Abstract
Background
The tryptophan hydroxylase 1 (TPH1) gene is reported to be associated with suicidal behavior. This has not been confirmed by prospective studies of suicide and clinical or biological mediators of this genetic risk have not been identified.
Methods
343 subjects (Caucasian, African-American, Hispanic) presenting with a Major Depressive Episode were genotyped for polymorphisms A218C in intron 7 and A-6526G in the promoter region of TPH1, and monitored for suicide attempts for up to one year. Clinical correlates of suicidal behavior and CSF-HIAA, HVA and MHPG levels were explored as possible mediators of genetic risk. Analyses were adjusted for ethnicity.
Results
The AA genotype on intron 7 and the GG genotype on the promoter (both more prevalent in Caucasians) predicted suicide attempts during the 1 year follow-up, and were-associated with past attempts of high medical lethality, regardless of ethnicity. The intron 7 genotype was associated with fewer reported reasons for living, and lower impulsivity. Haplotype analysis indicated significant increase in risk of suicide attempts for subjects with four risk alleles. TPH1 genotype was not associated with CSF metabolite levels.
Limitations
The TPH1 gene is likely one of several genes associated with suicidal behavior. Power to detect differential genotype effects by ethnicity is low.
Conclusions
Polymorphisms of TPH1 may assist in identifying a subgroup of mood disorder patients that is at higher risk for suicidal behavior.
Keywords: suicide attempt, prediction, major depressive episode, haplotype analysis
Background
Altered brain serotonin system function is associated with suicide and suicide attempts in depressed patients (Roy et al., 1989) and therefore serotonin-related genes are plausible candidate genes for suicidal behavior (Arango et al., 2003; Bondy et al., 2006; Courtet et al., 2005). The tryptophan hydroxylase isoform 1 (TPH1) gene is of interest with respect to the risk of suicidal behavior as it expresses a biosynthetic enzyme for serotonin in the brain during development (Nakamura et al., 2006) and may have long-lasting downstream trophic effects on the brain. The intronic polymorphisms A218C and the promoter polymorphism A-6526G of the TPH1 gene were reported to be associated with past suicidal behavior in association and linkage studies (Nielsen et al., 1998; Rotondo et al., 1999). The only prospective study investigating if the TPH1 polymorphism predicts suicide attempts found no relationship (Courtet et al., 2004). Prospective studies provide more reliable data on suicide attempts during follow-up because of the diminished chance for recall bias, and are more likely to lead to the discovery of possible biological and clinical mediators of the genetic risk of suicide attempt than retrospective studies, as temporal sequence is hard to establish in the latter.
In this study, we used prospective data to examine the association of the intronic polymorphisms A218C and the promoter polymorphism A-6526G in the TPH1 gene with suicide attempt following presentation with a Major Depressive Episode (MDE). We hypothesized that the “A” allele in intron 7 and the G allele in the promoter region will be associated with increased risk of suicide attempt during the first year following a major depressive episode. We also explored possible clinical and biological intermediate phenotypes for genetic risk in suicide attempt, including severity of psychopathology, clinical traits of aggression, impulsivity and pessimism, and levels of 5-hydroxyindoleacetic acid (5-HIAA), homovanillic acid (HVA) and 3-methoxy-4-hydroxyphenylglycol (MHPG) in cerebrospinal fluid (CSF). For increased generalizability we used a multiracial sample and adjusted our analyses for ethnicity.
Methods
Patients (N= 343) with Major Depressive Disorder or Bipolar Disorder, based on the Structured Clinical Interview for DSM IV (SCID-I) with a current Major Depressive Episode provided written informed consent, were admitted to the genetic study. The methodology for the clinical evaluation and follow-up of participants has been previously described (Oquendo et al., 2004). Polymorphisms A218C in intron 7 and A-6526G in the promoter region of the TPH1 gene were genotyped as previously described (Huang et al., 2003). Intron 7 genotype was available for all subjects and the promoter genotype for only 230 subjects. Availability of promoter genotype was not associated with ethnic group or suicidal behavior and was independent of intron genotype.
The sample was 20% Hispanic, 11% African-American, and 69% Caucasian. Average age was 39.8 ± 11.8 years, with a range between 18 and 79 years and the sample was predominantly female (65%). 29% (N=99) had a DSM-IV diagnosis of Bipolar Disorder and 71% (N=244) Major Depressive Disorder. 47% (N=160) of the sample reported a history of past suicide attempt.
Clinical assessment
Baseline clinical assessment included the collection of data on demographics, childhood development and history of suicide attempts, substance and tobacco use, severity of psychopathology and personality traits using the Hamilton Depression Rating Scale, Beck Depression Index, Beck Hopelessness Scale, Scale for Suicidal Ideation, Reasons For Living Inventory, Brown-Goodwin Lifetime Aggression History, Buss-Durkee Hostility Index, and Barratt Impulsivity Scale. Axis II diagnosis were made using the SCID II.
Suicide attempts were scored 0-8 for medical damage on the Medical Lethality Scale (MLS), with 0 representing no medical harm and 8 representing death (Beck et al., 1975), and classified as high-lethality (MLS>3) or low-lethality attempts (MLS≤3) where any score above 3 means that hospitalization for the medical sequelae of the attempt was needed. For multiple attempts, the score from the most lethal attempt was used to provide a subject-specific measure of severity of suicidal behavior.
Follow-up interviews at the 3 month and 1 year time-points included assessment of suicide attempt history since the last assessment. 297 subjects (87%) had at least some follow-up data and 48 (16%) of these patients left the study before the 1 year follow-up visit, thus only censored data is available for these patients. Two subjects died by suicide in the follow-up period and were included in all analyses.
CSF monoamine metabolites
Levels of cerebrospinal fluid 5HIAA, HVA and MHPG were assayed in 125 subjects as previously described (Placidi et al., 2001). Not all subjects in the study consented to the lumbar puncture, and that resulted in missing CSF monoamine levels. While subjects who consented to lumbar puncture were not a random subset, consent is not related to TPH1 intron genotype.
Statistical Methods
Baseline
Associations between genotype and ethnicity were tested using the chi-square test of independence. To separate group differences due to genotype from those due to ethnicity, subsequent tests of association between genotype and demographic, clinical variables, and baseline history of suicide attempt were adjusted for ethnicity using logistic regression or two-way ANOVA models as appropriate, with ethnicity an additive factor. Medical severity scores of suicide attempts were compared by paired and unpaired t-test with unequal variances.
Follow-up
Survival analysis was used to model the time to first attempt. Nonparametric Kaplan-Meier estimates of the survival curves were graphed for each genotype group, and the association between genotype and attempts during follow-up was tested using the log-rank test. Genotypes with similar survival curves were combined and Cox proportional hazard regression was used to estimate the hazard ratio for the final two genotypes. The assumption of proportional hazard was checked by tests of the scaled Schoenfeld residuals by time (Therneau et al., 2000). Variables associated with genotype and ethnic group were individually added to the Cox model to determine whether their effects were independent from genotype. Interactions between genotype and all other variables were also tested to identify possible moderators of the genotype effect. As this was an exploratory part of the study, p-values were not adjusted for multiple testing.
Number of risk alleles
To determine whether additional information regarding risk can be gained by using both SNPs, haplotype imputation of the two SNPs was performed in the statistical software R (http://cran.r-project.org) using the function haplo.em from the package haplo.stats. Due to genotype differences between ethnic groups, the imputation was done separately for each ethnic cohort. Best-guess haplotype pairs (those with the highest posterior probability) were used to compute the number of risk alleles in the haplotype pair for each subject. This measure was then used as predictor in a Cox proportional hazard model for attempts during follow-up, and in logistic regression for baseline history of suicide attempt. The resulting odds ratio estimates for medically serious past attempts and hazard ratios for future attempts were plotted against the number of risk alleles.
Results
TPH1 genotype distribution
Genotypes in both SNPs were in Hardy-Weinberg equilibrium regardless of ethnicity, gender or diagnosis. Genotype distributions are displayed in Table 1. There were significant differences between the three ethnic groups on the genotype distribution of both polymorphisms (intron 7: χ2=10.3, df=4, p =.036, promoter: χ2=21.4, df=4, p <.001) (Table 1). Caucasians and African-Americans had different genotype distributions, and Hispanics were intermediate. Sex and genotype were not associated (p>.3 for both intron 7 and promoter genotype).
Table 1.
TPH1 genotype distribution by group.
| Group | TPH1 Intron 7 Genotype | TPH1 Promoter Genotype | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AA | AC | CC | P-val | AA | AG | GG | P-val | ||
| Race/Ethnicity | AfrAm. | 7.9% | 42.1% | 50.0% | .036 | 54.2% | 33.3% | 12.5% | <.001 |
| Cauc. | 20.8% | 51.7% | 27.6% | 14.2% | 53.1% | 32.7% | |||
| Hisp. | 14.5% | 47.8% | 37.7% | 22.7% | 47.7% | 29.6% | |||
| Past att. (severe) | Yes | 29.4% | 42.6% | 27.9% | .025 | 18.0% | 38.0% | 44.0% | .046 |
| No | 15.3% | 51.6% | 33.1% | 20.6% | 53.3% | 26.1% | |||
| Follow-up att. | Yes | 39.3% | 39.3% | 9.4% | .007 | 19.2% | 26.9% | 53.8% | .012 |
| No | 15.6% | 50.9% | 33.5% | 20.8% | 52.8% | 26.4% | |||
Demographic differences between racial/ethnic groups
There was a higher proportion of females in both the African American and the Hispanic groups than in the Caucasian group (74% vs. 60%, χ2=6.0, df=2, p=.049). There were no differences in the proportion of married subjects, proportion of currently employed or personal income. There were ethnic group differences in religion (proportion Catholic:AA:13%, Hispanics: 53%, Caucasians: 28%), age at baseline (AA:39, Hispanics:37, Caucasians: 41 years, F= 3.4, df= (2, 340), p= 0.033) and education (average number of years in education: AA:14, Hispanics:13, Caucasians:16, F= 15.755, df=(2,336), p<.001).
Suicide attempts
47% (N=160) of the sample reported a history of past suicide attempt. The African-American cohort had the highest proportion of history of suicide attempt (66%), significantly higher than Caucasians (45%) and Hispanics (42%) (χ2= 6.5, df = 2, p = 0.039), however there was no ethnic group differences in medically serious suicide attempts (Caucasians 23%, African-Americans 13% and Hispanics 13%, χ2= 4.4, df = 2, p = .108).
28 (9.5%) of the 297 subjects who participated in the follow-up phase of the study attempted suicide in the first year after baseline assessment, three of whom had no history of past suicide attempt. For follow-up attempters who made multiple attempts in the one year follow-up period (N=12, 40%), only the first attempt was included in the analysis. The proportion of patients with follow-up suicide attempt was highest in African Americans (n=5, 16%), followed by Caucasians (n=22, 10%) and lowest in Hispanics (n=1, 2%).
Among the 25 subjects with both baseline and follow-up attempts, medical lethality of the most severe baseline and follow-up attempts did not differ (paired t = -0.2, df = 25, p = .853). Subjects with a follow-up attempt had higher lethality past suicide attempts than those with no attempt during follow-up (4.2 vs. 3.1, t = 2.4, df = 134, p = 0.017).
TPH1 genotype and past suicide attempt
The intron 7 AA genotype was associated with past suicide attempt at a weak trend level (OR=1.7, 95%CI: 0.9-3.2, p=.071), however, it was significantly associated with high-lethality attempts (OR=2.3, 95%CI: 1.2-4.4, p=.011) after adjusting for ethnic group, age, sex, religion and education. The promoter genotype GG was also significantly associated with a history of high-lethality suicide attempt (OR=2.4, 95%CI: 1.2-4.4, p=.0109) but not with history of attempt in general (OR=1.4, p=.308), adjusted for the same demographic variables.
TPH1 genotype and follow-up suicide attempt
Participation in the follow-up phase was not associated with either polymorphism (intron 7: χ2=0.1, df=2, p=.947; Promoter: χ2=0.4, df=2, p=.817), nor was time in study (Kruskall-Wallis test intron 7: χ2 = 0.8, df = 2, p= 0.668; promoter: χ2 = 0.4, df = 2, p= 0.813). TPH1 genotypes for both polymorphisms were significantly associated with the risk of future suicide attempts. The log-rank tests for association of the time to first attempt by genotype of both SNPs were significant (intron 7: χ2=11.0, df=2, p=.004, promoter: χ2=9.4, df=2, p=.009). Intron genotypes AC and CC were combined and promoter genotypes AG and AA were combined as they showed similar survival curves (Figure 1). In Cox proportional hazards regression, the intron 7 AA genotype and the promoter GG genotype predicted suicide attempt in the 1 year follow-up (intron 7: HR=3.4, 95% CI: 1.6-7.4, p=.002; promoter: HR=3.8, 95%CI:1.7-8.6, p=.001).
Figure 1.
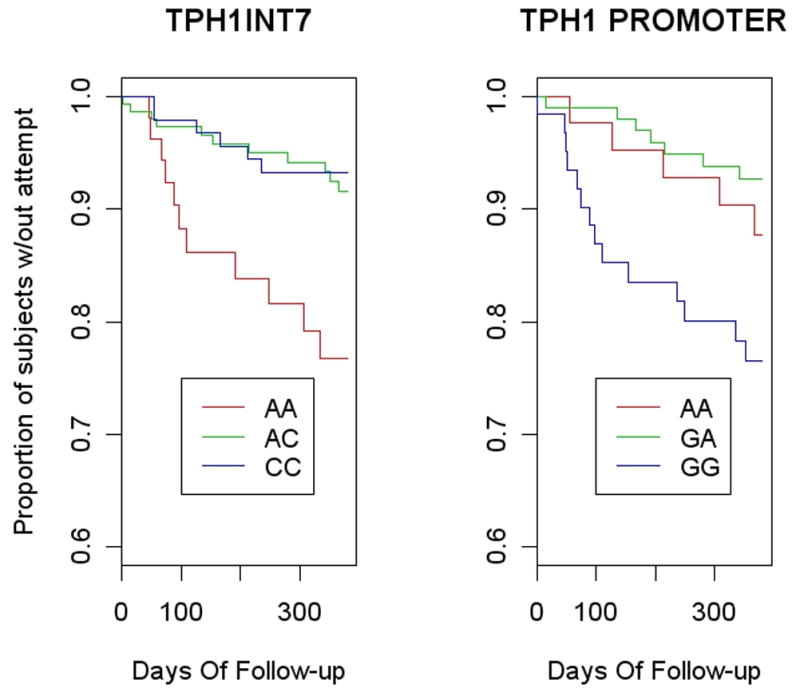
Kaplan-Meier estimates of the survival curves for the three genotypes of A218C in intron 7 of TPH1 (Log-rank test p=.004) and for A-6526G on the promoter region (Log-rank test p=.009).
Haplotype analysis
The two SNPs were in linkage disequilibrium (D′=0.72) with the AA genotype and the GG genotype significantly associated (χ2=60.0, df=1, p <.001). 53 (15.5%) of subjects were assigned the combined GG-AA promoter/intron 7 genotype (two copies of the AG haplotype) by the EM algorithm. This group had significantly higher risk for follow-up attempts and for high-lethality past attempts compared with other haplotype groups (HR=3.5, 95%CI: 1.5-8.0, p=.003, and OR=2.3, 95%CI: 1.2-4.5, p=.011; respectively).
Dose-response curves and tests for the association between risk of suicide attempt and number of “risk alleles” (A in intron 7 and G in the promoter) in the TPH1 haplotype show that the risk is significantly increased only when all 4 alleles are “risk alleles”, although subjects with 3 risk alleles show a trend to increased risk (Table 3 and Figure 2).
Table 3.
Cox proportional hazard regression estimates of hazard ratios of future suicide attempts by number of risk alleles and ethnic group.
| Variable | Value | Hazard Ratio | 95%CI for HR | Z | P-value |
|---|---|---|---|---|---|
| Number of risk alleles | No risk alleles | 1.0 | |||
| 1 allele | 0.8 | 0.2-4.2 | -0.2 | .800 | |
| 2 alleles | 1.1 | 0.3-4.4 | 0.2 | .860 | |
| 3 alleles | 3.1 | 0.7-14.3 | 1.4 | .160 | |
| 4 alleles | 4.2 | 1.1-17.2 | 2.0 | .043 | |
| Race/Ethnicity | Caucasian | 1.0 | |||
| Afr. Am. | 2.1 | 0.8-6.7 | 1.5 | .190 | |
| Hispanic | 0.1 | .02-1.3 | -1.7 | .036 | |
| Age | 0.97 | .089 | |||
| Sex | Male | 0.37 | .045 | ||
| Education(years) | 0.95 | .500 | |||
| Religion | Catholic | 1.1 | .640 |
Figure 2.
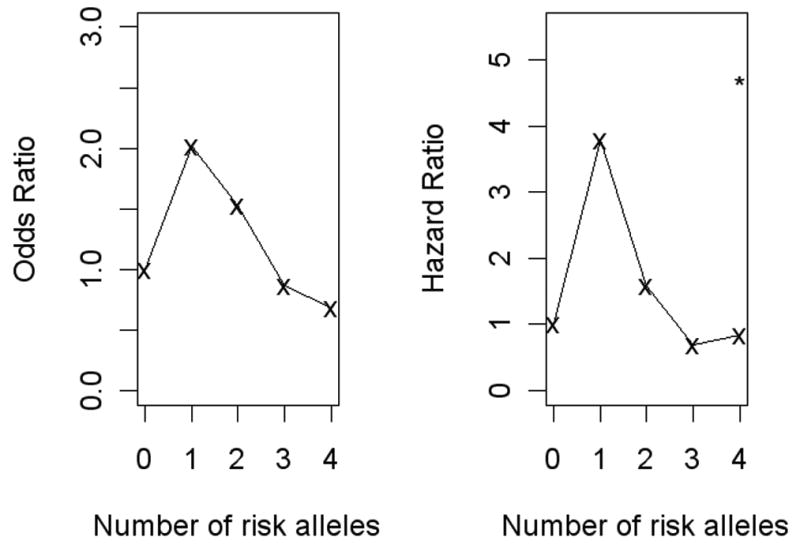
Dose-response curves of odds ratios for medically serious past suicide attempt and hazard ratios of future suicide attempt by number of TPH1 risk alleles.
Genotype and clinical and biological characteristics
Neither intron nor promoter genotype was associated with primary diagnosis (bipolar disorder vs. major depressive disorder) (intron 7: χ2=.01, df=1, p=.974, promoter χ2=.43, df=1, p=.512).
The intron 7 AA genotype was associated with fewer reported reasons for living (RFL) (135 vs. 157, t=-2.9, df=258, p=.004)where more reasons were protective against future suicide attempt (HR=0.8 for each 10 point increase, 95%CI= 0.7-0.9, p<.001)); and with lower impulsivity scores (50 vs. 56:, t=-2.1, df=303, p=.039), the latter was not associated with future attempts (p=.310). After adjusting for RFL, the AA genotype remained a significant predictor of future suicide attempt, with somewhat decreased power (HR=2.6, 95% CI: 1.1-6.4, p=.029), and RFL also remained a predictor (data not shown). Intron 7 genotype was also a moderator for the effect of depression severity (HDRS) on risk of future suicide attempt. Although depression score per se did not differ by genotype, there was a differential effect by genotype on the risk of suicide attempt whereby higher depression scores were associated with increased suicide risk in subjects with AA genotype, but not in the other two genotypes (AA genotype: HR=2.2 for a 5-point HDRS score increase vs. others: HR=0.82, z= 2.6, p=.010).
The GG promoter genotype was not associated with any clinical or demographic variables examined and no moderators of the genotype effect were found.
The number of high-risk alleles in intron 7 – promoter haplotype correlated negatively with Reasons For Living score (p-value= .016), showing a dose-response relationship between genetic risk and clinical phenotype. When RFL was added to the Cox model with number of risk alleles and ethnicity as predictors, both the number of risk alleles and RFL stayed significant, indicating that the genetic risk is only partially mediated by RFL.
Neither genotype was significantly associated with CSF 5-HIAA, HVA or MHPG levels in this sample (p-values ranged between .46 and .74, data available upon request), so mediation of the genetic risk of suicide through CSF metabolite levels is not supported by the data.
Discussion
We found that the AA genotype in intron 7 and the GG genotype in the promoter region of the TPH1 gene were associated with increased risk of suicide attempt in the year following a Major Depressive Episode, and with a history of medically severe suicide attempts. In addition, in this sample, subjects who attempted suicide during the 1 year follow-up period were more likely to have a history of high-lethality suicide attempts. These findings support previous studies linking the “A” allele on intron 7 of the TPH1 gene and/or the “G” allele on the promoter SNP to a history of violent (Nielsen et al., 1994) and medically severe (Nielsen et al., 1998) suicide attempts. Moreover, they extend that finding to increased risk for future attempts. The only other published prospective study of short-term risk of suicide attempt and the A218C TPH genotype we are aware of found no genotype effect (Courtet et al., 2004). This lack of agreement may be due to the heterogeneity in primary diagnosis in that sample which comprised subjects with different diagnoses after a suicide attempt, or the lower sample size (103 subjects followed-up vs. 297 here).
In this study we analyzed both intron 7 and promoter genotype because we wanted to examine whether the respective risks of suicide attempt conferred by them are multiplicative (in which case the risk of suicide increases with the number of risk alleles) or whether they are overlapping risk factors (in that case, they would explain approximately the same risk and could be substituted for each other). We did not find significantly increased risk with the presence of only one A or G allele per SNP; rather, all four alleles (AA and GG) were necessary for a significant risk increase – possible evidence for the existence of a risk haplotype. This may indicate that using the two SNP genotypes together is a stronger classifier of risk than either alone. Subjects with four high-risk alleles had four-fold higher hazard of future suicide attempt than those with no risk allele, and constituted 32% of the follow-up attempters (Figure 2).
TPH1 genotype is a marker for a subgroup of suicide attempters who are at risk for more serious suicidal behavior characterized by more highly-lethal attempts and a greater likelihood of making future attempts in the short term following a depressive episode. Moreover, although this high-risk group is overwhelmingly Caucasian, the individual SNP genotypes as well as the combined risk haplotype also confer some increased risk for other ethnic groups: in Hispanics with 4 high-risk alleles accounted for 22% of all medically serious baseline attempts (30% in Caucasians), compared to their 13% presence in the Hispanic cohort. In contrast, although African-Americans made more suicide attempts neither of the two African-Americans with the 4 risk alleles made an attempt during follow-up or had a medically serious baseline attempt. In the combined model (Table 3) TPH1 genotype confers a specific risk of suicide attempt in addition to the overall risk/protection offered by self-identified race/ethnicity, and its possible confounders age, race, education and religion. In addition, Hispanics, but not African Americans, are at lower risk of suicide attempt, even after adjustment for age, sex, religion and education.
We have proposed a model of risk for future suicide attempts in mood disorder patients wherein pessimism and aggression-impulsivity trait factors provided multiplicative risks for future attempt (Oquendo et al., 2004). In this sample, intron 7 AA genotype was associated with both fewer reasons for living and greater impulsivity that have shown some predictive utility for suicide attempt in prospective studies (Oquendo et al., 2006). However, our data showed that TPH1 was associated with risk of suicide attempt even after adjusting the model for lower RFL, suggesting that pessimism is at best a partial mediator of this genetic risk.
The AA genotype on intron 7 was also associated with lower impulsivity scores in this study. Higher impulsivity is associated with increased likelihood of making a suicide attempt, but lower likelihood of a high-lethality attempt (Baca-Garcia et al., 2005). Consistent with this observation, in this study the high-risk genotype group has both lower-impulsivity and a greater likelihood to have made a high-lethality past attempt. or a future attempt. We found no genotype association with aggression scores, although at least two studies (Manuck et al., 1999; Rujescu et al., 2002) linked intron 7 genotype to aggressive behavior.
Stress-diathesis interaction
We have found that higher depression severity on the HDRS scale is associated with an increased risk of subsequent suicide attempt in subjects with the AA genotype on intron 7, but not in other subjects. This finding supports the stress-diathesis hypothesis of suicidal behavior (Mann et al., 1999), where subjects react to the same stress (similar level of depression, in this case) according to their diathesis (in this case, TPH1 genotype).
Monoamine metabolites and TPH1 genotype
The A allele of intron 7 is reported to be associated with lower serotonin activity in the brain (Zhang et al., 2004), and related to risk of future suicide (Roy et al., 1989). We have previously reported lower 5-HT activity to be associated with increased aggression/impulsivity and with high lethality suicide attempts but not low lethality ones (Placidi et al., 2001). TPH1 is expressed during early brain development and not in the adult brain (Nakamura et al., 2006), therefore, TPH1 functional genetic variants may affect development of the serotonin system and contribute to the genetic effect on the risk for suicidal behavior via associated traits such as pessimism and/or aggression/impulsivity (Brown et al., 1988). In this study, TPH1 intro 7 or promoter genotype was not associated with CSF measures of 5-HIAA, HVA or MHPG, so we did not detect a biological intermediate phenotype.
Limitations
Suicidal behavior is complex and the TPH1 gene is unlikely to be the only one associated with suicidal behavior. The relatively low sample size in the African-American and Hispanic groups means that the power to detect differential genotype effects by ethnicity on the risk of suicidal behavior may be too low.
Conclusions
Individuals with 4 risk alleles from two SNPs on the TPH1 gene have a 4 fold greater risk of suicide attempt during a one-year follow-up for patients in a major depressive episode compared with those with no risk alleles. The high-risk population is predominantly Caucasian and characterized by greater pessimism, lower impulsivity and higher lethality suicide attempts. Future studies are needed to confirm that the risk conferred by the presence of the 4 alleles is similar across ethnic groups.
Table 2.
Psychiatric scales and CSF measures by TPH1 genotype
| TPH1 Intron 7 Genotype | TPH1 Promoter Genotype | |||||||
|---|---|---|---|---|---|---|---|---|
| AA | AC+CC | t | P-val | GG | AG+AA | t | P-val | |
| Reasons For Living | 135±36 | 157± 44 | 3.7 | <.001 | 152±46 | 154±41 | 0.3 | .794 |
| Brown-Goodwin Aggression | 18±5 | 18±5 | -.2 | .805 | 17±5 | 18±5 | 0.6 | .553 |
| Barratt Impulsivity | 50±16 | 55±17 | 2.2 | .033 | 52±17 | 55±16 | 1.0 | .310 |
| Beck Depression | 28±10 | 29±10 | 0.4 | .725 | 29±10 | 28±11 | -.6 | .542 |
| Hamilton Depression | 19±6 | 19±6 | 0.1 | .892 | 19±6 | 19±6 | -.1 | .900 |
| Suicidal Ideation | 13±10 | 11±10 | 1.4 | .163 | 10±9 | 12±10 | 1.1 | .284 |
| CSF-HIAA | 95±36 | 100±31 | 0.7 | .515 | 99±31 | 96±32 | -0.4 | .703 |
| CSF-MHPG | 48±24 | 46±19 | -.4 | .719 | 45±23 | 43±19 | -.5 | .601 |
| CSF-HVA | 195±66 | 210±87 | 0.9 | .368 | 193±67 | 199±86 | 0.4 | .706 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Arango V, Huang YY, Underwood MD, Mann JJ. Genetics of the serotonergic system in suicidal behavior. J Psychiatr Res. 2003;37(5):375–386. doi: 10.1016/s0022-3956(03)00048-7. [DOI] [PubMed] [Google Scholar]
- Baca-Garcia E, Diaz-Sastre C, Garcia RE, Blasco H, Braquehais CD, Oquendo MA, Saiz-Ruiz J, de Leon J. Suicide attempts and impulsivity. Eur Arch Psychiatry Clin Neurosci. 2005;255(2):152–156. doi: 10.1007/s00406-004-0549-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beck R, Kovacs M. Classification of suicidal behaviors: I. Quantifying intent and medical lethality. Am J Psychiatry. 1975;132(3):285–287. doi: 10.1176/ajp.132.3.285. [DOI] [PubMed] [Google Scholar]
- Bondy B, Buettner A, Zill P. Genetics of suicide. Mol Psychiatry. 2006;11(4):336–351. doi: 10.1038/sj.mp.4001803. [DOI] [PubMed] [Google Scholar]
- Brown CS, Kent TA, Bryant SG, Gevedon RM, Campbell JL, Felthous AR, Barratt ES, Rose RM. Blood platelet uptake of serotonin in episodic aggression. Psychiatry Res. 1988;27:5–12. doi: 10.1016/0165-1781(89)90003-6. [DOI] [PubMed] [Google Scholar]
- Courtet P, Jollant F, Castelnau D, Buresi C, Malafosse A. Suicidal behavior: relationship between phenotype and serotonergic genotype. Am J Med Genet C Semin Med Genet. 2005;133(1):25–33. doi: 10.1002/ajmg.c.30043. [DOI] [PubMed] [Google Scholar]
- Courtet P, Picot MC, Bellivier F, Torres S, Jollant F, Michelon C, Castelnau D, Astruc B, Buresi C, Malafosse A. Serotonin transporter gene may be involved in short-term risk of subsequent suicide attempts. Biol Psychiatry. 2004;55(1):46–51. doi: 10.1016/j.biopsych.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Huang YY, Oquendo MA, Friedman JM, Greenhill LL, Brodsky B, Malone KM, Khait V, Mann JJ. Substance abuse disorder and major depression are associated with the human 5-HT1B receptor gene (HTR1B) G861C polymorphism. Neuropsychopharmacology. 2003;28(1):163–169. doi: 10.1038/sj.npp.1300000. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999;156(2):181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Dent KM, Mann JJ, Muldoon MF. Aggression and anger-related traits associated with a polymorphism of the tryptophan hydroxylase gene. Biol Psychiatry. 1999;45:603–614. doi: 10.1016/s0006-3223(98)00375-8. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sugawara Y, Sawabe K, Ohashi A, Tsurui H, Xiu Y, Ohtsuji M, Lin QS, Nishimura H, Hasegawa H, Hirose S. Late developmental stage-specific role of tryptophan hydroxylase 1 in brain serotonin levels. J Neurosci. 2006;26(2):530–534. doi: 10.1523/JNEUROSCI.1835-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Goldman D, Virkkunen M, Tokola R, Rawlings R, Linnoila M. Suicidality and 5-hydroxyindoleacetic acid concentration associated with a tryptophan hydroxylase polymorphism. Arch Gen Psychiatry. 1994;51(1):34–38. doi: 10.1001/archpsyc.1994.03950010034005. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Virkkunen M, Lappalainen J, Eggert M, Brown GL, Long JC, Goldman D, Linnoila M. A tryptophan hydroxylase gene marker for suicidality and alcoholism. Arch Gen Psychiatry. 1998;55(7):593–602. doi: 10.1001/archpsyc.55.7.593. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Currier D, Mann JJ. Prospective studies of suicidal behavior in major depressive and bipolar disorders: what is the evidence for predictive risk factors? Acta Psychiatr Scand. 2006;114(3):151–158. doi: 10.1111/j.1600-0447.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, Mann JJ. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. Am J Psychiatry. 2004;161(8):1433–1441. doi: 10.1176/appi.ajp.161.8.1433. [DOI] [PubMed] [Google Scholar]
- Placidi GP, Oquendo MA, Malone KM, Huang YY, Ellis SP, Mann JJ. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol Psychiatry. 2001;50(10):783–791. doi: 10.1016/s0006-3223(01)01170-2. [DOI] [PubMed] [Google Scholar]
- Rotondo A, Schuebel K, Bergen A, Aragon R, Virkkunen M, Linnoila M, Goldman D, Nielsen D. Identification of four variants in the tryptophan hydroxylase promoter and association to behavior. Molecular Psychiatry. 1999;4(4):360–368. doi: 10.1038/sj.mp.4000578. [DOI] [PubMed] [Google Scholar]
- Roy A, De Jong J, Linnoila M. Cerebrospinal fluid monoamine metabolites and suicidal behavior in depressed patients. A 5-year follow-up study. Arch Gen Psychiatry. 1989;46:609–612. doi: 10.1001/archpsyc.1989.01810070035005. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Giegling I, Bondy B, Gietl A, Zill P, Moller HJ. Association of anger-related traits with SNPs in the TPH gene. Mol Psychiatry. 2002;7(9):1023–1029. doi: 10.1038/sj.mp.4001128. [DOI] [PubMed] [Google Scholar]
- Therneau TM, Grambsch PM. Modeling Survival Data: extending the Cox model. Springer Verlag; New York Berlin Heidelberg: 2000. [Google Scholar]
- Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305(5681):217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]


