Abstract
Background
High-grade upper tract urothelial carcinoma (UTUC) is frequently upstaged after surgery and is associated with uniformly poor survival. Neoadjuvant chemotherapy may offer a way to improve clinical outcomes. We compare the survival rates of UTUC patients who received neoadjuvant chemotherapy prior to surgery with patients who did not.
Methods
Retrospective review of patients with high-risk UTUC who received neoadjuvant chemotherapy followed by surgery in 2004–2008 (study group), compared to a matched cohort who underwent initial surgery in 1993–2003 (control group). The Fisher exact, Wilcoxon rank-sum, and Kaplan-Meier methods were used. The log-rank test and Cox proportional hazards model were used to evaluate association of these two outcomes with patient, treatment, and tumor characteristics in univariate and multivariate models.
Results
Of 112 patients, 31 were in the study group and 81 in the control group. Patients who received neoadjuvant chemotherapy had improved OS and DSS with a 5-year DSS of 90.1% and 5-year OS rate 80.2%, versus a 5-year DSS and OS of 57.6% for those treated with initial surgery (p = 0.0204 and p = 0.0015, respectively). In multivariate analyses the neoadjuvant group had a lower risk of mortality (OS hazard ratio 0.42 [p = 0.035]; DSS hazard ratio 0.19 [p = 0.006]).
Conclusions
Neoadjuvant chemotherapy improves survival in patients with UTUC compared with a matched historical cohort of patients treated with initial surgery. Patients with high-risk UTUC should be considered for neoadjuvant chemotherapy, in view of the limited opportunity to administer effective cisplatin-based chemotherapy after nephroureterectomy.
Keywords: ureteral cancer, renal pelvis cancer, urothelial carcinoma, survival, chemotherapy, surgical treatment
Introduction
The current standard treatment of upper tract urothelial carcinoma (UTUC) is radical nephroureterectomy. Results from single-institution 1, 2, multi-institutional 3, and population-based 4–6 studies consistently show poor survival for patients with muscle-invasive and non-organ-confined UTUC or for patients found to have lymph node involvement after extirpative treatment. Initial support for the use of perioperative chemotherapy for UTUC was provided by studies of bladder cancer showing improved survival in patients receiving neoadjuvant cisplatin-based chemotherapy 7, 8. The strongest argument for the use of neoadjuvant, as opposed to adjuvant, chemotherapy in UTUC patients is based on the high incidence of baseline and subsequent decline in renal function following a nephroureterectomy. The use of adjuvant chemotherapy in this population is limited by the significant loss of renal function that occurs after surgery. Studies have shown that over 50% of patients presenting with UTUC have chronic kidney disease, which worsens after nephroureterectomy and precludes post-nephroureterectomy cisplatin-based chemotherapy in the majority of patients, with only 19–22% of patients remaining eligible based on current renal function standards 9, 10.
We previously showed significant rates of disease downstaging and 14% rate of complete response after neoadjuvant chemotherapy in high-risk UTUC patients who underwent a nephroureterectomy compared with those rates for a matched historical control group of UTUC patients who underwent surgery without neoadjuvant chemotherapy 11. An earlier study by Igawa et al of 15 patients with locally advanced UTUC found a 13% complete remission rate and suggested improved survival in that small study 12. The objective of the current study was to determine whether neoadjuvant chemotherapy confers a demonstrable survival benefit in comparison to initial surgery, in patients with high-risk disease.
Patients and Methods
On the basis of our finding that patient survival had not improved over several decades 1 and in light of the limitations of postoperative chemotherapy, in 2004 we began uniformly offering neoadjuvant chemotherapy to patients with UTUC presenting with high-risk features at our institution. The criteria used to identify high-risk UTUC patients to be considered for neoadjuvant chemotherapy were biopsy specimen showing high-grade tumor 13–15, sessile tumor architecture 16, 17, and large tumor burden (measurable on axial imaging) 18, 19. This became our standard practice based on the few retrospective studies available and expert opinion based on experience with urothelial cell carcinoma of the bladder. Patients who elect to undergo initial surgery despite the recommendation of neoadjuvant chemotherapy are not explicitly accounted for in the present analysis, but form a very small proportion of patients offered the neoadjuvant approach.
The study group thus comprised UTUC patients who received neoadjuvant chemotherapy followed by radical nephroureterectomy at our institution from 2004 to 2008. These patients are the same as those in our initial report 11, with the exception that only clinically node negative (cN0)patients were included in the current analysis.
The control group consisted of UTUC patients who underwent a nephroureterectomy at our institution in 1993–2003, a period during which nephroureterectomy was offered to >90% of UTUC patients at our institution 1. For the current retrospective study, we re-reviewed the initial biopsy findings and included only those with truly high-grade disease on the basis of the 2004 update to the World Health Organization tumor classification system 20, and as well ensuring all patients were also cN0.
For the current study, we obtained from the patients’ records and evaluated multiple clinical and pathologic features, including patient data (age, gender, Eastern Cooperative Oncology Group (ECOG) performance status), tumor data (laterality, radiographic tumor size, prior history of bladder cancer, location of tumor, tumor architecture), treatment (type and courses of neoadjuvant chemotherapy, lymphadenectomy performed), pathology (pathologic classification, pathologic nodal classification, number of lymph nodes removed; and presence of extranodal extension, lymphovascular invasion, carcinoma in situ (CIS), and multifocality), and survival (disease specific (DSS) and overall survival (OS)). Pathologic complete remission was defined as the absence of any identifiable malignancy in all resected specimens. This retrospective study was approved by our institutional review board with waiver of informed consent.
The Fisher exact test and Wilcoxon rank-sum test were used to compare categorical and continuous patient characteristics, respectively. The Kaplan-Meier method was used to estimate the probability of overall survival and disease-specific survival rates starting from surgery. Patients who were alive at the last follow-up or lost-to-follow up or died due to other reasons were censored. The log-rank test and Cox proportional hazards model were used to evaluate the association of these two time-to-event outcomes with patient characteristics, treatments, and tumor characteristics.
Clinically relevant variables and variables that were significant in univariate analysis were included in the multivariate model. Age, neoadjuvant chemotherapy and sessile architecture were significant on univariate analysis (Supplementary Table 1a–d) for either/both OS and DSS. Number of lymph nodes was added to the model as surrogate to control differences over time in surgical management and principles of lymphadenectomy for upper tract disease. The mere performance of a lymphadenectomy as well as total number of lymph nodes removed was not significantly associated with DSS or OS on univariate analysis. Roscigno et al, recently reported in a retrospective study that at least 8 lymph nodes are necessary to consider lymphadenectomy in UTUC sufficient21 and therefore we used this cut-off in our analysis as a surrogate to account for differences in surgical technique. Lymphadenectomy in the study group included the paracaval or paraaortic lymph nodes, and interaortocaval lymph nodes in those with tumors above the mid ureter, while pelvic lymphadenectomy was performed for those with distal ureteral tumors.
SAS software 9.3 (SAS Institute Inc., Cary, NC) and S Plus software 8.2 (TIBCO Software Inc., Palo Alto, CA) were used for statistical analyses. P-values <0.05 were considered statistically significant.
Results
The study group consisted of 31 patients who received neoadjuvant chemotherapy, and the control group consisted of 81 patients who underwent surgery without receiving neoadjuvant chemotherapy. Table 1 lists results of baseline, surgery, and tumor characteristics of the two groups based on fisher exact tests and Wilcoxon rank-sum tests. Differences in laterality seemed spurious, and the lower incidence of lymphadenectomy in the study group reflects changing trends in the treatment of the disease. Twenty (24.7%) of patients in the control group received adjuvant chemotherapy while in the study group none received adjuvant chemotherapy. There was no statistically difference (p=0.416) in tumor size (mean, +/− SD) in 42/81 patients who underwent initial surgery (4.1cm +/− 2.1) compared with 19/31 patients who received neoadjuvant chemotherapy (3.7 +/− 1.3).
Table 1.
Differences in patient and tumor characteristics in those who received neoadjuvant chemotherapy and patients who did not. (Fisher exact and Wilcoxon rank-sum tests)
| Characteristic | No. of patients (%) undergoing initial surgery or neoadjuvant chemotherapy | p value | ||
|---|---|---|---|---|
|
| ||||
| Surgery (n=81) | Neoadjuvant (n=31) | |||
|
|
||||
| Age (median, range) | 68 (41–85) | 70 (32–85) | 0.9663 | |
| Race | Nonwhite | 6(7.4) | 5(16.1) | 0.1739 |
| White | 75(92.6) | 26(83.9) | ||
| Sex | Female | 33(40.7) | 12(38.7) | 1 |
| Male | 48(59.3) | 19(61.3) | ||
| ECOG performance status | 0 | 41(50.6) | 18(60) | 0.4009 |
| 1 | 40(49.4) | 12(40) | ||
| Laterality | Left | 47(58) | 11(35.5) | 0.037 |
| Right | 34(42) | 20(64.5) | ||
| Location | Renal pelvis | 47(58) | 21(67.7) | 0.3551 |
| Ureter | 24(29.6) | 9(29) | ||
| Ureteroenteric anastomosis | 10(12.3) | 1(3.2) | ||
| History of bladder cancer | No | 36(44.4) | 16(51.6) | 0.531 |
| Yes | 45(55.6) | 15(48.4) | ||
| Lymphadenectomy | No | 31(38.3) | 5(16.1) | 0.0259 |
| Yes | 50(61.7) | 26(83.9) | ||
| Tumor Architecture | Papillary | 32(39.5) | 11(35.5) | 0.8287 |
| Sessile | 49(60.5) | 20(64.5) | ||
| Adjuvant chemotherapy | No | 61(75.3) | 31(100) | 0.0015 |
| Yes | 20(24.7) | 0(0) | ||
| Lymphovascular invasion | No | 38(46.9) | 21(67.7) | 0.0584 |
| Yes | 43(53.1) | 10(32.3) | ||
| Carcinoma in situ | No | 33(40.7) | 19(61.3) | 0.0592 |
| Yes | 48(59.3) | 12(38.7) | ||
| Multifocality | No | 42(51.9) | 21(67.7) | 0.1426 |
| Yes | 39(48.1) | 10(32.3) | ||
Neoadjuvant therapy consisted of a cisplatin-containing regimen in 21 patients (standard or dose-dense methotrexate-vinblastine-doxorubicin-cisplatin, gemcitabine-cisplatin, or cisplatin-gemcitabine-ifosfamide) or high-dose ifosfamide–doxorubicin–gemcitabine in 3 patients. Kidney-sparing therapy (primarily gemcitabine-paclitaxel-doxorubicin) was given to 7 patients. All patients who were started on neoadjuvant chemotherapy were able to complete a median number of 4 cycles (IQR 4–5, range 2–6) prior to surgical extirpation. No patient was precluded from surgery because of preoperative chemotherapy.
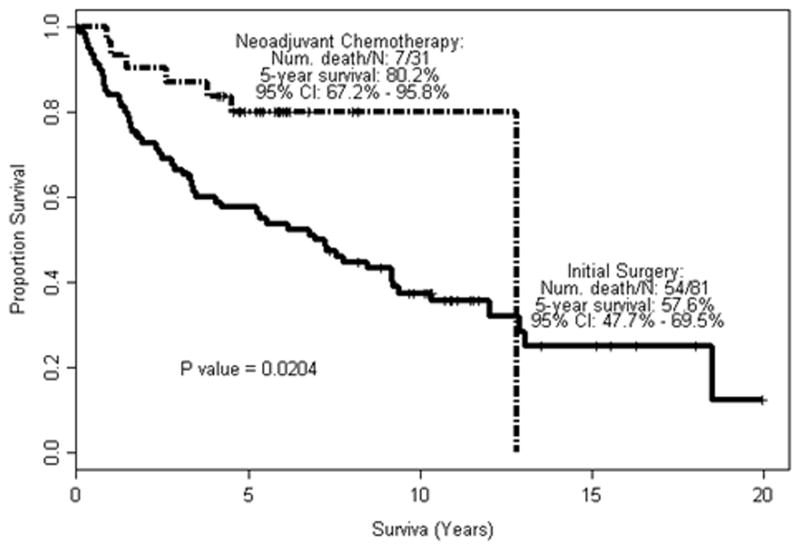
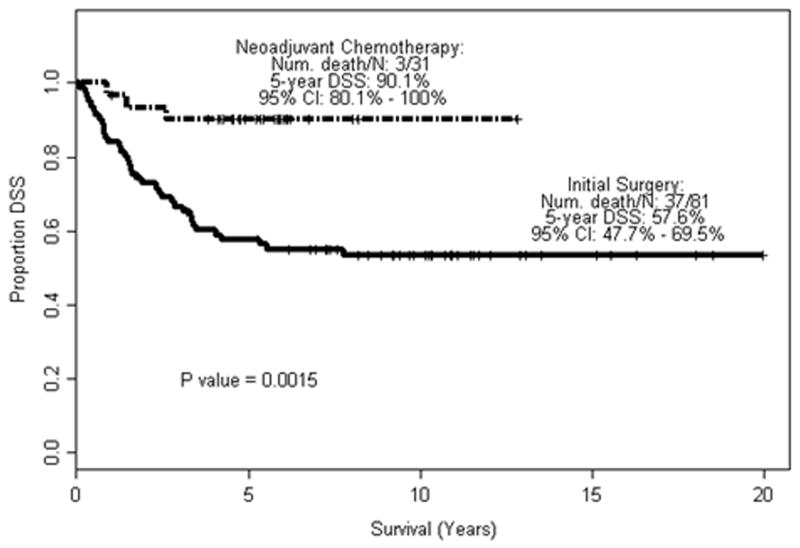
Significant differences in disease staging between the study population and the control group were observed when individual stages or various stage subgroupings. For example, there was a significantly lower rate of muscle-invasive (p = 0.0017), and organ-confined disease (p = 0.0024) in the study population versus the control group when evaluating only those with pN0 disease (Table 2). Downstaging remained significant when including pN+ patients (p = 0.0001 and 0.0005, respectively). There was no difference in rates of pN1–2 disease in control and study groups (18.5% and 6.5%, respectively, p = 0.2218). The 5-year OS and DSS rate was 80.2% and 90.1% in the neoadjuvant group, versus 57.6% and 57.6% in the initial surgery group (Fig 1, 2).
Table 2.
Pathologic stage classification in patients without nodal disease (pN0) who received neoadjuvant chemotherapy and patients who did not.
| Pathologic stage classification | No. of patients (%) undergoing initial surgery or neoadjuvant chemotherapy | p value | ||
|---|---|---|---|---|
|
| ||||
| Surgery (n=66) | Neoadjuvant (n=29) | |||
| pT-classification | T0 | 0 | 4(13.8) | 0.0011 |
| Ta | 5(7.6) | 4(13.8) | ||
| Tis | 6(9.1) | 2(6.9) | ||
| T1 | 9(13.6) | 9(31) | ||
| T2 | 15(22.7) | 6(20.7) | ||
| T3 | 29(43.9) | 3(10.3) | ||
| T4 | 2(3) | 1(3.4) | ||
| Non invasive | T0 Tis Ta | 11(16.7) | 10(34.5) | 0.0049 |
| Invasive (any) | T1–4 | 55(83.3) | 19(65.5) | |
| Non muscle-invasive | T0 Tis Ta T1 | 20(30.3) | 19(65.5) | 0.0017 |
| Muscle invasive | T2–4 | 46(69.7) | 10(34.5) | |
| Organ confined | T0 Tis Ta T1–2 | 35(53) | 25(86.2) | 0.0024 |
| Non organ confined | T3 T4 | 31(47) | 4(13.8) | |
Fig. 1.
Overall survival in relation to neoadjuvant chemotherapy.
Fig. 2.
Disease-specific survival in relation to neoadjuvant chemotherapy.
Multivariate analyses were performed to assess the effects of individual factors on outcomes. Variables that were significant and clinically relevant in univariate analyses were included in multivariate models as described in the methods section. Cox regression models for overall survival showed neoadjuvant chemotherapy to have significant influence on overall survival (Table 3). Age, number of lymph nodes removed and tumor architecture were not significant in the model. Similar analyses for disease-specific survival showed significant influence of neoadjuvant chemotherapy and tumor architecture (Table 3).
Table 3.
Multivariate Cox model for 5-year overall survival and disease-specific survival.
| Variable | HR (95% CI) | p value |
|---|---|---|
|
| ||
| Overall survival | ||
| Age | 1.02 (0.998–1.05) | 0.075 |
| Neoadjuvant chemotherapy | 0.42 (0.19–0.94) | 0.035 |
| ≥8 lymph nodes removed | 0.75 (0.40–1.40) | 0.370 |
| Sessile tumor architecture | 1.16 (0.69–1.96) | 0.580 |
| Disease-specific survival | ||
| Age | 1.01 (0.98–1.04) | 0.560 |
| Neoadjuvant chemotherapy | 0.19 (0.06–0.61) | 0.006 |
| ≥8 lymph nodes removed | 0.54 (0.24–1.23) | 0.140 |
| Sessile tumor architecture | 2.77 (1.30–5.89) | 0.008 |
Discussion
Neoadjuvant chemotherapy is associated with an improved overall and disease-specific survival as compared to a matched historical cohort of patients treated with initial surgery. Our results validate the initial observations reported by Igawa et al 12 and confirm the validity of using pathologic outcomes as a reasonable surrogate for outcomes in UTUC patients. Prior studies showed that the majority of UTUC patients present with chronic kidney disease, and an even greater proportion cannot receive effective postoperative chemotherapy when adverse pathologic features are found 9, 10. This finding may explain the conflicting and largely inconclusive reports from previous studies on the utility of adjuvant therapy for UTUC patients 22–27.
Despite significant improvements in imaging and technological advances in ureteroscopy (both presumably enabling earlier disease detection and treatment), not only have survival rates for UTUC patients not improved 1, but they may be worsening 28. These developments indicate that the treatment paradigm for UTUC may need to shift from reflexive initial surgery to more accurate thoughtful risk stratification with consideration neoadjuvant chemotherapy for patients classified as high risk.
Accurate clinical risk stratification becomes essential to avoid overtreatment and identify patients most likely to benefit from neoadjuvant chemotherapy. To aid with clinical risk stratification, two preoperative nomograms using a combination of various clinically available factors—such as biopsy grade, tumor architecture, results of selective cytology, and imaging findings such as hydronephrosis—have been shown to provide independent prognostic value 29, 30. These tools can help make the selection of the most appropriate treatment for a patient more systematic and more accurate than has historically been possible.
The limitations of the current study included the retrospective nature of the analysis. We performed pathologic reanalysis of any equivocal biopsy result and excluded those found to not be high-grade tumor (see Patients and Methods). The survival rates for our control population were similar to survival rates in other published studies of UTUC patients, supporting use of this group as a historical matched control. In a study of 1,363 UTUC patients who underwent a radical nephroureterectomy, Margulis et al. reported that patients with high-grade disease had a 5-year survival rate of 57.2%, very similar to our findings 3. Likewise, in a study of 252 patients with UTUC treated surgically, Hall et al. reported a disease-specific survival rate of 40.5–72.6% for patients with T2–4 disease 2. Those data support the validity of our control population. In contrast to pathologic outcomes reported in bladder cancer neoadjuvant trials, documented downstaging for each individual UTUC patient is not possible, given the inaccuracy of initial clinical staging. Clinical stage is thought to have a misclassification bias of approximately 45% and current expert opinion as well as many retrospective studies have shown tumor grade as a stronger predictor of high-risk disease with strong association to advance pathologic stage, recurrence, and outcomes1, 3. It is therefore difficult to control for true clinical stage in our analyses, which adds to the limitations of our findings and conclusions. Additionally, a small number of patients did have a history of bladder cancer, and although this was not significantly different between groups, it may confound findings and outcomes related to disease specific survival. Thus, short of a randomized trial, matched historical cohorts, such as the one used in this study, provide the best available data for assessing patient outcomes.
Nevertheless, only a prospective, and ideally randomized, study can definitively validate these findings. The design of such trials is made complicated by variations in the chemotherapy regimens used, as seen in our study. Given the relatively advanced age of UTUC patients and the high rates of comorbidity (including baseline renal dysfunction and cardiac disease), the current dogma of devising narrow inclusion criteria for the sake of study population homogeneity would render recruitment of patients with this rare disease even more difficult and the subsequent results inapplicable to a large proportion of patients. It is on this basis that a call has been made to consider in future trials more practical designs that allow for the diverse comorbidities frequently seen in patients with UTUC. In the meantime, the results of this study provide a strong foundation for urologists and medical oncologists looking to improve the outcomes of patients with UTUC to consider applying accurate clinical risk stratification and offering neoadjuvant chemotherapy to those with high-risk features.
Conclusions
Neoadjuvant chemotherapy in high-risk UTUC patients resulted in significantly higher survival rates than did surgery without neoadjuvant chemotherapy in a matched historical group.
Acknowledgments
Funding/Support and role of the sponsor: This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672
Arthur Gelmis reviewed the manuscript for readability.
Footnotes
Disclosures: None
References
- 1.Brown GA, Busby JE, Wood CG, Pisters LL, Dinney CP, Swanson DA, et al. Nephroureterectomy for treating upper urinary tract transitional cell carcinoma: Time to change the treatment paradigm? BJU Int. 2006;98(6):1176–80. doi: 10.1111/j.1464-410X.2006.06524.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17125474 http://onlinelibrary.wiley.com/store/10.1111/j.1464-410X.2006.06524.x/asset/j.1464-410X.2006.06524.x.pdf?v=1&t=gk4h4cvp&s=5d4fac22250794051c0efb3781a13c7811726824. [DOI] [PubMed] [Google Scholar]
- 2.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52(4):594–601. doi: 10.1016/s0090-4295(98)00295-7. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9763077. [DOI] [PubMed] [Google Scholar]
- 3.Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115(6):1224–33. doi: 10.1002/cncr.24135. Available from http://www.ncbi.nlm.nih.gov/pubmed/19156917. [DOI] [PubMed] [Google Scholar]
- 4.Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int. 2011;107(7):1059–64. doi: 10.1111/j.1464-410X.2010.09675.x. Available from http://www.ncbi.nlm.nih.gov/pubmed/20825397. [DOI] [PubMed] [Google Scholar]
- 5.Lughezzani G, Jeldres C, Isbarn H, Sun M, Shariat SF, Alasker A, et al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: a population-based study of 2299 patients. Eur J Cancer. 2009;45(18):3291–7. doi: 10.1016/j.ejca.2009.06.016. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19615885. [DOI] [PubMed] [Google Scholar]
- 6.Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164(5):1523–5. Available from http://www.ncbi.nlm.nih.gov/pubmed/11025695. [PubMed] [Google Scholar]
- 7.Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48(2):202–5. doi: 10.1016/j.eururo.2005.04.006. discussion 05-6. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15939524. [DOI] [PubMed] [Google Scholar]
- 8.Millikan R, Dinney C, Swanson D, Sweeney P, Ro JY, Smith TL, et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol. 2001;19(20):4005–13. doi: 10.1200/JCO.2001.19.20.4005. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11600601. [DOI] [PubMed] [Google Scholar]
- 9.Kaag MG, O’Malley RL, O’Malley P, Godoy G, Chen M, Smaldone MC, et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol. 2010;58(4):581–7. doi: 10.1016/j.eururo.2010.06.029. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20619530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane BR, Smith AK, Larson BT, Gong MC, Campbell SC, Raghavan D, et al. Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer. 2010;116(12):2967–73. doi: 10.1002/cncr.25043. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20564402 http://onlinelibrary.wiley.com/doi/10.1002/cncr.25043/abstract http://onlinelibrary.wiley.com/doi/10.1002/cncr.25043/abstract;jsessionid=3893280830D3424F26F080D0D3C09F21.d02t03 http://onlinelibrary.wiley.com/store/10.1002/cncr.25043/asset/25043_ftp.pdf?v=1&t=grrzryx0&s=93e95ddf6c1539e5a05f80034612f84acf2b2835. [DOI] [PubMed] [Google Scholar]
- 11.Matin SF, Margulis V, Kamat A, Wood CG, Grossman HB, Brown GA, et al. Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer. 2010;116(13):3127–34. doi: 10.1002/cncr.25050. Available from http://www.ncbi.nlm.nih.gov/pubmed/20564621. [DOI] [PubMed] [Google Scholar]
- 12.Igawa M, Urakami S, Shiina H, Kishi H, Himeno Y, Ishibe T, et al. Neoadjuvant chemotherapy for locally advanced urothelial cancer of the upper urinary tract. Urol Int. 1995;55(2):74–7. doi: 10.1159/000282755. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8533199. [DOI] [PubMed] [Google Scholar]
- 13.Brown GA, Matin SF, Busby JE, Dinney CP, Grossman HB, Pettaway CA, et al. Ability of clinical grade to predict final pathologic stage in upper urinary tract transitional cell carcinoma: implications for therapy. Urology. 2007;70(2):252–6. doi: 10.1016/j.urology.2007.03.051. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17826484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keeley FX, Kulp DA, Bibbo M, McCue PA, Bagley DH. Diagnostic accuracy of ureteroscopic biopsy in upper tract transitional cell carcinoma. J Urol. 1997;157(1):33–7. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8976209. [PubMed] [Google Scholar]
- 15.Williams SK, Denton KJ, Minervini A, Oxley J, Khastigir J, Timoney AG, et al. Correlation of upper-tract cytology, retrograde pyelography, ureteroscopic appearance, and ureteroscopic biopsy with histologic examination of upper-tract transitional cell carcinoma. J Endourol. 2008;22(1):71–6. doi: 10.1089/end.2007.9853. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18315477. [DOI] [PubMed] [Google Scholar]
- 16.Fritsche HM, Novara G, Burger M, Gupta A, Matsumoto K, Kassouf W, et al. Macroscopic sessile tumor architecture is a pathologic feature of biologically aggressive upper tract urothelial carcinoma. Urol Oncol. 2010 doi: 10.1016/j.urolonc.2010.07.010. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20933445. [DOI] [PubMed]
- 17.Remzi M, Haitel A, Margulis V, Karakiewicz P, Montorsi F, Kikuchi E, et al. Tumour architecture is an independent predictor of outcomes after nephroureterectomy: a multi-institutional analysis of 1363 patients. BJU Int. 2009;103(3):307–11. doi: 10.1111/j.1464-410X.2008.08003.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18990163. [DOI] [PubMed] [Google Scholar]
- 18.Cho KS, Hong SJ, Cho NH, Choi YD. Grade of hydronephrosis and tumor diameter as preoperative prognostic factors in ureteral transitional cell carcinoma. Urology. 2007;70(4):662–6. doi: 10.1016/j.urology.2007.06.1106. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17991533. [DOI] [PubMed] [Google Scholar]
- 19.Simone G, Papalia R, Loreto A, Leonardo C, Sentinelli S, Gallucci M. Independent prognostic value of tumour diameter and tumour necrosis in upper urinary tract urothelial carcinoma. BJU Int. 2009;103(8):1052–7. doi: 10.1111/j.1464-410X.2008.08134.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18990140. [DOI] [PubMed] [Google Scholar]
- 20.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. [Google Scholar]
- 21.Roscigno M, Shariat SF, Margulis V, Karakiewicz P, Remzi M, Kikuchi E, et al. The extent of lymphadenectomy seems to be associated with better survival in patients with nonmetastatic upper-tract urothelial carcinoma: how many lymph nodes should be removed? Eur Urol. 2009;56(3):512–8. doi: 10.1016/j.eururo.2009.06.004. Available from http://www.ncbi.nlm.nih.gov/pubmed/19559518. [DOI] [PubMed] [Google Scholar]
- 22.Kwak C, Lee SE, Jeong IG, Ku JH. Adjuvant systemic chemotherapy in the treatment of patients with invasive transitional cell carcinoma of the upper urinary tract. Urology. 2006;68(1):53–7. doi: 10.1016/j.urology.2006.01.053. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16806415 http://www.mdconsult.com/das/article/body/230003802-2/jorg=journal%26source=%26sp=16311164%26sid=0/N/540488/s0090429506001233.pdf?issn=0090-4295. [DOI] [PubMed] [Google Scholar]
- 23.Lee SE, Byun SS, Park YH, Chang IH, Kim YJ, Hong SK. Adjuvant chemotherapy in the management of pT3N0M0 transitional cell carcinoma of the upper urinary tract. Urol Int. 2006;77(1):22–6. doi: 10.1159/000092930. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16825811 http://content.karger.com/ProdukteDB/produkte.asp?doi=10.1159/000092930. [DOI] [PubMed] [Google Scholar]
- 24.Soga N, Arima K, Sugimura Y. Adjuvant methotrexate, vinblastine, adriamycin, and cisplatin chemotherapy has potential to prevent recurrence of bladder tumors after surgical removal of upper urinary tract transitional cell carcinoma. Int J Urol. 2008;15(9):800–3. doi: 10.1111/j.1442-2042.2008.02114.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18651862 http://onlinelibrary.wiley.com/doi/10.1111/j.1442-2042.2008.02114.x/abstract http://onlinelibrary.wiley.com/store/10.1111/j.1442-2042.2008.02114.x/asset/j.1442-2042.2008.02114.x.pdf?v=1&t=grrzu4n0&s=db13d9c548f22748e1f5e50fd99d35e0aca64541. [DOI] [PubMed] [Google Scholar]
- 25.Hellenthal NJ, Shariat SF, Margulis V, Karakiewicz PI, Roscigno M, Bolenz C, et al. Adjuvant chemotherapy for high risk upper tract urothelial carcinoma: results from the Upper Tract Urothelial Carcinoma Collaboration. J Urol. 2009;182(3):900–6. doi: 10.1016/j.juro.2009.05.011. Available from http://www.ncbi.nlm.nih.gov/pubmed/19616245. [DOI] [PubMed] [Google Scholar]
- 26.Vassilakopoulou M, de la Motte Rouge T, Colin P, Ouzzane A, Khayat D, Dimopoulos MA, et al. Outcomes after adjuvant chemotherapy in the treatment of high-risk urothelial carcinoma of the upper urinary tract (UUT-UC): Results From a Large Multicenter Collaborative Study. Cancer. 2011 doi: 10.1002/cncr.26172. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21638278 http://onlinelibrary.wiley.com/doi/10.1002/cncr.26172/abstract;jsessionid=5C16DACE12CA97263097EFBC086321EE.d03t02. [DOI] [PubMed]
- 27.Bamias A, Deliveliotis C, Fountzilas G, Gika D, Anagnostopoulos A, Zorzou MP, et al. Adjuvant chemotherapy with paclitaxel and carboplatin in patients with advanced carcinoma of the upper urinary tract: a study by the Hellenic Cooperative Oncology Group. J Clin Oncol. 2004;22(11):2150–4. doi: 10.1200/JCO.2004.09.043. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15169801 http://jco.ascopubs.org/content/22/11/2150.full.pdf. [DOI] [PubMed] [Google Scholar]
- 28.Eylert MF, Hounsome L, Verne J, Bahl A, Jefferies ER, Persad RA. Prognosis is deteriorating for upper tract urothelial cancer: data for England 1985–2010. BJU Int. 2012;112(2):E107–13. doi: 10.1111/bju.12025. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23470094. [DOI] [PubMed] [Google Scholar]
- 29.Favaretto RL, Shariat SF, Savage C, Godoy G, Chade DC, Kaag M, et al. Combining imaging and ureteroscopy variables in a preoperative multivariable model for prediction of muscle-invasive and non-organ confined disease in patients with upper tract urothelial carcinoma. BJU Int. 2012;109(1):77–82. doi: 10.1111/j.1464-410X.2011.10288.x. Available from http://www.ncbi.nlm.nih.gov/pubmed/21631698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zigeuner R, Shariat SF, Margulis V, Karakiewicz PI, Roscigno M, Weizer A, et al. Tumour necrosis is an indicator of aggressive biology in patients with urothelial carcinoma of the upper urinary tract. Eur Urol. 2010;57(4):575–81. doi: 10.1016/j.eururo.2009.11.035. Available from http://www.ncbi.nlm.nih.gov/pubmed/19959276. [DOI] [PubMed] [Google Scholar]