Abstract
Background
Fanconi Anemia (FA) is a rare genetic disorder resulting in a loss of function of the FA-related DNA repair pathway. Individuals with FA are predisposed to some cancers including oropharyngeal and gynecological cancers with known associations with human papillomavirus (HPV) in the general population. Since individuals with FA respond poorly to chemotherapy and radiation, prevention of cancer is critical.
Methods
To determine if individuals with FA are particularly susceptible to oral HPV infection, we analyzed survey-based risk factor data and tested DNA isolated from oral rinses from 126 individuals with FA and 162 unaffected first-degree family members for 37 HPV types.
Results
Fourteen individuals (11.1%) with FA tested positive, significantly more (p=0.003) than family members (2.5%). While HPV prevalence was even higher for sexually active individuals with FA (17.7% vs. 2.4% in family; p=0.003), HPV positivity also tended to be higher in the sexually inactive (8.7% in FA vs. 2.9% in siblings). Indeed, having FA increased HPV positivity 4.9 fold (95%CI: 1.6–15.4) considering age and sexual experience, but did not differ by other potential risk factors.
Conclusion
Our studies suggest that oral HPV is more common in individuals with FA. It will be essential to continue to explore associations between risk factors and immune dysfunction on HPV incidence and persistence over time.
Impact
HPV vaccination should be emphasized in those with FA as a first step to prevent oropharyngeal cancers, although additional studies are needed to determine if the level of protection it offers in this population is adequate.
Keywords: Fanconi anemia, oral human papillomavirus, head and neck squamous cell carcinoma, oropharyngeal cancer, HPV vaccine
Introduction
Fanconi anemia (FA) is an inherited autosomal recessive or rare X-linked disorder characterized by genome instability, progressive bone marrow failure and predisposition to gynecological and head and neck squamous cell carcinomas (HNSCC). The risk of HNSCC is 1,000-fold greater compared to the general population, remains high even after bone marrow transplantation (BMT), and occurs at strikingly early ages with poor prognoses (1–4). Human papillomavirus (HPV) infection, particularly HPV16, is known to be associated with HNSCC in the general population; specifically oropharyngeal cancers (5). Interestingly, HPV positivity was significantly higher in oral samples from individuals with FA living in Brazil than in non-FA controls (6) suggesting a role for HPV in FA-associated cancer. This report was consistent with an earlier United States (US)-based study that found HPV DNA prevalence to be significantly greater in 25 FA-related HNSCC tumors compared to non-FA controls (7). However, a Dutch study failed to detect HPV DNA in any of their FA-related tumors tested (8) and HPV was not detected in 5 HNSCC or 4 anogenital squamous cell carcinoma samples from subjects with FA in a more recent study (9). Therefore, the extent to which HPV is associated with FA or HNSCC in individuals with FA remains unclear. While HPV is commonly sexually transmitted, it has been detected in healthy children and virgin adults in a number of studies (10–12), likely as a result of horizontal (non-sexual, perinatal or parenteral) transmission. Better characterization of the natural history of oral HPV in the FA population is therefore needed.
FA is a consequence of mutations in one of 16 genes whose respective protein products assemble in the nucleus to repair DNA damage (13, 14). Our recent studies indicate that under normal circumstances, activation of this important DNA repair pathway limits the HPV life cycle and prevents malignant transformation. Specifically, loss of function of the FA-related DNA repair pathway in HPV positive keratinocytes stimulates cellular and viral DNA replication (15). Conversely, rescue experiments in cells obtained from individuals with FA reduced HPV-mediated DNA damage and suppressed tumor growth (16) indicating that FA DNA repair pathway deficient keratinocytes uniquely support HPV infection and/or replication. These findings are reinforced by studies in mice demonstrating that deficiencies in the FA DNA repair pathway are associated with an increased incidence of HNSCCs, and with the ability of the HPV E7 protein to induce DNA damage (17–19). In addition, recent immunophenotyping and immune function studies in ten children with FA suggest heterogeneous immune defects (20). The extent to which FA DNA repair pathway deficiency in keratinocytes and variable or inadequate immune cell function contributes to early HPV acquisition, maintenance and/or susceptibility to cancer remains unknown. Given that HPV vaccination has been shown to offer protection against some high risk HPV types in the general population, now is a critical time to investigate HPV in these vulnerable individuals to determine the need for earlier vaccination and to develop targeted treatments.
In this study, we examined the prevalence of HPV infection in a sample of individuals with FA together with their parents who are obligate heterozygotes for the FA mutation, and siblings who are possible heterozygotes for the FA mutation. Study of family members helped to control for possibly non-sexual routes of exposure to HPV. Importantly, we observed a higher prevalence of HPV in individuals with FA as compared to their family members, particularly those that had been sexually exposed.
Materials and Methods
Informed Consent
This study was approved by Cincinnati Children’s Hospital Medical Center (CCHMC) Institutional Review Board. Briefly, following a detailed verbal description of the study, written informed consent/parental permission was obtained by a trained clinical research coordinator from participants’ ≥18 years old or participants’ parent(s)/legal authorized representatives. Participants that were ≥8 years old were asked to sign the consent document along with their parents/guardians to indicate their assent. Younger children or children unable to provide written assent indicated their assent by willingly providing their sample(s).
Participant Recruitment
Patients visiting the Cincinnati Children’s FA Comprehensive Care Center (CCFACCC) were approached to participate. Further, attendees of the Fanconi Anemia Research Fund (FARF) Adult and Family Meetings were invited to participate. Individuals of all ages were eligible to participate if they reported a diagnosis of FA or were a parent or sibling of an individual with FA, and were willing to complete study-related surveys and provide oral rinse samples. A subset (N=68) also provided a venous blood sample for HPV serology and immune studies. A total of 150 subjects with FA have been enrolled in the study as well as 247 family members (biological siblings and parents). Of these, 126 individuals with FA, 41 siblings and 121 parents currently have at least one HPV test result.
Oral Sample Collection, Nucleic Acid Isolation and HPV Testing
Oral exfoliated cells or oral-rinse and gargle samples were collected in 15 mL normal saline and 2.5 mL of 100% ethanol was added immediately after collection. Samples were stored for no more than 4 days at room temperature and then spun at 3,200 RPM for 10 minutes at 4°C, the pellet washed in 10 mL of phosphate-buffered saline (PBS), spun again and resuspended in 1 mL PBS and stored in 500 µl aliquots at −80°C. In the laboratory of Darron Brown at Indiana University, DNA was isolated using the 5 Prime ArchivePure DNA kit (5 Prime Inc., Gaithersburg, MD) according to the manufacturer’s protocol. DNA samples were genotyped by The Linear Array HPV Genotyping Test (Roche Molecular Diagnostics, Indianapolis, Indiana) (21). Reactions were amplified in a PerkinElmer TC9600 Thermal Cycler (PerkinElmer), and positive and negative controls (included in the assay) were performed with every polymerase chain reaction (PCR) run. The GH20/PC04 human β-globin target was co-amplified to determine sample adequacy, and detection of specific HPV types was performed as previously described (22). The 37 individual HPV types detected are comprised of high risk HPV types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, 82, and Subtype 82 W13B) and low-risk HPV types (6, 11, 40, 42, 54, 55, 61, 62, 64, 72, 81, 83, 84, and 89).
Blood Collection, Processing and HPV Antibody Testing
Venous blood was collected observing appropriate aseptic techniques and universal precautions by trained personnel. Fifty percent were collected at the CCFACCC and immediately placed into storage at −80 degrees Celsius. Samples collected at FARF Meetings were typically kept at room temperature and centrifuged at 2,400 × g within 8 hours of collection, aliquoted and then stored on dry ice until they were shipped via Same-Day FedEx (N=20). Otherwise, they were shipped via Same-Day FedEx to CCHMC, centrifuged within 24 hours, aliquoted and stored at −80 degree Celsius (N=14). An aliquot of each serum (0.5–1mL typically) was sent via Next-Day FedEx for HPV serology testing in the HPV laboratory at the Centers for Disease Control and Prevention. The HPV4-plex virus-like particle (VLP)-based IgG enzyme-linked immunosorbent assay (ELISA) for HPV 6, 11, 16 and 18 was performed as previously described (23). Cut-off values of Median+2 standard deviations (SD) were calculated using children’s sera (Gift from Joakim Diller Karolinksa Institutet, Sweden) for each type. The pseudovirion neutralization assay (PBNA) was performed as described (24).
Immune assays
Humoral immune function was analyzed to assess effective or ineffective immune responses to HPV infection and or vaccination. Patient sera were tested for lymphocyte subsets, B cell panel, immunoglobulin levels (IgA, IgG, and IgM) along with tetanus and diphtheria titers. Immunoglobulin levels were determined by standard methods in the CCHMC clinical laboratory. Tetanus and diphtheria titers were determined by Quantitative Multiplex Bead Assay in the clinical diagnostic laboratory at CCHMC. Remaining assays were performed at the Diagnostic Immunology Laboratories at CCHMC. Results were interpreted with respect to age-appropriate reference ranges established in the laboratory. Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by centrifugation with lymphocyte separation media (MP Biomedicals, Solon, OH, USA). Evaluation of patient lymphocyte subsets was performed via routine four-color flow cytometric analysis of EDTA preserved whole blood using fluorochrome-labelled monoclonal antibodies to lineage-specific cell surface markers for T cells (CD3, CD4, CD8), B cells (CD19), and natural killer (NK) cells (CD16, CD56). All antibodies were obtained from BD Biosciences (San Jose, CA, USA). Briefly, erythrocytes were lysed by incubation in FACSLyse (BD Biosciences). Samples were then stained with antibody and analyzed on a FACSCalibur flow cytometer (BD Biosciences) using multiset software (BD Biosciences). Similar flow based approach was applied for assessment of B-Cell Panel (25).
Risk Factor Survey
Survey completion was done either by paper or by electronic survey (Research electronic data capture (REDCap) software) (26). Participants ages 15 years and older were asked to complete surveys for themselves unless there was a developmental delay, in which case a parent or guardian was asked to complete the survey. For children ages 12 to 15 years, a parent was asked either to complete the survey or assist the child in completing the survey. However, answers to the sexual questions were ascertained by study staff interview whenever possible. Surveys collected demographic and clinical data including FA complementation group, disease severity, and date/age at bone marrow transplant (if any). In addition, other potential risk factors for HPV-related disease such as vaginal versus Cesarean section mode of delivery at birth, gestational age at birth, tobacco smoke exposure, alcohol consumption and sexual history (including questions about oral sex and kissing), family history of FA and cancer, history of personal immunization and/or HPV testing, and presence and frequency of oral lesions were ascertained by survey and/or staff interview. When available, biological mothers of participants were asked whether they have had genital or anal warts, a dysplastic Pap smear, or had a positive HPV test within a 3-year period before the birth of the individual(s) with FA and siblings.
Statistical Analysis
Statistical analyses were performed using Statistical Analysis Software (SAS), version 9.3 (SAS Institute Inc., Cary, NC). Prior to analyses, data qualities were examined. Demographic variables and potential risk factors of HPV were first summarized as median and interquartile range (IQR) or frequencies (proportions) and compared in FA, siblings and parents. These factors were then compared between HPV negative and positive individuals with FA. Using Fisher’s exact tests, the impact of FA status on HPV prevalence was stratified by sexual experience or HPV vaccination status. Using a multi-variable logistic regression, we further tested the effects of HPV risk factors on the prevalence of HPV, adjusting for sexual experience, HPV vaccination status, and FA status in the model. Finally, the association between BMT and oral HPV was assessed considering HPV infection and sexual experience. Effects were considered significant if p-value ≤ 0.05.
Results
Population characteristics
A total of 288 individuals with oral rinse samples and risk factor questionnaires were included in this study. Of these, 126 individuals had FA, 121 were parents of individuals with FA (74 mothers and 47 fathers), and 41 were siblings of the individuals with FA (Table 1). Participants with FA ranged in age from 6 months to 55 years. Family members’ ages ranged from 4 months to 79 years. As expected, ages were significantly different between subjects with FA and family members (p<0.0001). There were more females in each group than males, and more Caucasians than any other race. Individuals with FA were living in families across all income levels (26% <40K, 36% 40–90K, 39%>90K) and most had health insurance (32% public, 54% private and 11% had both public and private insurance) (Table 1).
Table 1.
Demographic and Socioeconomic Characteristics.
| Fanconi Anemia | Siblings | Parents | |||||
|---|---|---|---|---|---|---|---|
| All N=126 |
All N=41 |
N=121 All |
p | ||||
| Age (years) | 12 | (9, 22) | 9 | (5, 16) | 43 | (37, 50) | <0.0001 |
| Age Range (years) | <0.0001 | ||||||
| 0–4 | 13 | (10%) | 6 | (15%) | 0 | (0%) | |
| 5–12 | 51 | (40%) | 19 | (46%) | 0 | (0%) | |
| 13–18 | 25 | (20%) | 10 | (24%) | 0 | (0%) | |
| 19+ | 37 | (29%) | 6 | (15%) | 121 | (100%) | |
| Sex | 0.034 | ||||||
| Male | 56 | (44%) | 9 | (22%) | 47 | (39%) | |
| Female | 70 | (56%) | 32 | (78%) | 74 | (61%) | |
| Race | 0.020 | ||||||
| White/Caucasian | 107 | (86%) | 34 | (94%) | 113 | (95%) | |
| Black/African American | 6 | (5%) | 2 | (6%) | 5 | (4%) | |
| Other/Mixed | 11 | (9%) | 0 | (0%) | 1 | (1%) | |
| Income | 0.14 | ||||||
| Under $39,999 | 28 | (26%) | 3 | (8%) | 21 | (18%) | |
| $40,000–$89,999 | 39 | (36%) | 16 | (40%) | 42 | (37%) | |
| Over $90,000 | 42 | (39%) | 21 | (53%) | 52 | (45%) | |
| Insurance/Medical | 0.001 | ||||||
| Self-Pay/Other | 4 | (3%) | 0 | (0%) | 10 | (9%) | |
| Public/Government | 37 | (32%) | 7 | (21%) | 19 | (16%) | |
| Private | 63 | (54%) | 26 | (79%) | 83 | (72%) | |
| Private and Public | 13 | (11%) | 0 | (0%) | 4 | (3%) | |
Age was tested using Kruskal-Wallis test; other variables were tested by Fisher's exact tests. All analyses were performed on non-missing data. Information on race, income and health insurance was missing in 9, 24 and 22 subjects, respectively. In bold are those P values that are less than 0.05.
HPV prevalence in FA
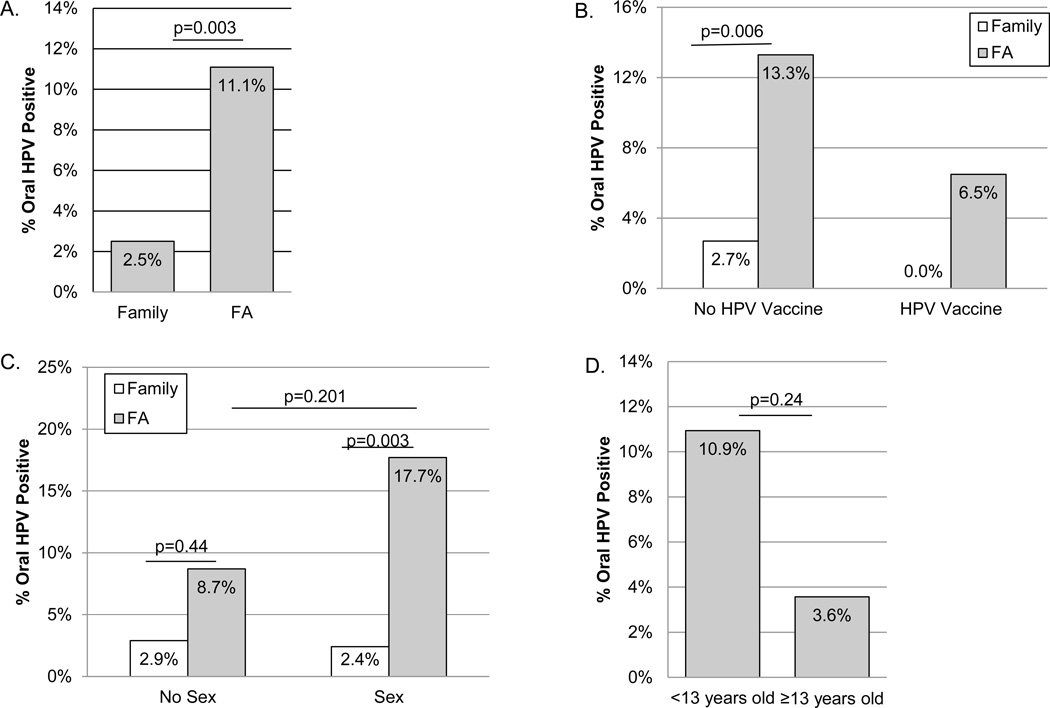
Among those individuals with FA, 14 (11.1%) tested positive for one or more HPV types. Indeed, oral HPV positivity in FA was significantly greater than in non-FA family members (parents and siblings; 2.5%; p-value=0.003; Fisher’s exact test; Figure 1A), whose prevalence’s were consistent with published levels in the general population (range from 1.9% to 7% (10–12)). Also consistent with published studies, the most common types of HPV detected among those with FA were HPV 6 (50%) and HPV 16 (64%), although HPV 18, 51, 66, 84 and CP6 were also detected. Only three of the HPV positive individuals with FA had previously been vaccinated for HPV (all receiving the GARDASIL Quadrivalent HPV Vaccine, Merck & Co., Inc., West Point, PA, USA) and of these three vaccinated HPV positive individuals, only a single person with FA tested positive for an HPV type included in the vaccine (HPV16). We also examined HPV positivity in people based on HPV vaccine history. As shown in Figure 1B, in both vaccinated and unvaccinated groups, individuals with FA tested positive for HPV more often. Importantly, in our study population, we observed significantly higher rates of vaccination in individuals with FA compared to family members (38% vs. 21% in siblings and 1% of parents, p<0.0001, Fisher’s exact test, Table 2). It is therefore interesting that HPV prevalence is higher in FA despite greater rates of vaccination.
Figure 1.
Associations between oral HPV positivity in individuals with FA vs. their family members. A) Markedly greater oral HPV positivity considering all HPV-tested individuals with FA vs. their family members. B) Oral HPV positivity stratified by HPV vaccination (Gardasil). C) Oral HPV positivity stratified by sexual experience. Sex indicates that the participant reported having had sexual contact (oral, genital, and/or anal) via paper or online survey at the time of oral sampling. Those not having reported sexual contact are in the no sex group. D) Oral HPV positivity among those in the no sex group stratified by age. For all comparisons, Oral HPV positivity was determined by Roche Linear Array and the percentage of positive individuals are shown. A fisher’s exact test was used to assess statistical differences (2-sided p-values).
Table 2.
Differences in HPV Risk Factors Between Individuals with Fanconi Anemia and Their Family Members
| Risk Factors | FA N=126 |
Siblings N=41 |
Parents N=121 |
P-value | |||
|---|---|---|---|---|---|---|---|
| HPV Vaccination (N=279) | 46 | (38%) | 8 | (21%) | 1 | (1%) | <0.0001 |
| Sexual Experience (N=288) | 34 | (27%) | 6 | (15%) | 121 | (100%) | <0.0001 |
| Vaginal Birth/Delivery (N=276) | 87 | (71%) | 22 | (54%) | 101 | (90%) | <0.0001 |
| Primary Tobacco User (N=285) | 5 | (4%) | 1 | (2%) | 21 | (18%) | 0.0003 |
| Secondhand Smoke Exposure (N=283) | 14 | (11%) | 3 | (7%) | 14 | (12%) | 0.8120 |
| Alcohol Use (N=285) | 14 | (11%) | 2 | (5%) | 43 | (36%) | <0.0001 |
| Genital Warts (N=282) | 4 | (3%) | 1 | (2%) | 2 | (2%) | 0.8733 |
| Common Warts (N=64) | 9 | (30%) | 2 | (22%) | 9 | (36%) | 0.8123 |
| Oral Sores (N=224) | 14 | (18%) | 3 | (8%) | 5 | (4%) | 0.006 |
| Breastfed as an infant (N=217) | 60 | (71%) | 30 | (81%) | 46 | (48%) | 0.0003 |
| Mother Abnormal Pap/ HPV/STD at Birth (N=109) | 8 | (11%) | 5 | (14%) | - | - | 0.7554 |
All variables were binary. The fisher’s exact test was used to assess differences (2-sided p-values). Pap- Papanicolaou, STD – other sexually transmitted disease (trichomoniasis, chlamydia, gonorrhea, and genital herpes). As some variables have missing data, frequencies are provided for each. In bold are those p-values that are less than 0.05.
Typically transmission of high risk carcinogenic HPV types in the general population occurs predominantly via sexual contact including oral sex and open-mouthed kissing outside of vaginal sex (27). When we examined only those individuals who were sexually experienced, we observed significantly more oral HPV infections in those with FA (17.7%; p-value =0.003) compared to family members (2.4%; Figure 1C). In participants that were not yet sexually experienced, individuals with FA also tested positive for oral HPV (8.7%) more often compared to siblings (2.9%), though the difference did not reach statistical significance. We next assessed the effect of sex on the association between oral HPV positivity and FA. While having sex did not seem to increase HPV positivity in family members, individuals with FA exhibited a higher prevalence of HPV when they reported having had sexual experience (17.7% vs 8.7%), but this association again was not statistically significant at the 5% level. In multivariable logistic regression analysis including sexual experience, HPV vaccination and FA status, only having FA was significantly associated with oral HPV positivity (p=0.002). Indeed, having FA increased the odds of HPV positivity by 4.9 fold (95% CI: 1.6 –15.4). Greater HPV prevalence was observed in those with FA compared to family members regardless of age. Importantly, among those individuals with FA that were not sexually active, 7 children less than 13 years of age (10.9%) also tested HPV positive (Figure 1D).
Other potential between-group confounding or risk factors
We next evaluated several other factors associated with HPV positivity or HNSCC in the general population and compared those with FA to their family members (Table 2). We observed that 71% of individuals with FA compared to 54% of siblings and 90% of parents were delivered vaginally (p=0.0001; Table 2). The biological mothers of those with FA compared to sibling group as a whole were not more likely to have reported having an abnormal Pap smear, positive HPV test or other sexually transmitted disease within 3 years of their children’s birth (p=0.75). Not surprising given the differences in age as well as strength of the anti-smoking and anti-alcohol guidelines and education provided to those with FA by the Fanconi Anemia Research Fund (28), primary smoking and alcohol use were lower for those with FA and their siblings compared to parents (p=0.0003 and 0.0001, respectively; Table 2). While the frequency of genital warts and common warts did not significantly differ between groups (p=0.87 and p=0.81, respectively; Table 2), significantly more individuals with FA reported oral sores (18%) compared to 8% of siblings and 4% of parents (p=0.006).
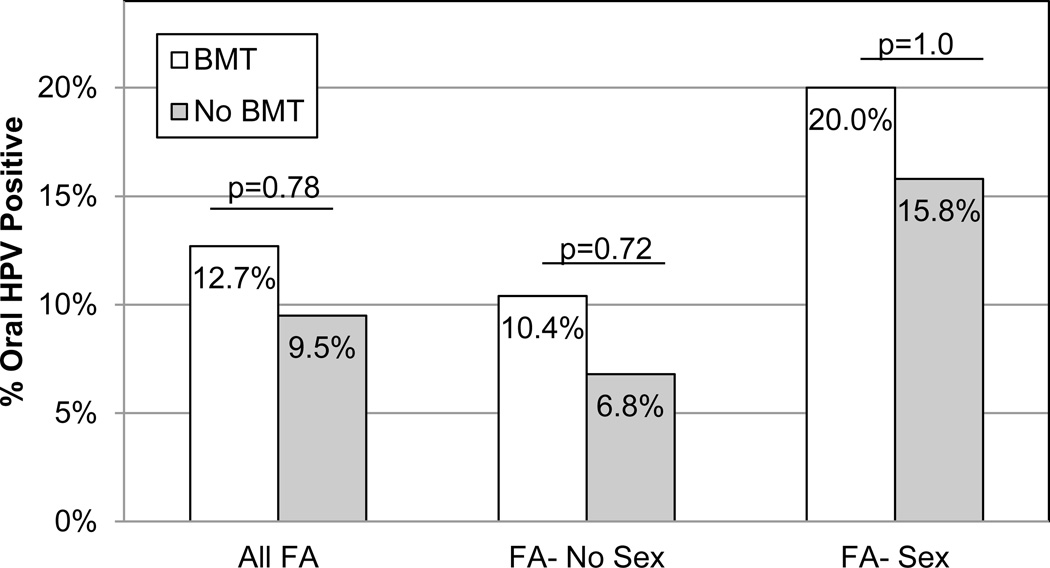
Examining only those individuals with FA, no other factor related to FA (complementation group, having had a transfusion or having a family history of cancer) or those known to contribute to HPV or HNSCCs (i.e. tobacco and alcohol use, oral, genital and common warts, and dental hygiene) were found to be significantly different between oral HPV positive or oral HPV negative individuals with FA in our study (Table 3). While some have hypothesized that BMT might be associated with the increased risk of HNSCC in individuals with FA, we found that HPV positivity was only slightly higher (12.7%) post-BMT compared to pre-BMT (9.5%) and this association was not statistically significant (p=0.78; Figure 2). When we considered BMT and sexual experience simultaneously, we again observed higher levels of oral HPV positivity in those post-BMT particularly if they had had sexual experience, but again these differences were not statistically significant (p=1.0; Figure 2). Secondhand smoke exposure, however, was marginally statistically significantly higher in those that were oral HPV positive (29%) compared to oral HPV negative (9%; p=0.052).
Table 3.
Associations with HPV In Individuals with Fanconi Anemia
| Potential Risk Factors | FA HPV− N=112 |
FA HPV+ N=14 |
P-value | ||
|---|---|---|---|---|---|
| Age ≥ 13 years (N=126) | 55 | (49%) | 7 | (50%) | 1.000 |
| Male Sex (N=126) | 48 | (43%) | 8 | (57%) | 0.396 |
| White/Caucasian (N=124) | 95 | (86%) | 12 | (86%) | 1.000 |
| Income < $39,999/year (N=109) | 25 | (26%) | 3 | (23%) | 1.000 |
| Private Medical Insurance (N=117) | 51 | (50%) | 12 | (86%) | 0.0195 |
| HPV Vaccination (N=121) | 43 | (40%) | 3 | (23%) | 0.366 |
| Sex Experience (N=126) | 28 | (25%) | 6 | (43%) | 0.201 |
| Delivered Vaginally (N=123) | 77 | (71%) | 10 | (71%) | 1.000 |
| Primary Tobacco User (N=126) | 4 | (4%) | 1 | (7%) | 0.451 |
| Secondhand Smoke Exposure (N=125) | 10 | (9%) | 4 | (29%) | 0.052 |
| Alcohol User (N=126) | 12 | (11%) | 2 | (14%) | 0.655 |
| Genital Warts (N=124) | 3 | (3%) | 1 | (8%) | 0.362 |
| Common Warts (N=30) | 7 | (26%) | 2 | (67%) | 0.207 |
| Oral Sores (N=76) | 13 | (19%) | 1 | (17%) | 1.000 |
| Breastfed (N=85) | 53 | (68%) | 7 | (100%) | 0.100 |
| Family History of Cancer (N=119) | 48 | (46%) | 7 | (50%) | 0.783 |
| Mother Abnormal Pap/HPV/STD at Birth (n=73) | 7 | (11%) | 1 | (13%) | 1.000 |
| Bone Marrow Transplant (n=126) | 55 | (49%) | 8 | (57%) | 0.778 |
| Blood Transfusions (n=121) | 67 | (63%) | 9 | (64%) | 1.000 |
All variables were dichotomized. The Fisher’s exact test was used to assess group differences (2-sided p-values). Among the 14 HPV+ individuals, 1 person was missing data on HPV vaccination and genital warts, 11 were missing common warts, 8 missing oral sores, 7 subjects were missing breastfeeding data and 6 subjects did not have biological mother Pap/sexually transmitted disease (STD) data at the participant’s birth available. Those with private medical insurance were compared to those with no-pay/self-pay/public policies. In bold are those p-values that are less than 0.05.
Figure 2.
Oral HPV positivity stratified by both history of bone marrow transplant (BMT) history and sexual experience. Oral HPV positivity was determined by the Roche Linear Array and the percentage of positive individuals are shown. BMT history was reported via paper or online survey at the time of oral sampling. Sex indicates that the participant reported having had sexual contact (oral, genital, and/or anal). Those not having reported sexual contact are in the no sex group. A fisher’s exact test was used to assess statistical differences (2-sided p-values).
Characterization of individuals with FA who are HPV positive
There was a broad range of ages (from 6 to 51 years of age) among the 14 individuals with FA who tested oral HPV positive (Table 3). Importantly, 7 (50%) were under the age of 13 years and 8 individuals with FA indicated that they were not sexually active. The level of oral HPV positivity was similar for males and females and there were no differences by whether or not they had a BMT (57%). While 9 (64%) had the FANCA complementation group, C, F, I, and D2 complementation groups were also represented in those that tested oral HPV positive (Table 4). Only a single oral HPV positive participant (HPV 84) reported having had a diagnosis of oropharyngeal cancer prior to sampling.
Table 4.
Individual Characteristics of HPV+ Individuals with Fanconi Anemia
| # | Age (years) |
Sex | Sexual Exposure |
BMT | FANC Group |
HPV Vaccine | HPV Types+ |
|---|---|---|---|---|---|---|---|
| 1 | 32 | F | + | − | A | − | 6, 16 |
| 2 | 7 | F | − | + | A | − | 66 |
| 3 | 23 | F | + | + | Unknown | − | 84 |
| 4 | 20 | F | + | − | A | + | 84 |
| 5 | 14 | F | − | + | A | + | 16 |
| 6 | 12 | F | − | + | A | + | CP6 |
| 7 | 7 | M | − | − | Other | − | 6, 16 |
| 8 | 27 | M | + | + | Other | − | 6, 16 |
| 9 | 10 | M | − | − | A | − | 6, 16 |
| 10 | 6 | M | − | + | Other | − | 6, 16 |
| 11 | 51 | M | + | − | A | − | 16 |
| 12 | 6 | M | − | − | Other | − | 6, 16 |
| 13 | 12 | M | − | + | A | − | 6, 16, 18 |
| 14 | 35 | M | + | + | A | − | 51 |
| Sum | 7 <13y | 8 M's | 6+'s | 8+'s | 8 A's | 3 +'s | 9 HPV16's |
M – Male sex, F-female sex, BMT – history of Bone Marrow Transplant, FANC- FA complementation group, + indicates presence of exposure/history of BMT or HPV vaccine. Other complementation groups represented include C, F, I, and D2. BMT, bone marrow transplant
Further, among all 8 oral HPV positive individuals with FA who had corresponding immune data, we observed 6 study participants to have normal IgG levels and 5 participants with normal absolute and/or memory B cells (Table 5). Most tetanus and diphtheria titers were also normal. Only 3 of the 8 subjects had been vaccinated for HPV prior to blood sampling. All vaccinated individuals had positive titers to all four HPV types included in the vaccine (all Gardasil) as well as normal tetanus and diphtheria levels (Table 5). Two of the oral HPV positive individuals who indicated that they had not yet been vaccinated for HPV were also serologically positive to types other than identified in their oral sample suggesting prior natural infections with these types. Three of the oral HPV positive participants had no detectable HPV titers. Two of these individuals tested positive for both oral HPV6 and HPV16. Interestingly, these individuals were either deficient in their absolute B cell count or memory B cells. Overall, there were no unifying characteristics that were shared by those that were oral HPV or serologically positive with FA. In fact, these data support our previous report that individuals with FA have heterogeneous immune responses (20).
Table 5.
Immune and HPV-serological Responses
| # | Age (years) at: | General Immune Response | Oral HPV Types + |
GARDASIL HPV Vaccine (#shots) |
HPV-Specific Immune Responses | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMT | Oral rinse |
Blood | Absolute B-cell Count |
Memory B-cells |
IgG | Tet | Dip | HPV6 VLP- ELISA |
HPV11 VLP- ELISA |
HPV16 VLP- ELISA |
HPV18 VLP- ELISA |
HPV16 PBNA |
HPV18 PBNA |
|||
| 1 | 7 | 10 | Normal | Normal | Down | ++ | + | 6,16 | −/+ (2)* | + | + | + | + | + | + | |
| 2 | 18 | 27 | 30 | Normal | Normal | Normal | + | − | 6, 16 | −/+ (3)* | + | + | + | + | + | − |
| 3 | 10 | 13 | Normal | Normal | Normal | + | ++ | 6,16 | − | + | + | + | + | + | + | |
| 4 | 4 | 6 | 9 | Down | Up | Normal | ++ | ++ | 6, 16 | − | − | − | − | − | − | − |
| 5 | 32 | 35 | Normal | Down | Normal | + | + | 6, 16 | − | − | − | − | − | − | − | |
| 6 | 5 | 7 | 7 | Up | Normal | Normal | + | + | 66 | − | − | − | − | − | − | − |
| 7 | 11 | 23 | 23 | Normal | Normal | Up | + | − | 84 | − | + | − | + | + | + | + |
| 8 | 20 | 23 | Down | Up | Normal | ++ | + | 84 | 3 | + | + | + | + | + | + | |
Did not have the human papillomavirus (HPV) vaccine at time of oral rinse collection, but had received the vaccine at time of blood collection; tetanus (Tet)/diphtheria (Dip) responses >=1.0 IU/mL is considered protective (++); 0.1 to 0.99 IU/mL (+); <0.1 IU/mL (−) is considered a non-responder; absolute CD3 and absolute CD16/56 were normal for all 8 participants. BMT-bone marrow transplant; VLP- Virus-Like Particles; ELISA- enzyme-linked immunosorbent assay; PBNA-pseudovirion-based neutralization assay.
Discussion
Our cross sectional study demonstrates a higher frequency of oral HPV positivity in individuals with FA compared to family members. Further, while HPV prevalence was significantly higher for those with FA who reported sexual exposure compared to family members, HPV prevalence tended to also be higher in those who were not yet exposed to sex.
A possible role for HPV in FA (29) has been previously suggested following the report of higher prevalence of HPV in FA-related head and neck tumors (7). In contrast, another equally small study reported the absence of HPV in FA-related HNSCC tumors and cell lines derived from them (8). These opposing results may be due to experimental variability such as types of primers used, quality and content of DNA tested, analysis of tumors or tumor-derived cell lines, or geographic differences. More recently, Alter et al were unable to detect HPV in HNSCCs from individuals with FA (9). However, de Araujo et al. have shown an increased prevalence of oral HPV isolated from 42 oral cytobrush samples from individuals with FA without oral malignant lesions (6). One possibility is that the Brazilian population spanned a younger age range comparable to our study population. A second explanation for the discrepancy could be that some/many individuals with FA successfully clear the virus or suppress its replication before tumors develop, thus limiting detection in the tumors themselves. These studies, together with our data presented here, suggest that studies of the association between HPV and FA might have to be conducted on younger individuals, and call for continued longitudinal studies across the lifespan to determine if early life exposure to HPV impacts the development of HNSCCs in individuals with FA later in life. Further, longitudinal studies would better address potential routes of transmission outside of sexual exposure. We have currently not detected significant differences in breastfeeding, birthing method (c-section vs. vaginal), BMT/blood transfusions, and alcohol and tobacco exposure, although in some cases, the data collected was incomplete and likely underpowered to ascertain a true association.
While studies in human subjects remain controversial, there is growing evidence from cell lines, animal studies, expression analysis and computational biology approaches that support a strong association between the consequences of HPV infection in the context of deficiency in the FA proteins and development of cancer. Organotypic raft cultures of HPV-oncogene immortalized, FANCA deficient patient-derived keratinocytes demonstrate increased DNA damage, and excessive hyperplasia compared to FANCA complemented cell populations (16). In addition, the FA pathway seems to limit the ability of HPV to replicate in cultured keratinocytes (15). Indeed, FANCD2 deficient mice overexpressing the HPV E7 oncoprotein have increased DNA damage and are more likely to develop HNSCCs (17) and cervical and vaginal cancer (18). These effects of E7 are due to inactivation of the Rb family of tumor suppressors that limit DNA damage (19). Microarray analysis of vulvar tissue infected with both low-risk and/or high-risk HPV types indicated that FANCA, FANCD2, and other DNA damage markers were significantly induced following high-risk HPV infection, along with increased DNA damage in the tissues (30). And finally, using a computational biology approach, a link between HPV16 and the FA DNA repair pathway was identified and then validated experimentally by evaluating the impact of overexpressing E6 or E7 proteins in primary fibroblasts and keratinocytes using global gene expression analysis (31).
Together, these diverse approaches point to a common theme. It appears that HPV infection results in elevated DNA damage that then triggers the FA pathway to repair this DNA damage. In individuals where this pathway is defective, it is likely that the DNA damage will not be repaired, and as a consequence increases the likelihood of tumor development in the long term. It becomes important to consider these studies again in the context of the conflicting human data. Even if HPV is suppressed or cleared, and is undetectable by PCR assays, one might speculate that the resulting DNA damage is the trigger for increased HNSCCs and anogenital carcinomas many years later. Additional studies will be required to support this hypothesis.
Similar to the Brazilian study (6), we found that oral HPV16 was the most common type detected. We also detected less common HPV types in those with FA (36% of oral samples). It is plausible, although speculative, that less common high-risk types, or even low-risk HPV types, might unusually initiate a cancer cascade in those with FA who have both a DNA repair defect and heterogeneous immune deficiency, suggesting a need to evaluate the broad spectrum of HPV types rather than HPV16 and 18 alone in oral rinse and possibly other sample types. Indeed, among 67 throat swabs from individuals with FA collected at the same time as our oral rinse samples in this report, we identified 5 individuals positive for HPV 33, HPV 84, HPV 66, 51, 52, 62 82 and HPV 51; a 60% agreement between sample types (32).
An important variable to consider is the immune response to HPV infection in individuals with FA. We have previously demonstrated that individuals with FA have fewer B cells and NK cells, as well as decreased NK cell function and cytotoxic T cell activity (20). It is conceivable that in the absence of a fully functional FA pathway, the ability to suppress or clear HPV is also delayed. Based on the data from Table 5, there seems to be variability in the immune responses in HPV positive individuals with FA. While some subjects with FA had normal immune responses, others seem to be unable to mount a strong response (based on B cell and memory B cell numbers, IgG levels, and response to tetanus and diphtheria vaccines). These data indicate that while not all individuals with FA have measurably impaired immune responses, those that do could conceivably be at higher risk. It is reasonable to speculate that viral persistence due to the inability of the immune system to clear the infection or due to fluctuations in host immunity as a result of progressive bone marrow failure or FA-related illnesses, further increases the duration of HPV infection and extent of DNA damage, thereby resulting in an increased risk for carcinomas. Our data showed no differences in the type and distribution of factors known to cause cancers in individuals with Fanconi anemia regardless of their HPV infection status. This would further suggest that it might be the ability of the individual to suppress or clear the infection that most likely determines HPV prevalence, incidence, and susceptibility to HNSCCs.
In conclusion, continued longitudinal collection of oral samples and broad testing for HPV types along with immunological studies to better characterize response remains critical to address these questions. In addition, studies are needed to determine prospectively if HPV infection is associated with increased HNSCCs in individuals with FA, and if vaccination at a younger age could be effective in preventing early infection.
Key Points.
-
–
Increased oral HPV prevalence, particularly of HPV16, in individuals with Fanconi Anemia
-
–
No obvious risk factors for oral HPV positivity among individuals with FA
-
–
Those with FA have heterogeneous general immune responses and positive HPV serology
Acknowledgments
This study was funded through the Fanconi Anemia Research Fund (P.I. S.W), a CCHMC-sponsored Translational Research Initiative Grant (P.I. S.W) and by an NIH R01 HL108102 (P.I. MBK). REDCap was hosted at CCHMC and supported by the Center for Clinical and Translational Science and Training grant UL1-RR026314-01 NCRR/NIH. We also would like to thank the CCHMC Fanconi Anemia Comprehensive Care Center staff, Fanconi Anemia Research Fund staff and Camp Sunshine staff for their support of this study. Most importantly, we want to thank all the patients, campers and their families who participated and continue to participate in the study!
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Abbreviations
- HPV
human papillomavirus
- HNSCC
head and neck squamous cell carcinoma
- FA
Fanconi anemia
- BMT
bone marrow transplant
- CCHMC
Cincinnati Children’s Hospital Medical Center
- CCFACCC
Cincinnati Children’s FA Comprehensive Care Center
- REDCap
Research Electronic Data Capture
- FARF
Fanconi Anemia Research Fund
- IQR
interquartile range
- PBS
phosphate-buffered saline
- PBNA
pseudovirion neutralization assay
- VLP
virus-like particle
- ELISA
enzyme-linked immunosorbent assay
- DNA
deoxyribonucleic acid
- PCR
polymerase chain reaction
- SD
standard deviation
- STD
sexually transmitted disease
- IgG
immunoglobulin G
- Tet
tetanus
- Dip
diphtheria
Footnotes
Conflict of Interests: The authors do not have any conflicts of interest or financial disclosures related to this work, including declarations of financial interest, to report.
References
- 1.Lin J, Kutler DI. Why otolaryngologists need to be aware of Fanconi anemia. Otolaryngol Clin North Am. 2013;46:567–577. doi: 10.1016/j.otc.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Tamary H, Nishri D, Yacobovich J, Zilber R, Dgany O, Krasnov T, et al. Frequency and natural history of inherited bone marrow failure syndromes: the Israeli Inherited Bone Marrow Failure Registry. Haematologica. 2010;95:1300–1307. doi: 10.3324/haematol.2009.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101:1249–1256. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 4.Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129:106–112. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 5.Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 6.de Araujo MR, Rubira-Bullen IR, Santos CF, Dionisio TJ, Bonfim CM, De Marco L, et al. High prevalence of oral human papillomavirus infection in Fanconi's anemia patients. Oral Dis. 2011;17:572–576. doi: 10.1111/j.1601-0825.2011.01803.x. [DOI] [PubMed] [Google Scholar]
- 7.Kutler DI, Wreesmann VB, Goberdhan A, Ben-Porat L, Satagopan J, Ngai I, et al. Human papillomavirus DNA and p53 polymorphisms in squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2003;95:1718–1721. doi: 10.1093/jnci/djg091. [DOI] [PubMed] [Google Scholar]
- 8.van Zeeburg HJ, Snijders PJ, Wu T, Gluckman E, Soulier J, Surralles J, et al. Clinical and molecular characteristics of squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2008;100:1649–1653. doi: 10.1093/jnci/djn366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alter BP, Giri N, Savage SA, Quint WG, de Koning MN, Schiffman M. Squamous cell carcinomas in patients with Fanconi anemia and dyskeratosis congenita: a search for human papillomavirus. Int J Cancer. 2013;133:1513–1515. doi: 10.1002/ijc.28157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith EM, Swarnavel S, Ritchie JM, Wang D, Haugen TH, Turek LP. Prevalence of human papillomavirus in the oral cavity/oropharynx in a large population of children and adolescents. Pediatr Infect Dis J. 2007;26:836–840. doi: 10.1097/INF.0b013e318124a4ae. [DOI] [PubMed] [Google Scholar]
- 12.Turner DO, Williams-Cocks SJ, Bullen R, Catmull J, Falk J, Martin D, et al. High-risk human papillomavirus (HPV) screening and detection in healthy patient saliva samples: a pilot study. BMC Oral Health. 2011;11:28. doi: 10.1186/1472-6831-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kottemann MC, Smogorzewska A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature. 2013;493:356–363. doi: 10.1038/nature11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H, D'Andrea AD. Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev. 2012;26:1393–1408. doi: 10.1101/gad.195248.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoskins EE, Morreale RJ, Werner SP, Higginbotham JM, Laimins LA, Lambert PF, et al. The fanconi anemia pathway limits human papillomavirus replication. J Virol. 2012;86:8131–8138. doi: 10.1128/JVI.00408-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoskins EE, Morris TA, Higginbotham JM, Spardy N, Cha E, Kelly P, et al. Fanconi anemia deficiency stimulates HPV-associated hyperplastic growth in organotypic epithelial raft culture. Oncogene. 2009;28:674–685. doi: 10.1038/onc.2008.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JW, Pitot HC, Strati K, Spardy N, Duensing S, Grompe M, et al. Deficiencies in the Fanconi anemia DNA damage response pathway increase sensitivity to HPV-associated head and neck cancer. Cancer Res. 2010;70:9959–9968. doi: 10.1158/0008-5472.CAN-10-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JW, Shin MK, Lambert PF. High incidence of female reproductive tract cancers in FA-deficient HPV16-transgenic mice correlates with E7's induction of DNA damage response, an activity mediated by E7's inactivation of pocket proteins. Oncogene. 2014 Jun 26;33(26):3383–3391. doi: 10.1038/onc.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JW, Shin MK, Pitot HC, Lambert PF. High incidence of HPV-associated head and neck cancers in FA deficient mice is associated with E7's induction of DNA damage through its inactivation of pocket proteins. PLoS One. 2013;8:e75056. doi: 10.1371/journal.pone.0075056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers KC, Bleesing JJ, Davies SM, Zhang X, Martin LJ, Mueller R, et al. Impaired immune function in children with Fanconi anaemia. Br J Haematol. 2011;154:234–240. doi: 10.1111/j.1365-2141.2011.08721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castle PE, Porras C, Quint WG, Rodriguez AC, Schiffman M, Gravitt PE, et al. Comparison of two PCR-based human papillomavirus genotyping methods. J Clin Microbiol. 2008;46:3437–3445. doi: 10.1128/JCM.00620-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shew ML, Fortenberry JD, Tu W, Juliar BE, Batteiger BE, Qadadri B, et al. Association of condom use, sexual behaviors, and sexually transmitted infections with the duration of genital human papillomavirus infection among adolescent women. Arch Pediatr Adolesc Med. 2006;160:151–156. doi: 10.1001/archpedi.160.2.151. [DOI] [PubMed] [Google Scholar]
- 23.Kumar D, Unger ER, Panicker G, Medvedev P, Wilson L, Humar A. Immunogenicity of quadrivalent human papillomavirus vaccine in organ transplant recipients. Am J Transplant. 2013;13:2411–2417. doi: 10.1111/ajt.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 25.Bleesing JJ. Assays for B cell and germinal center development. Curr Protoc Immunol. 2004;Chapter 7(Unit 7):35. doi: 10.1002/0471142735.im0735s63. [DOI] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263–1269. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eiler ME, Frohnmayer D, Frohnmayer L, Larsen K, Owen J, editors. Fanconi Anemia: Guidelines for Diagnosis and Management. 3rd ed. Eugene, Oregon: Fanconi Anemia Research Fund; 2008. [Google Scholar]
- 29.Lowy DR, Gillison ML. A new link between Fanconi anemia and human papillomavirus-associated malignancies. J Natl Cancer Inst. 2003;95:1648–1650. doi: 10.1093/jnci/djg125. [DOI] [PubMed] [Google Scholar]
- 30.Santegoets LA, van Baars R, Terlou A, Heijmans-Antonissen C, Swagemakers SM, van der Spek PJ, et al. Different DNA damage and cell cycle checkpoint control in low- and high-risk human papillomavirus infections of the vulva. Int J Cancer. 2012;130:2874–2885. doi: 10.1002/ijc.26345. [DOI] [PubMed] [Google Scholar]
- 31.Gulbahce N, Yan H, Dricot A, Padi M, Byrdsong D, Franchi R, et al. Viral perturbations of host networks reflect disease etiology. PLoS Comput Biol. 2012;8:e1002531. doi: 10.1371/journal.pcbi.1002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winer R, Huang C, Cherne S, Stern J, Butsch Kovacic M, Mehta P, et al. Detection of human papillomavirus in the oral cavities of persons with Fanconi anemia. Oral Dis. 2015 Apr;21(3):349–354. doi: 10.1111/odi.12286. Epub 2014 Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]