol II, RNA polymerase II.
An important aspect of cooperative action of RBPs on their respective binding sites is protein–protein and protein–RNA interactions. Interestingly, many splicing factors, especially those involved in early spliceosome assembly steps, seem to have intrinsically disordered regions (that is, segments that lack a stable protein structure in solution) — a property that may allow these proteins to adopt functional confirmations after they are engaged in interactions with their binding partners127. Recent studies show that low-complexity domains that are characteristic of numerous RBPs can nucleate protein– protein networks in the form of hydrogels128,129, which might underlie the organization of not only various types of RNA granules but also the splicing machinery, as well as the formation of pathological aggregates in neurological diseases, such as amyotrophic lateral sclerosis and Parkinson’s disease17,130,131.
Tissue specificity and regulation by family members
Different RBP family members bind to the same RNA target sequence but have distinct biochemical properties that might create distinct splicing regulatory outcomes in different tissues or developmental stages. The best-studied example of this is PTB and neural PTB (nPTB): PTB is expressed in most cell types and tissues, whereas nPTB expression is highly restricted to the nervous system132. Such tight tissue specificity is maintained by PTB autoregulation and by PTB-mediated repression of nPTB in non-neuronal cells133–135. Interestingly, the regulated expression of PTB seems to have a central role in neuronal induction134,136, and the induced nPTB expression is eventually diminished in matured neurons137. These programmed switches are likely to create a sequentially regulated splicing programme during development of the nervous system122,123.
Context-dependent cooperation and competition
As distinct splicing factors can have overlapping sequence recognition specificity, they may collaborate or compete in different contexts. Context-dependent splice site recognition has been extensively studied in a neuron-specific exon at which a splicing regulator selectively operates in combination with certain 3′ splice site features to achieve regulation138. A more recent study knocked down one SR protein and monitored binding of another SR protein in the mouse genome. On certain regulated exons, loss of one SR protein was found to cause reduced binding of another SR protein but, at other locations, loss of one SR protein allowed another SR protein to bind more efficiently59. These observations indicate that SR proteins act both cooperatively and competitively in the regulation of alternative splicing in mammalian cells (FIG. 4a,b). Such cooperativity and competition may also apply to various hnRNPs and other splicing regulators. For example, a recent study showed that hnRNP C competes with U2AF65 on some Alu-associated 3′ splice sites and, as a result of hnRNP C removal, U2AF65 gained the ability to activate a large set of exons from expressed Alu transcripts139.
Cross-regulation among splicing regulators
Given such complex relationships in controlling splicing, it is not surprising that RBP expression is frequently autoregulated and cross-regulated, thus creating extensive regulatory networks in the cell140–142. SR proteins have long been known to regulate their own expression at the level of splicing, which seems to be a key mechanism for homeostatic expression of most SR proteins in mammalian cells and tissues143–145. A network of potential cross-regulatory interactions has also been observed in an integrated study of hnRNPs, in which different hnRNP-encoding genes were found to contain binding sites for other hnRNPs; in conjunction with protein–protein interactions, it is conceivable that it is the cross-regulation that gave rise to the complex functional response in the cell78. Interestingly, in many loss of function studies, regulatory RBPs seem to be among the most affected, which may underlie the function and regulation of many RBPs, such as disease-associated FUS (also known as TLS) and TAR DNA-binding protein 43 (TDP 43), the mutations of which may be manifested into broad alterations in gene expression146,147.
Besides the regulation of RBPs by RBPs at the splicing level, altered transcription elongation rates might create windows of opportunity for different RBPs to gain increased kinetic access to their regulatory sequences on nascent transcripts during co transcriptional splicing7 (FIG. 4c). Conversely, many RBPs that control splicing also seem to function at other steps in the gene expression pathway. Of particular interest is the finding that the SR protein SRSF2 seems to be directly involved in regulating RNA polymerase II pause release from promoters and elongation in the gene body148,149, which further complicates the interpretation of the splicing responses upon depletion of this SR protein. Similarly, an increasing amount of evidence suggests that various hnRNPs are widely involved in the regulation of mRNA stability, subcellular localization and translational control through several mechanisms, including direct binding to mRNA 3′UTRs and regulating microRNA processing and function. It is therefore conceivable that splicing regulation in vivo represents a system-wide response to the composition of RBPs in different cell types or during development and disease processes. As transcription is thought to have various influences on mRNA splicing and other post-transcriptional regulatory events7,9, dissection of gene networks with an aim to understand the splicing regulation involved has now become a systems biology problem and represents a major new challenge in the research field.
Modulating splicing regulators
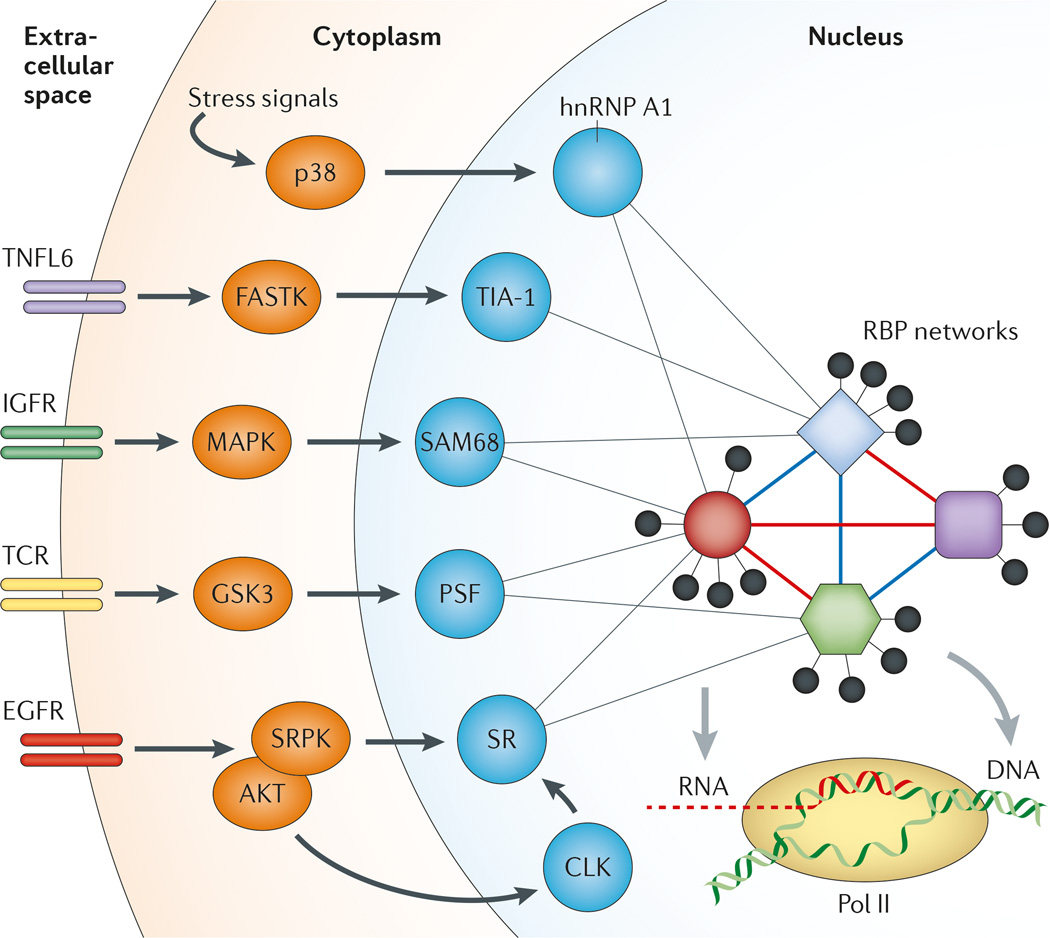
Splicing regulatory networks are subject to signalling events that control alternative splicing. This has been best illustrated by the Ca2+ ion-mediated regulation of alternative splicing of a calcium-activated potassium channel by calcium/calmodulin-dependent protein kinase type IV (CaMK IV)150. In response to membrane depolarization, an influx of Ca2+ ions triggers a splicing response through an RNA element called the CaRRE motif near the 3′ splice site. The process not only involves CaMK IV phosphorylation-regulated binding of hnRNP L151 but also requires some undefined transcription-induced splicing regulators. Most studies of signal-regulated splicing have so far been focused on the identification of specific signal-induced post-transcriptional modifications (mostly phosphorylation) on some known splicing regulators152,153. Key mechanistic insights have been gained through dissection of signal-induced splicing events on model genes (FIG. 5), including cellular sequestration of splicing regulators (for example, hnRNP A1 and EWS) in response to osmotic stress or DNA damage154,155, enhanced RNA-binding activity of an RBP (for example, SAM68) upon activation of the mitogen-activated protein kinase (MAPK) pathway156 or in response to depolarization157, conversion from an activator to a repressor in response to heat shock158, release from inhibitory protein complexes (for example, PTB-associated splicing factor (PSF) and hnRNP L)97,159 and/ or transcriptional induction of a cofactor during T cell signalling75,76. In general, however, we only have little understanding of how a specific signalling event might trigger system-wide changes in control of splicing regulators and how such changes might in turn induce global changes in alternative splicing.
Figure 5. Signal transduction to regulate alternative splicing in the nucleus.
Both internal and external signals can influence alternative splicing through post-transcriptional modification of specific splicing regulators as illustrated, which may modulate both protein–protein and protein–RNA interactions to produce a network response to affect many regulated splicing events. In terms of signal transduction pathways, the SR protein system seems to have a dedicated pathway through SR protein kinases (SRPKs) and CDC-like kinases (CLKs) in the cytoplasm and the nucleus, respectively. These kinases are responsible for controlling the phosphorylation state of SR proteins to modulate their activities in splicing. Various phosphatases may be also involved to antagonize the activity of these splicing kinases. EGFR, epidermal growth factor receptor; FASTK, Fas-activated serine/threonine kinase; GSK3, glycogen synthase kinase 3; hnRNP A1, heterogeneous nuclear ribonucleoprotein A1; IGFR, insulin-like growth factor receptor; MAPK, mitogen-activated protein kinase; p38 is a MAPK involved in the DNA damage response; Pol II, RNA polymerase II; PSF, PTB (polypyrimidine-tract binding protein)-associated splicing factor; RBP, RNA-binding protein; TCR, T cell receptor; TIA 1, nucleolysin TIA 1 isoform p40; TNFL6, tumour necrosis factor ligand superfamily member 6 (also known as FasL).
SR proteins are well known to depend on phosphorylation to regulate their activities in different phases of spliceosome assembly and catalysis57,160. Two families of kinases are involved in the regulation of SR protein phosphorylation: SR protein kinases (SRPKs) in the cytoplasm and CDC-like kinases (CLKs) in the nucleus, both of which consist of multiple family members. Recent studies revealed that the SRPK family of kinases is regulated by molecular chaperones in the cytoplasm81. Upon activation by growth factors — such as epidermal growth factor (EGF), which activates AKT — these SRPKs translocate to the nucleus to cause widespread changes in alternative splicing161,162 (FIG. 5). Remarkably, this AKT– SRPK–SR protein pathway seems to be responsible for EGF-induced splicing to a large extent, as removal of SRPK1 and/or SRPK2 by RNAi abolishes most EGF-induced events162, and such functional interplay between SRPKs and AKT seems to be a key tumour-promoting event163. As inhibition of mammalian target of rapamycin (mTOR) has little effect, these findings suggest that SRPKs are major transducers of growth signals that impinge on the regulation of alternative splicing in the nucleus. Interestingly, during the recovery phase after cellular stress, CLKs seem to be responsible for restoring the proper phosphorylation state in SR proteins164. Therefore, the dual kinase systems for SR proteins might contribute in a coordinated manner to splicing regulation in response to different types of signals. In the future, large-scale splicing profiling coupled with systematic RNAi against splicing regulators will help to dissect signalling-induced splicing in different cell types.
Conclusions and perspectives
Many regulatory principles have been deduced by studies on model genes, but these principles require global analyses to establish their scope and general applicability. In this regard, the elegant studies on the sex determination pathway in Drosophila melanogaster have provided many guiding principles for understanding regulatory mechanisms in alternative splicing, including the negative effect of intronic binding of the Sex-lethal (Sxl) protein on spliceosome assembly, the positive enhancement of splice site selection by Transformer 2 (Tra2) on the doublesex (dsx) pre-mRNA to dictate sex-specific alternative splicing, as well as the requirement for Tra (which does not bind directly to RNA) to cooperate with Tra2 in dsx splicing165. Many of these regulatory principles have been extended in whole-genome studies.
Recent progress in understanding this area, particularly using global approaches, has revealed new sets of rules for context-sensitive and position-dependent splicing. Currently, some of these rules are still unclear, and the molecular mechanisms that underlie these phenomena await detailed biochemical dissection. Thus, we are left with a set of fundamental questions that will take some time to address. Given the diversity of cis-acting elements and the large array of trans-acting factors that recognize them in cooperative and antagonistic ways, what is the code for the control of alternative splicing? If an RBP has multiple tasks in the cell, then how are its different functions reconciled and controlled? How can we dissect and disentangle such complex and overlapping networks to understand their integrated function and regulation? Finally, how do specific alternative splicing events contribute to crucial phenotypes in development, behaviour and human disease? It is almost certain that the community will continue to rely on functional genomics to discover and generalize regulatory paradigms, and on biochemistry to tackle the direct effects and the mechanisms. This effort will be undoubtedly aided by systematic identification of RBPs that can be crosslinked to diverse RNA populations in the cell, large-scale decoding of binding specificities of RBPs using high-throughput methods; dissection of both RNA-independent and RNA-dependent protein–protein interactions involved in regulated splicing; computational analyses of the effect of RNA secondary structure on RBP access to RNA sequence information; genome engineering to study cis-acting elements in their native context; and deconvolution of gene networks through genome-wide loss and gain of function studies.
Acknowledgements
This Review has benefitted from numerous discussions with colleagues in the field. The work in the authors’ laboratories were supported by grants GM040478 (to M.A.Jr), and GM049369 and GM052872 (to X.-D.F.) from the US National Institutes of Health.
Glossary
- Spliceosome
A macromolecular RNA–protein complex that is responsible for intron removal and that consists of U1, U2, U4, U5 and U6 small nuclear ribonucleoproteins (snRNPs) and many auxiliary protein factors.
- Alternative splicing
Differential inclusion of exons in the final processed RNA product by splicing of a precursor RNA segment.
- Transcriptomes
Complete sets of RNA transcripts in a cell.
- mRNA isoforms
Different mRNAs produced from the same precursor mRNA.
- Cis-acting RNA elements
RNA sequences in precursor mRNA that are important for both constitutive and regulated splicing.
- Splicing signals
Essential sequences in the pre-mRNA for recognition by the core splicing machinery.
- Branchpoint
A sequence as part of the 3′ splice site that is recognized by the spliceosome and that reacts with the 5′ splice site in the first step of the splicing reaction to form the lariat.
- Splicing regulatory elements
(SREs). RNA motifs in precursor RNA that have regulatory roles in splice site selection.
- Trans-acting factors
Proteins that interact with cis-acting regulatory RNA elements.
Footnotes
Competing interests statement
The authors declare no competing interests.
Contributor Information
Xiang-Dong Fu, Email: xdfu@ucsd.edu.
Manuel Ares, Jr, Email: ares@ucsc.edu.
References
- 1.Penny D, Hoeppner MP, Poole AM, Jeffares DC. An overview of the introns-first theory. J. Mol. Evol. 2009;69:527–540. doi: 10.1007/s00239-009-9279-5. [DOI] [PubMed] [Google Scholar]
- 2.Ast G. How did alternative splicing evolve? Nature Rev. Genet. 2004;5:773–782. doi: 10.1038/nrg1451. [DOI] [PubMed] [Google Scholar]
- 3.Keren H, Lev-Maor G, Ast G. Alternative splicing and evolution: diversification, exon definition and function. Nature Rev. Genet. 2010;11:345–355. doi: 10.1038/nrg2776. [DOI] [PubMed] [Google Scholar]
- 4.Brown JB, et al. Diversity and dynamics of the Drosophila transcriptome. Nature. 2014 doi: 10.1038/nature12962. http://dx.doi. org/10.1038/nature12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbosa-Morais NL, et al. The evolutionary landscape of alternative splicing in vertebrate species. Science. 2012;338:1587–1593. doi: 10.1126/science.1230612. [DOI] [PubMed] [Google Scholar]
- 6.Merkin J, Russell C, Chen P, Burge CB. Evolutionary dynamics of gene and isoform regulation in mammalian tissues. Science. 2012;338:1593–1599. doi: 10.1126/science.1228186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bentley DL. Coupling mRNA processing with transcription in time and space. Nature Rev. Genet. 2014;15:163–175. doi: 10.1038/nrg3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braunschweig U, Gueroussov S, Plocik AM, Graveley BR, Blencowe BJ. Dynamic integration of splicing within gene regulatory pathways. Cell. 2013;152:1252–1269. doi: 10.1016/j.cell.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornblihtt AR, et al. Alternative splicing: a pivotal step between eukaryotic transcription and translation. Nature Rev. Mol. Cell Biol. 2013;14:153–165. doi: 10.1038/nrm3525. [DOI] [PubMed] [Google Scholar]
- 10.Wahl MC, Will CL, Luhrmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010;463:457–463. doi: 10.1038/nature08909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu XD. Towards a splicing code. Cell. 2004;119:736–738. doi: 10.1016/j.cell.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 13.Barash Y, et al. Deciphering the splicing code. Nature. 2010;465:53–59. doi: 10.1038/nature09000. [DOI] [PubMed] [Google Scholar]
- 14.Havlioglu N, et al. An intronic signal for alternative splicing in the human genome. PLoS ONE. 2007;2:e1246. doi: 10.1371/journal.pone.0001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136:777–793. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes Dev. 2010;24:2343–2364. doi: 10.1101/gad.1973010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalsotra A, Cooper TA. Functional consequences of developmentally regulated alternative splicing. Nature Rev. Genet. 2011;12:715–729. doi: 10.1038/nrg3052. This is an excellent review from the perspective of how splicing regulation controls the function of gene products during development.
- 18.Poulos MG, Batra R, Charizanis K, Swanson MS. Developments in RNA splicing and disease. Cold Spring Harb. Perspect. Biol. 2011;3:a000778. doi: 10.1101/cshperspect.a000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robberson BL, Cote GJ, Berget SM. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol. Cell. Biol. 1990;10:84–94. doi: 10.1128/mcb.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo HC, Nasim FH, Grabowski PJ. Control of alternative splicing by the differential binding of U1 small nuclear ribonucleoprotein particle. Science. 1991;251:1045–1050. doi: 10.1126/science.1825520. [DOI] [PubMed] [Google Scholar]
- 21.McManus CJ, Graveley BR. RNA structure and the mechanisms of alternative splicing. Curr. Opin. Genet. Dev. 2011;21:373–379. doi: 10.1016/j.gde.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graveley BR. Mutually exclusive splicing of the insect Dscam pre-mRNA directed by competing intronic RNA secondary structures. Cell. 2005;123:65–73. doi: 10.1016/j.cell.2005.07.028. This paper shows the crucial contribution of RNA secondary structure to splice site selection.
- 23.Ding Y, et al. In vivo genome-wide profiling of RNA secondary structure reveals novel regulatory features. Nature. 2014;505:696–700. doi: 10.1038/nature12756. [DOI] [PubMed] [Google Scholar]
- 24.Rouskin S, Zubradt M, Washietl S, Kellis M, Weissman JS. Genome-wide probing of RNA structure reveals active unfolding of mRNA structures in vivo. Nature. 2014;505:701–705. doi: 10.1038/nature12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan Y, et al. Landscape and variation of RNA secondary structure across the human transcriptome. Nature. 2014;505:706–709. doi: 10.1038/nature12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang XH, Leslie CS, Chasin LA. Dichotomous splicing signals in exon flanks. Genome Res. 2005;15:768–779. doi: 10.1101/gr.3217705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lovci MT, et al. Rbfox proteins regulate alternative mRNA splicing through evolutionarily conserved RNA bridges. Nature Struct. Mol. Biol. 2013;20:1434–1442. doi: 10.1038/nsmb.2699. This study shows that a long-range RNA interaction and binding of a RBFOX protein control exon inclusion at a distance.
- 28.Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nature Methods. 2013;10:957–963. doi: 10.1038/nmeth.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 2003;72:291–336. doi: 10.1146/annurev.biochem.72.121801.161720. [DOI] [PubMed] [Google Scholar]
- 31. Guenther UP, et al. Hidden specificity in an apparently nonspecific RNA-binding protein. Nature. 2013;502:385–388. doi: 10.1038/nature12543. This paper reveals the unappreciated contribution of binding kinetics to sequence-specific RNA recognition.
- 32.Fairbrother WG, Yeh RF, Sharp PA, Burge CB. Predictive identification of exonic splicing enhancers in human genes. Science. 2002;297:1007–1013. doi: 10.1126/science.1073774. [DOI] [PubMed] [Google Scholar]
- 33.Xiao X, Wang Z, Jang M, Burge CB. Coevolutionary networks of splicing cis -regulatory elements. Proc. Natl Acad. Sci. USA. 2007;104:18583–18588. doi: 10.1073/pnas.0707349104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang XH, Chasin LA. Computational definition of sequence motifs governing constitutive exon splicing. Genes Dev. 2004;18:1241–1250. doi: 10.1101/gad.1195304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang XH, Leslie CS, Chasin LA. Computational searches for splicing signals. Methods. 2005;37:292–305. doi: 10.1016/j.ymeth.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Goren A, et al. Comparative analysis identifies exonic splicing regulatory sequences — the complex definition of enhancers and silencers. Mol. Cell. 2006;22:769–781. doi: 10.1016/j.molcel.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Tian H, Kole R. Selection of novel exon recognition elements from a pool of random sequences. Mol. Cell. Biol. 1995;15:6291–6298. doi: 10.1128/mcb.15.11.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coulter LR, Landree MA, Cooper TA. Identification of a new class of exonic splicing enhancers by in vivo selection. Mol. Cell. Biol. 1997;17:2143–2150. doi: 10.1128/mcb.17.4.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, et al. Systematic identification and analysis of exonic splicing silencers. Cell. 2004;119:831–845. doi: 10.1016/j.cell.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Ma M, Xiao X, Wang Z. Intronic splicing enhancers, cognate splicing factors and context-dependent regulation rules. Nature Struct. Mol. Biol. 2012;19:1044–1052. doi: 10.1038/nsmb.2377. This study reveals that multiple RBPs have the capacity to affect splicing through similar sequence motifs, which exemplifies the challenges of understanding context-dependent effects of splicing regulation.
- 41.Wang Y, et al. A complex network of factors with overlapping affinities represses splicing through intronic elements. Nature Struct. Mol. Biol. 2013;20:36–45. doi: 10.1038/nsmb.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ke S, et al. Quantitative evaluation of all hexamers as exonic splicing elements. Genome Res. 2011;21:1360–1374. doi: 10.1101/gr.119628.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castello A, et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149:1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 44.Kwon SC, et al. The RNA-binding protein repertoire of embryonic stem cells. Nature Struct. Mol. Biol. 2013;20:1122–1130. doi: 10.1038/nsmb.2638. [DOI] [PubMed] [Google Scholar]
- 45.Lunde BM, Moore C, Varani G. RNA-binding proteins: modular design for efficient function. Nature Rev. Mol. Cell Biol. 2007;8:479–490. doi: 10.1038/nrm2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ray D, et al. A compendium of RNA-binding motifs for decoding gene regulation. Nature. 2013;499:172–177. doi: 10.1038/nature12311. This is an important resource for keeping track of RBPs and their binding sites in diverse genomes.
- 47.Moore MJ, Wang Q, Kennedy CJ, Silver PA. An alternative splicing network links cell-cycle control to apoptosis. Cell. 2010;142:625–636. doi: 10.1016/j.cell.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park JW, Parisky K, Celotto AM, Reenan RA, Graveley BR. Identification of alternative splicing regulators by RNA interference in. Drosophila. Proc. Natl Acad. Sci. USA. 2004;101:15974–15979. doi: 10.1073/pnas.0407004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pashev IG, Dimitrov SI, Angelov D. Crosslinking proteins to nucleic acids by ultraviolet laser irradiation. Trends Biochem. Sci. 1991;16:323–326. doi: 10.1016/0968-0004(91)90133-g. [DOI] [PubMed] [Google Scholar]
- 50.Ule J, et al. CLIP identifies Nova-regulated RNA networks in the brain. Science. 2003;302:1212–1215. doi: 10.1126/science.1090095. [DOI] [PubMed] [Google Scholar]
- 51.Hafner M, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konig J, et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nature Struct. Mol. Biol. 2010;17:909–915. doi: 10.1038/nsmb.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambert N, et al. RNA Bind-n-Seq: quantitative assessment of the sequence and structural binding specificity of RNA binding proteins. Mol. Cell. 2014;54:887–900. doi: 10.1016/j.molcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Busch A, Hertel KJ. Evolution of SR protein and hnRNP splicing regulatory factors. Wiley Interdiscip. Rev. RNA. 2012;3:1–12. doi: 10.1002/wrna.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long JC, Caceres JF. The SR protein family of splicing factors: master regulators of gene expression. Biochem. J. 2009;417:15–27. doi: 10.1042/BJ20081501. [DOI] [PubMed] [Google Scholar]
- 56.Fu XD. The superfamily of arginine/serine-rich splicing factors. RNA. 1995;1:663–680. [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Z, Fu XD. Regulation of splicing by SR proteins and SR protein-specific kinases. Chromosoma. 2013;122:191–207. doi: 10.1007/s00412-013-0407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anko ML, et al. The RNA-binding landscapes of two SR proteins reveal unique functions and binding to diverse RNA classes. Genome Biol. 2012;13:R17. doi: 10.1186/gb-2012-13-3-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pandit S, et al. Genome-wide analysis reveals SR protein cooperation and competition in regulated splicing. Mol. Cell. 2013;50:223–235. doi: 10.1016/j.molcel.2013.03.001. This paper reveals what happens to the binding profile of an RBP when the presence of another RBP that contributes to context is eliminated.
- 60.Sanford JR, Gray NK, Beckmann K, Caceres JF. A novel role for shuttling SR proteins in mRNA translation. Genes Dev. 2004;18:755–768. doi: 10.1101/gad.286404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanford JR, et al. Splicing factor SFRS1 recognizes a functionally diverse landscape of RNA transcripts. Genome Res. 2009;19:381–394. doi: 10.1101/gr.082503.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayeda A, Krainer AR. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell. 1992;68:365–375. doi: 10.1016/0092-8674(92)90477-t. [DOI] [PubMed] [Google Scholar]
- 63.Mayeda A, Munroe SH, Caceres JF, Krainer AR. Function of conserved domains of hnRNP A1 and other hnRNP A/B proteins. EMBO J. 1994;13:5483–5495. doi: 10.1002/j.1460-2075.1994.tb06883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu J, Mayeda A, Krainer AR. Exon identity established through differential antagonism between exonic splicing silencer-bound hnRNP A1 and enhancer-bound SR proteins. Mol. Cell. 2001;8:1351–1361. doi: 10.1016/s1097-2765(01)00409-9. [DOI] [PubMed] [Google Scholar]
- 65.Okunola HL, Krainer AR. Cooperative-binding and splicing-repressive properties of hnRNP A1. Mol. Cell. Biol. 2009;29:5620–5631. doi: 10.1128/MCB.01678-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh R, Valcarcel J, Green MR. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science. 1995;268:1173–1176. doi: 10.1126/science.7761834. [DOI] [PubMed] [Google Scholar]
- 67.Llorian M, et al. Position-dependent alternative splicing activity revealed by global profiling of alternative splicing events regulated by PTB. Nature Struct. Mol. Biol. 2010;17:1114–1123. doi: 10.1038/nsmb.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xue Y, et al. Genome-wide analysis of PTB-RNA interactions reveals a strategy used by the general splicing repressor to modulate exon inclusion or skipping. Mol. Cell. 2009;36:996–1006. doi: 10.1016/j.molcel.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sharma S, Kohlstaedt LA, Damianov A, Rio DC, Black DL. Polypyrimidine tract binding protein controls the transition from exon definition to an intron defined spliceosome. Nature Struct. Mol. Biol. 2008;15:183–191. doi: 10.1038/nsmb.1375. This study describes a role for PTB in controlling an important transition in pre-mRNA assembly into spliceosomes.
- 70.Izquierdo JM, et al. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol. Cell. 2005;19:475–484. doi: 10.1016/j.molcel.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 71.Sharma S, Maris C, Allain FH, Black DL. U1 snRNA directly interacts with polypyrimidine tract-binding protein during splicing repression. Mol. Cell. 2011;41:579–588. doi: 10.1016/j.molcel.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chiou NT, Shankarling G, Lynch KW. hnRNP L and hnRNP A1 induce extended U1 snRNA interactions with an exon to repress spliceosome assembly. Mol. Cell. 2013;49:972–982. doi: 10.1016/j.molcel.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yu Y, et al. Dynamic regulation of alternative splicing by silencers that modulate 5′ splice site competition. Cell. 2008;135:1224–1236. doi: 10.1016/j.cell.2008.10.046. This paper shows that a modest difference in modulating a splice site in certain context leads to a much larger functional outcome in alternative splicing.
- 74. Warzecha CC, Sato TK, Nabet B, Hogenesch JB, Carstens RP. ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol. Cell. 2009;33:591–601. doi: 10.1016/j.molcel.2009.01.025. This study carries out a functional cDNA expression screen to capture tissue-specific RBP regulators of a given alternatively spliced gene.
- 75.Topp JD, Jackson J, Melton AA, Lynch KW. A cell-based screen for splicing regulators identifies hnRNP LL as a distinct signal-induced repressor of CD45 variable exon 4. RNA. 2008;14:2038–2049. doi: 10.1261/rna.1212008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oberdoerffer S, et al. Regulation of CD45 alternative splicing by heterogeneous ribonucleoprotein, hnRNPLL. Science. 2008;321:686–691. doi: 10.1126/science.1157610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calarco JA, et al. Regulation of vertebrate nervous system alternative splicing and development by an SR-related protein. Cell. 2009;138:898–910. doi: 10.1016/j.cell.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 78. Huelga SC, et al. Integrative genome-wide analysis reveals cooperative regulation of alternative splicing by hnRNP proteins. Cell Rep. 2012;1:167–178. doi: 10.1016/j.celrep.2012.02.001. This paper documents the overlapping responsibilities and crosstalk between a set of hnRNPs that contribute to splicing regulatory context.
- 79.Licatalosi DD, et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature. 2008;456:464–469. doi: 10.1038/nature07488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ule J, et al. Nova regulates brain-specific splicing to shape the synapse. Nature Genet. 2005;37:844–852. doi: 10.1038/ng1610. [DOI] [PubMed] [Google Scholar]
- 81.Zhong XY, Ding JH, Adams JA, Ghosh G, Fu XD. Regulation of SR protein phosphorylation and alternative splicing by modulating kinetic interactions of SRPK1 with molecular chaperones. Genes Dev. 2009;23:482–495. doi: 10.1101/gad.1752109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yeo GW, et al. An RNA code for the FOX2 splicing regulator revealed by mapping RNA-protein interactions in stem cells. Nature Struct. Mol. Biol. 2009;16:130–137. doi: 10.1038/nsmb.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang ET, et al. Transcriptome-wide regulation of pre-mRNA splicing and mRNA localization by muscleblind proteins. Cell. 2012;150:710–724. doi: 10.1016/j.cell.2012.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Du H, et al. Aberrant alternative splicing and extracellular matrix gene expression in mouse models of myotonic dystrophy. Nature Struct. Mol. Biol. 2010;17:187–193. doi: 10.1038/nsmb.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hall MP, et al. Quaking and PTB control overlapping splicing regulatory networks during muscle cell differentiation. RNA. 2013;19:627–638. doi: 10.1261/rna.038422.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Warzecha CC, Shen S, Xing Y, Carstens RP. The epithelial splicing factors ESRP1 and ESRP2 positively and negatively regulate diverse types of alternative splicing events. RNA Biol. 2009;6:546–562. doi: 10.4161/rna.6.5.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dittmar KA, et al. Genome-wide determination of a broad ESRP-regulated posttranscriptional network by high-throughput sequencing. Mol. Cell. Biol. 2012;32:1468–1482. doi: 10.1128/MCB.06536-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sugnet CW, Kent WJ, Ares M, Jr, Haussler D. Transcriptome and genome conservation of alternative splicing events in humans and mice. Pac. Symp. Biocomput. 2004;2004:66–77. doi: 10.1142/9789812704856_0007. [DOI] [PubMed] [Google Scholar]
- 89.Saltzman AL, Pan Q, Blencowe BJ. Regulation of alternative splicing by the core spliceosomal machinery. Genes Dev. 2011;25:373–384. doi: 10.1101/gad.2004811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Forch P, Puig O, Martinez C, Seraphin B, Valcarcel J. The splicing regulator TIA-1 interacts with U1-C to promote U1 snRNP recruitment to 5′ splice sites. EMBO J. 2002;21:6882–6892. doi: 10.1093/emboj/cdf668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Labourier E, Adams MD, Rio DC. Modulation of P-element pre-mRNA splicing by a direct interaction between PSI and U1 snRNP 70K protein. Mol. Cell. 2001;8:363–373. doi: 10.1016/s1097-2765(01)00311-2. [DOI] [PubMed] [Google Scholar]
- 92.Xiao R, et al. Nuclear matrix factor hnRNP U/SAF-A exerts a global control of alternative splicing by regulating U2 snRNP maturation. Mol. Cell. 2012;45:656–668. doi: 10.1016/j.molcel.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Munding EM, Shiue L, Katzman S, Donohue JP, Ares M., Jr Competition between pre-mRNAs for the splicing machinery drives global regulation of splicing. Mol. Cell. 2013;51:338–348. doi: 10.1016/j.molcel.2013.06.012. This study reveals how competition between pre-mRNAs for a limiting splicing machinery can regulate splicing.
- 94.Boon KL, et al. prp8 mutations that cause human retinitis pigmentosa lead to a U5 snRNP maturation defect in yeast. Nature Struct. Mol. Biol. 2007;14:1077–1083. doi: 10.1038/nsmb1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Z, et al. SMN deficiency causes tissue-specific perturbations in the repertoire of snRNAs and widespread defects in splicing. Cell. 2008;133:585–600. doi: 10.1016/j.cell.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshida K, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
- 97.Melton AA, Jackson J, Wang J, Lynch KW. Combinatorial control of signal-induced exon repression by hnRNP L and PSF. Mol. Cell. Biol. 2007;27:6972–6984. doi: 10.1128/MCB.00419-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rothrock CR, House AE, Lynch KW. hnRNP L represses exon splicing via a regulated exonic splicing silencer. EMBO J. 2005;24:2792–2802. doi: 10.1038/sj.emboj.7600745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hui J, et al. Intronic CA-repeat and CA-rich elements: a new class of regulators of mammalian alternative splicing. EMBO J. 2005;24:1988–1998. doi: 10.1038/sj.emboj.7600677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hui J, Stangl K, Lane WS, Bindereif A. hnRNP L stimulates splicing of the eNOS gene by binding to variable-length CA repeats. Nature Struct. Biol. 2003;10:33–37. doi: 10.1038/nsb875. [DOI] [PubMed] [Google Scholar]
- 101.Xiao X, et al. Splice site strength-dependent activity and genetic buffering by poly-G runs. Nature Struct. Mol. Biol. 2009;16:1094–1100. doi: 10.1038/nsmb.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chou MY, Rooke N, Turck CW, Black DL. hnRNP H is a component of a splicing enhancer complex that activates a c-src alternative exon in neuronal cells. Mol. Cell. Biol. 1999;19:69–77. doi: 10.1128/mcb.19.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen CD, Kobayashi R, Helfman DM. Binding of hnRNP H to an exonic splicing silencer is involved in the regulation of alternative splicing of the rat beta-tropomyosin gene. Genes Dev. 1999;13:593–606. doi: 10.1101/gad.13.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Crawford JB, Patton JG. Activation of alpha-tropomyosin exon 2 is regulated by the SR protein 9G8 and heterogeneous nuclear ribonucleoproteins H and F. Mol. Cell. Biol. 2006;26:8791–8802. doi: 10.1128/MCB.01677-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mauger DM, Lin C, Garcia-Blanco MA. hnRNP H and hnRNP F complex with Fox2 to silence fibroblast growth factor receptor 2 exon IIIc. Mol. Cell. Biol. 2008;28:5403–5419. doi: 10.1128/MCB.00739-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martinez-Contreras R, et al. Intronic binding sites for hnRNP A/B and hnRNP F/H proteins stimulate pre-mRNA splicing. PLoS Biol. 2006;4:e21. doi: 10.1371/journal.pbio.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garneau D, Revil T, Fisette JF, Chabot B. Heterogeneous nuclear ribonucleoprotein F/H proteins modulate the alternative splicing of the apoptotic mediator Bcl-x. J. Biol. Chem. 2005;280:22641–22650. doi: 10.1074/jbc.M501070200. [DOI] [PubMed] [Google Scholar]
- 108.Caputi M, Zahler AM. Determination of the RNA binding specificity of the heterogeneous nuclear ribonucleoprotein (hnRNP) H/H′/F/2H9 family. J. Biol. Chem. 2001;276:43850–43859. doi: 10.1074/jbc.M102861200. [DOI] [PubMed] [Google Scholar]
- 109. Erkelenz S, et al. Position-dependent splicing activation and repression by SR and hnRNP proteins rely on common mechanisms. RNA. 2013;19:96–102. doi: 10.1261/rna.037044.112. This paper presents some general rules of SR proteins and hnRNPs during splicing regulation.
- 110.Buratti E, Stuani C, De Prato G, Baralle FE. SR protein-mediated inhibition of CFTR exon 9 inclusion: molecular characterization of the intronic splicing silencer. Nucleic Acids Res. 2007;35:4359–4368. doi: 10.1093/nar/gkm444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang ZH, Zhang WJ, Rao Y, Wu JY. Regulation of Ich-1 pre-mRNA alternative splicing and apoptosis by mammalian splicing factors. Proc. Natl Acad. Sci. USA. 1998;95:9155–9160. doi: 10.1073/pnas.95.16.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Motta-Mena LB, Heyd F, Lynch KW. Context-dependent regulatory mechanism of the splicing factor hnRNP L. Mol. Cell. 2010;37:223–234. doi: 10.1016/j.molcel.2009.12.027. This paper presents an example of how sequence context can influence the activity of a splicing regulator.
- 113.Sun S, Zhang Z, Fregoso O, Krainer AR. Mechanisms of activation and repression by the alternative splicing factors RBFOX1/2. RNA. 2012;18:274–283. doi: 10.1261/rna.030486.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Damianov A, Black DL. Autoregulation of Fox protein expression to produce dominant negative splicing factors. RNA. 2010;16:405–416. doi: 10.1261/rna.1838210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Han H, et al. MBNL proteins repress ES-cell-specific alternative splicing and reprogramming. Nature. 2013;498:241–245. doi: 10.1038/nature12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Han J, et al. SR proteins induce alternative exon skipping through their activities on the flanking constitutive exons. Mol. Cell. Biol. 2011;31:793–802. doi: 10.1128/MCB.01117-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Han J, Xiong J, Wang D, Fu XD. Pre-mRNA splicing: where and when in the nucleus. Trends Cell Biol. 2011;21:336–343. doi: 10.1016/j.tcb.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bechara EG, Sebestyen E, Bernardis I, Eyras E, Valcarcel J. RBM5, 6, and 10 differentially regulate NUMB alternative splicing to control cancer cell proliferation. Mol. Cell. 2013;52:720–733. doi: 10.1016/j.molcel.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 119.Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nature Rev. Mol. Cell Biol. 2009;10:741–754. doi: 10.1038/nrm2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu X, et al. ASF/SF2-regulated CaMKIIδ alternative splicing temporally reprograms excitation-contraction coupling in cardiac muscle. Cell. 2005;120:59–72. doi: 10.1016/j.cell.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 121.Feng Y, et al. SRp38 regulates alternative splicing and is required for Ca2+ handling in the embryonic heart. Dev. Cell. 2009;16:528–538. doi: 10.1016/j.devcel.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li Q, et al. The splicing regulator PTBP2 controls a program of embryonic splicing required for neuronal maturation. Elife. 2014;3:e01201. doi: 10.7554/eLife.01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Licatalosi DD, et al. Ptbp2 represses adult-specific splicing to regulate the generation of neuronal precursors in the embryonic brain. Genes Dev. 2012;26:1626–1642. doi: 10.1101/gad.191338.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kuroyanagi H, Ohno G, Mitani S, Hagiwara M. The Fox-1 family and SUP-12 coordinately regulate tissue-specific alternative splicing in vivo. Mol. Cell. Biol. 2007;27:8612–8621. doi: 10.1128/MCB.01508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang C, et al. Integrative modeling defines the Nova splicing-regulatory network and its combinatorial controls. Science. 2010;329:439–443. doi: 10.1126/science.1191150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ohno G, et al. Muscle-specific splicing factors ASD-2 and SUP-12 cooperatively switch alternative pre-mRNA processing patterns of the ADF/cofilin gene in Caenorhabditis elegans. PLoS Genet. 2012;8:e1002991. doi: 10.1371/journal.pgen.1002991. This study provides evidence for cooperative behaviour between two splicing factors at a regulated splicing switch.
- 127.Korneta I, Bujnicki JM. Intrinsic disorder in the human spliceosomal proteome. PLoS Comput. Biol. 2012;8:e1002641. doi: 10.1371/journal.pcbi.1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Han TW, et al. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell. 2012;149:768–779. doi: 10.1016/j.cell.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 129. Kato M, et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149:753–767. doi: 10.1016/j.cell.2012.04.017. This paper describes how a chance observation made in pursuit of an unrelated question led to the discovery of a general biochemical behaviour of RBPs.
- 130.Kim HJ, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495:467–473. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li YR, King OD, Shorter J, Gitler AD. Stress granules as crucibles of ALS pathogenesis. J. Cell Biol. 2013;201:361–372. doi: 10.1083/jcb.201302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Keppetipola N, Sharma S, Li Q, Black DL. Neuronal regulation of pre-mRNA splicing by polypyrimidine tract binding proteins, PTBP1 and PTBP2. Crit. Rev. Biochem. Mol. Biol. 2012;47:360–378. doi: 10.3109/10409238.2012.691456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Spellman R, Llorian M, Smith CW. Crossregulation and functional redundancy between the splicing regulator PTB and its paralogs nPTB and ROD1. Mol. Cell. 2007;27:420–434. doi: 10.1016/j.molcel.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boutz PL, et al. A post-transcriptional regulatory switch in polypyrimidine tract-binding proteins reprograms alternative splicing in developing neurons. Genes Dev. 2007;21:1636–1652. doi: 10.1101/gad.1558107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xue Y, et al. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell. 2013;152:82–96. doi: 10.1016/j.cell.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zheng S, et al. PSD-95 is post-transcriptionally repressed during early neural development by PTBP1 and PTBP2. Nature Neurosci. 2012;15:381–388. doi: 10.1038/nn.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Modafferi EF, Black DL. Combinatorial control of a neuron-specific exon. RNA. 1999;5:687–706. doi: 10.1017/s1355838299990155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zarnack K, et al. Direct competition between hnRNP C and U2AF65 protects the transcriptome from the exonization of Alu elements. Cell. 2013;152:453–466. doi: 10.1016/j.cell.2012.12.023. This study reveals that hnRNP C competes with U2AF to keep intronic Alu sequences from being recognized as exons.
- 140.Wilbert ML, et al. LIN28 binds messenger RNAs at GGAGA motifs and regulates splicing factor abundance. Mol. Cell. 2012;48:195–206. doi: 10.1016/j.molcel.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kosti I, Radivojac P, Mandel-Gutfreund Y. An integrated regulatory network reveals pervasive cross-regulation among transcription and splicing factors. PLoS Comput. Biol. 2012;8:e1002603. doi: 10.1371/journal.pcbi.1002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ellis JD, et al. Tissue-specific alternative splicing remodels protein-protein interaction networks. Mol. Cell. 2012;46:884–892. doi: 10.1016/j.molcel.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 143.Ni JZ, et al. Ultraconserved elements are associated with homeostatic control of splicing regulators by alternative splicing and nonsense-mediated decay. Genes Dev. 2007;21:708–718. doi: 10.1101/gad.1525507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lareau LF, Inada M, Green RE, Wengrod JC, Brenner SE. Unproductive splicing of SR genes associated with highly conserved and ultraconserved DNA elements. Nature. 2007;446:926–929. doi: 10.1038/nature05676. [DOI] [PubMed] [Google Scholar]
- 145.Sureau A, Gattoni R, Dooghe Y, Stevenin J, Soret J. SC35 autoregulates its expression by promoting splicing events that destabilize its mRNAs. EMBO J. 2001;20:1785–1796. doi: 10.1093/emboj/20.7.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Arnold ES, et al. ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc. Natl Acad. Sci. USA. 2013;110:E736–E745. doi: 10.1073/pnas.1222809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lagier-Tourenne C, et al. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nature Neurosci. 2012;15:1488–1497. doi: 10.1038/nn.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ji X, et al. SR proteins collaborate with 7SK and promoter-associated nascent RNA to release paused polymerase. Cell. 2013;153:855–868. doi: 10.1016/j.cell.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin S, Coutinho-Mansfield G, Wang D, Pandit S, Fu XD. The splicing factor SC35 has an active role in transcriptional elongation. Nature Struct. Mol. Biol. 2008;15:819–826. doi: 10.1038/nsmb.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xie J, Black DL. A CaMK IV responsive RNA element mediates depolarization-induced alternative splicing of ion channels. Nature. 2001;410:936–939. doi: 10.1038/35073593. [DOI] [PubMed] [Google Scholar]
- 151.Yu J, et al. The heterogeneous nuclear ribonucleoprotein L is an essential component in the Ca2+/calmodulin-dependent protein kinase IV-regulated alternative splicing through cytidine-adenosine repeats. J. Biol. Chem. 2009;284:1505–1513. doi: 10.1074/jbc.M805113200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heyd F, Lynch KW. Degrade, move, regroup: signaling control of splicing proteins. Trends Biochem. Sci. 2011;36:397–404. doi: 10.1016/j.tibs.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shin C, Manley JL. Cell signalling and the control of pre-mRNA splicing. Nature Rev. Mol. Cell Biol. 2004;5:727–738. doi: 10.1038/nrm1467. [DOI] [PubMed] [Google Scholar]
- 154.van der Houven van Oordt W, et al. The MKK(3/6)–p38-signaling cascade alters the subcellular distribution of hnRNP A1 and modulates alternative splicing regulation. J. Cell Biol. 2000;149:307–316. doi: 10.1083/jcb.149.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Paronetto MP, Minana B, Valcarcel J. The Ewing sarcoma protein regulates DNA damage-induced alternative splicing. Mol. Cell. 2011;43:353–368. doi: 10.1016/j.molcel.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 156.Matter N, Herrlich P, Konig H. Signal-dependent regulation of splicing via phosphorylation of Sam68. Nature. 2002;420:691–695. doi: 10.1038/nature01153. [DOI] [PubMed] [Google Scholar]
- 157.Iijima T, et al. SAM68 regulates neuronal activity-dependent alternative splicing of neurexin-1. Cell. 2011;147:1601–1614. doi: 10.1016/j.cell.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shin C, Feng Y, Manley JL. Dephosphorylated SRp38 acts as a splicing repressor in response to heat shock. Nature. 2004;427:553–558. doi: 10.1038/nature02288. [DOI] [PubMed] [Google Scholar]
- 159.Heyd F, Lynch KW. Phosphorylation-dependent regulation of PSF by GSK3 controls CD45 alternative splicing. Mol. Cell. 2010;40:126–137. doi: 10.1016/j.molcel.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cho S, et al. Interaction between the RNA binding domains of Ser-Arg splicing factor 1 and U1-70K snRNP protein determines early spliceosome assembly. Proc. Natl Acad. Sci. USA. 2011;108:8233–8238. doi: 10.1073/pnas.1017700108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jang SW, et al. Interaction of Akt-phosphorylated SRPK2 with 14-3-3 mediates cell cycle and cell death in neurons. J. Biol. Chem. 2009;284:24512–24525. doi: 10.1074/jbc.M109.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Zhou Z, et al. The Akt-SRPK-SR axis constitutes a major pathway in transducing EGF signaling to regulate alternative splicing in the nucleus. Mol. Cell. 2012;47:422–433. doi: 10.1016/j.molcel.2012.05.014. This study elucidates how EGF signalling leads to global changes in alternative splicing.
- 163.Wang P, et al. Both decreased and increased SRPK1 levels promote cancer by interfering with PHLPP-mediated dephosphorylation of Akt. Mol. Cell. 2014;54:378–391. doi: 10.1016/j.molcel.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ninomiya K, Kataoka N, Hagiwara M. Stress-responsive maturation of Clk1/4 pre-mRNAs promotes phosphorylation of SR splicing factor. J. Cell Biol. 2011;195:27–40. doi: 10.1083/jcb.201107093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maniatis T. Mechanisms of alternative pre-mRNA splicing. Science. 1991;251:33–34. doi: 10.1126/science.1824726. [DOI] [PubMed] [Google Scholar]
- 166.Daughters RS, et al. RNA gain-of-function in spinocerebellar ataxia type 8. PLoS Genet. 2009;5:e1000600. doi: 10.1371/journal.pgen.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Charizanis K, et al. Muscleblind-like 2-mediated alternative splicing in the developing brain and dysregulation in myotonic dystrophy. Neuron. 2012;75:437–450. doi: 10.1016/j.neuron.2012.05.029. This paper shows that some splicing events regulated by MBNL1 in muscles are instead controlled by MBNL2 in the brain, which indicates division of labour among MBNL proteins depending on tissue context.
- 168.Weyn-Vanhentenryck SM, et al. HITS-CLIP and integrative modeling define the Rbfox splicing-regulatory network linked to brain development and autism. Cell Rep. 2014;6:1139–1152. doi: 10.1016/j.celrep.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang Z, et al. iCLIP predicts the dual splicing effects of TIA-RNA interactions. PLoS Biol. 2010;8:e1000530. doi: 10.1371/journal.pbio.1000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Uniacke J, et al. An oxygen-regulated switch in the protein synthesis machinery. Nature. 2012;486:126–129. doi: 10.1038/nature11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lebedeva S, et al. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol. Cell. 2011;43:340–352. doi: 10.1016/j.molcel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 172.Mukherjee N, et al. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol. Cell. 2011;43:327–339. doi: 10.1016/j.molcel.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ince-Dunn G, et al. Neuronal Elav-like (Hu) proteins regulate RNA splicing and abundance to control glutamate levels and neuronal excitability. Neuron. 2012;75:1067–1080. doi: 10.1016/j.neuron.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Corioni M, Antih N, Tanackovic G, Zavolan M, Kramer A. Analysis of in situ pre-mRNA targets of human splicing factor SF1 reveals a function in alternative splicing. Nucleic Acids Res. 2011;39:1868–1879. doi: 10.1093/nar/gkq1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rossbach O, et al. Crosslinking-immunoprecipitation (iCLIP) analysis reveals global regulatory roles of hnRNP L. RNA Biol. 2014;11:146–155. doi: 10.4161/rna.27991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shankarling G, Cole BS, Mallory MJ, Lynch KW. Transcriptome-wide RNA interaction profiling reveals physical and functional targets of hnRNP L in human T cells. Mol. Cell. Biol. 2014;34:71–83. doi: 10.1128/MCB.00740-13. [DOI] [PMC free article] [PubMed] [Google Scholar]