Abstract
Objective:
The aim of this study was to evaluate the regenerative potential of cell-laden and cell-free collagen matrices in comparison to microfracture treatment applied to full-thickness chondral defects in an ovine model.
Methods:
Animals (n = 30) were randomized into 5 treatment groups, and 7-mm full-cartilage-thickness defects were set at the trochlea and medial condyle of both knee joints and treated as follows: 2 scaffolds in comparison (collagen I/III, Chondro-Gide®; collagen II, Chondrocell®) for covering microfractured defects (autologous matrix-induced chondrogenesis), both scaffolds colonized in vitro with autologous chondrocytes (matrix-associated chondrocyte transplantation), or scaffold-free microfracture technique. One year after surgery, cartilage lesions were biomechanically (indentation test), histologically (O’Driscoll score), and immunohistochemically (collagen type I and II staining) evaluated.
Results:
All treatment groups of the animal model induced more repair tissue and showed better histological scores and biomechanical properties compared to controls. The average thickness of the repair tissue was significantly greater when a scaffold was used, especially the collagen I/III membrane. However, none of the index procedures surpassed the others from a biomechanical point of view or based on the histological scoring. Collagen type II expression was better in condylar defects compared to the trochlea, especially in those treated with collagen I/III membranes.
Conclusion:
Covering of defects with suitable matrices promotes repair tissue formation and is suggested to be a promising treatment option for cartilage defects. However, it failed to improve the biomechanical and histological properties of regenerated articular cartilage compared to microfracture alone in an ovine model under the given circumstances.
Keywords: cartilage repair, MACT, AMIC, microfracture, ovine model
Introduction
Articular cartilage is frequently injured, but because of its avascular nature, the capacity for repair is limited. Focal articular cartilage defects have been recognized to be progressive, leading to deterioration; therefore, early diagnosis and treatment are recommended prior to the development of more advanced osteoarthritis.1 Surgical treatment aims at formation of an entirely new articulating surface that essentially duplicates the original articular cartilage in its structure, composition, and function.
Tissue engineering may be a promising approach for the treatment of focal articular cartilage defects.2 Since the clinical introduction of autologous chondrocyte implantation (ACI) by Brittberg et al.,3 a variety of clinical studies have documented the clinical effectiveness of implanting autologous culture–expanded chondrocytes for the regeneration of cartilage.4 To overcome the intrinsic technical disadvantages of classical ACI using a periosteal flap (e.g., graft hypertrophy),5 3-dimensional scaffolds were launched.6 Because collagen is a naturally occurring component of skeletal tissues, collagen-based scaffolds favor the attachment of cells normally found in joint tissue, as well as exogenous cells embedded within a collagen delivery device.7 These scaffolds allow ingrowth of cells, stimulate matrix formation, and bind new cells and matrix to the host tissue.8,9 Encouraging results have been obtained using collagen I/III and II fiber scaffolds in vitro.10-13 In the present study, we compared 2 different, off-the-shelf collagen matrices (Chondro-Gide and Chondro-Cell).
Two different concepts can be followed in matrix-associated cartilage regeneration that are compared in the present study: Either the matrices are seeded in vitro before implantation (tissue engineering) or the scaffolds are implanted as acellular matrices for intrinsic seeding in vivo (guided tissue regeneration). Against this background, animals were randomized into different treatment groups: scaffold alone for covering microfractured defects,14 scaffold colonized in vitro with autologous chondrocytes,15 and scaffold-free microfracture (MF) technique.16
Although the repair of articular cartilage defects has been studied in many species, including rabbits, goats, and sheep, there is no consensus on the most appropriate animal model.17 Nevertheless, the ovine stifle joint is an entrenched experimental model for studying a range of orthopedic conditions.18 In a former study, we established a sheep model for evaluation of cartilage repair procedures in chondral and osteochondral lesions.19
In cartilage repair procedures, usually the graft is secured to the surrounding native cartilage by sutures, which is a technically demanding and time-consuming procedure and may further damage the native tissue.6 In a goat model, suturing of articular cartilage induced severe local damage, which was progressive and reminiscent of that associated with the early stages of osteoarthritis.20 In the present study, the matrix was fixed with fibrin glue. Fibrin can be used to adhere other engineered cartilage onto the recipient site, as a stand-alone scaffold or as a growth factor.6 In this series, we used a semiautologous fibrin glue that offers superior properties compared with commercial fibrin glue, as we published previously.21
For many decades, biomechanical investigation focused on the mechanical properties of cartilage tissue and on the relationship between the mechanical behavior and the structural composition.22 Nowadays, biomechanical analysis is also increasingly applied to characterize the mechanical properties of cartilage repair tissue, because although the repaired tissue may histologically resemble the normal articular cartilage, its mechanical properties will determine the functional competence of the repair tissue.23 In the present study, we used a measuring apparatus for indentation tests on cartilage repair tissue using a standard displacement transducer, load cell, and laboratory equipment, as published previously.24
In recent years, the field of cartilage tissue engineering has seen a sharp increase in published studies using various histological analysis methods. A broad range of histological scoring systems is used to examine cartilage quality. In the present study, we used the O’Driscoll score, as it is estimated as a reliable semiquantitative cartilage scoring system with a low intraobserver variability of 0.05 and an interobserver reliability of 0.001.25
Based on the experimental design, we did not intend to conclude that a particular treatment was superior. Rather, we hypothesized that our approach using a cell-free collagen scaffold in combination with MF might generally improve common problems associated with repair techniques, namely, graft hypertrophy (1st-generation autologous chondrocyte transplantation), MF (fibrous repair tissue), and matrix-associated chondrocyte transplantation (MACT; 2nd surgery after in vitro cell expansion). We tested these hypotheses in a sheep model by creating defects in 2 locations at which focal defects frequently occur in patients.
Materials and Methods
The study was approved by the Ministry of Environment, Nature and Forest (MUNF) of Schleswig Holstein, Germany (V 252-72241.122-15; 38-5/02).
All operations were performed by the same surgeon during a time window of 4 wk. Randomization of animals into treatment groups was performed. Cartilage defects were treated in right knees; contralateral knee joints served as controls.
Surgical Model
Thirty healthy female common German sheep, between 12 and 18 mo old and 30 and 62 (46 ± 9.6) kg weight, were randomly divided into 5 experimental groups of 6 sheep each.
Treatment included MACT, MF, and autologous matrix-induced chondrogenesis (AMIC) based on MF using a collagen I/III (Chondro-Gide) or a collagen II matrix (Chondrocell; both Geistlich Pharma AG, Wolhusen, Switzerland; Table 1).
Table 1.
Experimental Group and Treatment
| Experimental Group | Treatment |
|---|---|
| MACT I/III | MACT with collagen I/III matrix |
| MACT II | MACT with collagen II matrix |
| MF | Microfracture |
| AMIC I/III | AMIC with collagen I/III matrix |
| AMIC II | AMIC with collagen II matrix |
Note: A total of 30 sheep were randomly divided into 5 experimental groups. The matrices were either seeded in vitro before implantation (matrix-associated chondrocyte transplantation [MACT]) or the scaffolds were implanted as acellular matrices for intrinsic seeding in vivo (autologous matrix-induced chondrogenesis [AMIC]). Two different collagen scaffolds were used (collagen I/III, Chondro-Gide; collagen II, Chondrocell).
The sheep underwent the surgical procedure after premedication and spinal anesthesia (bupivacaine-HCl 0.5%; 1.4-1.6 mL, maintenance midazolam: 1-2 mg) as described before.15 A single dose of penicillin V (10 mega) served as antibiotic prophylaxis. After shaving and sterile prepping both lower extremities, a medial parapatellar arthrotomy was carried out, and the patella was luxated laterally to expose the trochlea and medial condyle in knee flexion.18 In all knees, 1 defect was created in the central load-bearing region of the medial femoral condyle and 1 defect was made in the lateral distal facet of the trochlea (Facies patellaris femoris). With a dermal punch (Ø7 mm), defects were circumscribed and as much calcified cartilage removed with a curette as possible without damaging the subchondral bone. Previous studies have demonstrated that such defects fail to repair spontaneously.26
The created cartilage defects were treated according to the assigned protocol; the contralateral knee served as control.
For the MACT groups, chondral biopsies were taken prior from the left stifle joint in the course of defect creation for controls. Articular chondrocytes were isolated from the biopsied tissue, expanded in monolayer culture, seeded on the collagen scaffolds at a concentration of 105 cells/mL, and cultured for 3 additional days (experimental group 1 and 2) according to previously published protocols.11 The seeded cell constructs were trimmed to defect size and sealed into the defect with semiautologous fibrin glue (Tissucol, Baxter, Germany), as previously published.21
In the sheep of group 3, microfracturing was the only intervention. In every defect, 9 MF perforations were introduced using a chondropick until bleeding was observed.
Microfracturing was also performed for experimental groups 4 and 5, but the defects were additionally covered with a collagen I/III or II matrix, which was glued in the defects as described in groups 1 and 2.
When a matrix was used, the knee joint was mobilized in full range of motion several times after reposition of the patella to ensure the fit of the implant. The knee joint was closed with Vicryl suturing, and the wound was closed in layers with resorbable sutures; a plaster spray dressing was applied. The hind leg was immobilized with a plaster cast for 7 d. The animals were returned to the field after removal of the cast and ambulated freely.
Animals were euthanized by intravenous administration of a lethal dose of T-61 (Hoechst, Germany) after 1 y.
Gross Examination
The retrieved samples were observed for signs of inflammation, such as tissue reddening, hypertrophy of the villous part of the synovial membrane, tissue adhesions, and clarity and color of the synovial fluid. Synovial biopsies (0.5 cm3) were taken from each joint and prepared for histological evaluation.
The knee joints were excised, inspected for osteophyte formation indicating degeneration, photo documented, and kept in 0.9% NaCl solution until biomechanical testing was performed.
Biomechanical Testing
The prepared knee was fixed with 4 screws onto the lifting platform, which allowed free motion, permitting precise alignment of the 4-mm-diameter ball indenter perpendicular to the test surface, as shown in Figure 1. Data were collected by DIADEM (National Instruments, Austin, TX). Within 0.2 s after release of the adjustment, a constant force of 0.8 N was applied, and displacement was recorded continuously for 35 s. The integrated force sensor ensured that no predeformation of cartilage took place. The indentation data were evaluated using start deformation and 25-s creep indentation, considering cartilage thickness to determine the 25-s creeping index and the Elastic Modulus E (Young’s modulus), a measure of the stiffness of a given material, as parameters. It is defined as the ratio of the rate of change of stress with strain. The measurement was repeated twice at an interval of 10 min to allow the cartilage to recover. The tissue was moistened with 0.9% NaCl solution throughout the biomechanical testing to avoid any tissue damage. Data smaller than 0.02 (25-s creeping index) and more than 15 Mpa (Young’s modulus) were excluded from statistical analysis.22 Immediately after biomechanical investigation, specimens were processed for histology by fixation in 4% buffered formalin.
Figure 1.

Experimental setup for biomechanical testing. Specimens were fixed with 4 screws onto the lifting platform, which allowed free motion, permitting precise alignment of the 4-mm-diameter ball indenter perpendicular to the test surface.
Histological Evaluation
The specimens from the cartilage repair and control sites were decalcified with EDTA for about 4 wk and processed for histology. After embedding in paraffin, 6-µm sections were made using a Microm HM340E (Microm International GmbH, Heidelberg, Germany). Serial sections were made throughout the diameter of the defects, after removing the first 2 mm of tissue. Then, another 800 µm of tissue was removed, and again serial sections were cut. This procedure was repeated 3 times, and finally 5 transverse sections (1 from each defect area) were evaluated for proteoglycan content and tissue structure. Proteoglycans were visualized by staining with Alcian Blue 8GS (Roth, Karlsruhe, Germany) at pH 2.5, followed by counterstaining with nuclear fast red (Sigma, St. Louis, MO). Hematoxylin-eosin staining was performed to evaluate cartilage and repair tissue thickness, as previously published.15 Tissue sections were immunohistochemical stained with anti–type I and II collagen antibodies (DAKO, Hamburg, Germany). All histological samples were examined by 2 blinded observers under standard light microscopy (Carl Zeiss GmbH, Jena, Germany) and evaluated by a scoring system according to O’Driscoll et al.27 The O’Driscoll score includes several parameters, which are evaluated separately, and at the end, the values for each parameter are added to give a final sum, ranging from 0 (the worst tissue structure) to 24 (identical to intact articular cartilage). The parameters according to O’Driscoll et al. are nature of the predominant tissue: cellular morphology (hyaline articular cartilage, 4; incompletely differentiated mesenchyme, 2; fibrous tissue or bone, 0) and Alcian blue staining of the matrix (normal or nearly normal, 3; moderate, 2; slight, 1; none, 0). Structural characteristics are surface regularity (smooth and intact, 3; superficial horizontal lamination, 2; fissures −25% to 100% of the thickness, 1; severe disruption, including fibrillation, 0); structural integrity (normal, 2; slight disruption. including cysts, 1; severe disintegration, 0); thickness (100% of normal adjacent cartilage, 2; 50%-100% of normal cartilage, 1; 0%-50% of normal cartilage, 0); bonding to the adjacent cartilage (bonded at both ends of graft, 2; bonded at 1 end or partially at both ends, 1; not bonded, 0). Freedom from cellular changes of degeneration: hypercellularity (normal cellularity, 3; slight hypercellularity, 2; moderate hypercellularity, 1; severe hypercellularity, 0); chondrocyte clustering (no clusters, 2; <25% of the cells, 1; 25%-100% of the cells, 0). Freedom from degenerative changes in adjacent cartilage: (normal cellularity, no clusters, normal staining, 3; normal cellularity, mild clusters, moderate staining, 2; mild or moderate hypercellularity, slight staining, 1; severe hypercellularity, poor or no staining, 0). Two variations of the original score have been made: O’Driscoll et al. used a Safranin-O staining for detection of proteoglycans, and cellular changes were changed into hypercellularity instead of hypocellularity. The original score was used for rabbit tissue, which has a very high cellular density. In sheep, cellular density is low, and all repair tissues had a higher cell density than intact cartilage.
For collagen staining, paraffin sections were deparaffinated with xylol and transferred into Aqua Dest using decreasing concentrations of ethanol. Sections were treated with pepsin (3.9 kU per milliliter 0.5% acetic acid; Sigma) for 30 min. Unspecific peroxidases were blocked by treatment with 0.6% hydrogen peroxide in methanol for 20 min; samples were rinsed with Tris-buffered saline (TBS) and treated with the primary antibody (collagen type I mouse anti–collagen-type I, C-2456 Sigma; or collagen type II mouse anti–collagen-type II, Clone CII C1; DSHB, Iowa City, IA; both 1:1,000 in TBS) for 1 h (controls were incubated with TBS without the 1st antibody). After rinsing (TBS), the samples were treated with the 2nd antibody (rabbit anti–mouse IgG, HRP-conjugated; Dako P-0260, Hamburg, Germany, 1:200 in TBS containing 1% bovine serum, 30-min incubation), rinsed again, and incubated with the 3rd antibody (goat anti–rabbit IgG, HRP-conjugated; Dako P-0448, 1:100 in TBS containing 1% bovine serum, 30-min incubation). The samples were stained with diaminobenzidine (DAB-Kit, Vector Laboratories, Burlingame, VT). Cell nuclei were counterstained using Meyer’s hemalum (Merck, Darmstadt, Germany). The stained samples were embedded with Aquatex (Merck).
The semiquantitative assessment of collagen staining was performed by scoring the intensity of staining in 3 locations of each defect site (edges and center) using the following scale: 0 = no noticeable staining, 1 = moderate staining, and 2 = strong staining. Each area was scored separately, and the mean value was calculated for the corresponding defect. Because in some cases values were zero, it was not possible to calculate the ratio of collagen type II versus type I staining. Therefore, the difference of staining was calculated by subtraction of the collagen type I values from corresponding collagen type II values, giving positive values if collagen type II staining was stronger than type I staining and vice versa.
In addition, cartilage thickness was determined using a light microscope connected to an image-analyzing system at histological sections (Leica S6 E, Wetzlar, Germany). Measurements were performed on 4 points in the knee joint: the defect sites condylus medialis and trochlea (facies patellaris femoris) and 2 reference points at remote area cartilage. Cartilage thickness was measured interactively with a mouse board by determining the extension of the cartilage layer on the monitor screen and calculating the distance between the 2 given marks. The mean cartilage thickness of each sample was calculated from 5 repeated measurements of the distance from tidemark to surface.
Synovial biopsies (each 0.5 cm3) were stained with Mayer’s hematoxylin-eosin and Masson-Goldner. Results were rated by the following criteria: villous transformation, cover cell transformation, fibrosis, fibroblast proliferation, and inflammatory cells. Each parameter of the criteria was graded from 0 to +++ by 2 blinded observers. Higher values indicate a higher level of the variable.
Data Analysis
No more than 6 sheep in each group were allowed by the MUNF of Schleswig Holstein, Germany (V 252-72241.122-15; 38-5/02). Because of the small sample size, all statistical calculations should be considered with caution.
Data from histological scoring were calculated using the Kruskal-Wallis test (SPSS, version 15) with a significance level at P < 0.05.
Values of cartilage thickness were calculated using the Student t test, and differences were considered significant at P < 0.05.
For biomechanical testing, values smaller than 0.02 µm/mm relating to the 25-s creeping index and values larger than 15 Mpa concerning the Young’s module were excluded from statistical analysis.22 Because of the exclusion criteria, in groups MACT II and MF with regard to condylar defects, only 1 or 2 measured values could be counted. However, it is justifiable to analyze trends on the basis of means. For descriptive statistics, a boxplot was used for depicting groups of numerical data. Even in groups <2, this graphic approach was performed, although they were not considered for statistical analysis.
Results
Gross Findings
All animals tolerated surgery well, and their gaits were normal, without any severe limps. The joints appeared to be stable at physical examination. After sacrifice, the boundaries of the defects in all knees were clearly demarcated and macroscopically differentiable on sacrifice. A complete reconstruction of the articular cartilage was not evident. After MF repair, tissue appeared with an irregular texture and often depressed topology (Fig. 2 a). Almost no repair tissue was seen in control defects. Regenerated tissue of matrix-based treatment groups appeared white, smooth, glistening, and uniform in texture (Fig. 2 b). None of the specimens showed signs of degeneration, such as sclerosis or osteophyte. There were no significant differences in the macroscopic appearance of the synovial tissue analysis between treated and control joints.
Figure 2.
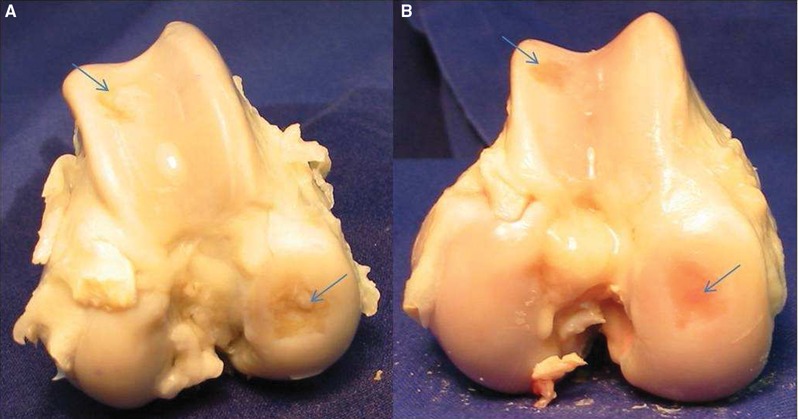
Examples for gross findings at sacrifice, 1 y after defect setting and treatment. The defect sites are marked with an arrow. (a) Inhomogenous defect filling after microfracture at the medial femoral condyle and trochlear groove. (b) Incomplete defect filling after autologous matrix-induced chondrogenesis at the femoral condyle and trochlear groove.
Cartilage Thickness
As shown in Table 2, none of the repair tissues was as thick as that found in remote area specimens. In addition, remote area cartilage was thicker at the femoral condyle compared with the trochlear groove. MF without additional matrix induced slightly more repair tissue at the femoral condyle and at the trochlear groove compared with controls. However, the average thickness of repair tissue was greater in treated groups, when a scaffold was used. Here, most repair tissue was seen in treated defects at the trochlea, when the AMIC procedure was performed and when a collagen type I/III membrane was implanted. At the femoral condyle, most repair tissue was seen when a collagen type I/III was used, independent on the index procedure (AMIC v. MACI). Significant more repair tissue was induced when using a collagen I/III matrix compared with controls and MF; results for a collagen II membrane did not reveal these significant differences.
Table 2.
Quantitative Evaluation of Repair Tissue Formation at Treated Cartilage Defects, Controls, and Cartilage Thickness at Remote Area Specimens
| Femoral Condyle | Trochlear Groove | |
|---|---|---|
| Remote area specimens | 1,125 ± 290 | 667 ± 160 |
| Control group | 302 ± 205 | 212 ± 121 |
| MACT I/III | 510 ± 161 | 387 ± 178 |
| MACT II | 335 | 304 ± 128 |
| MF | 310 ± 126 | 268 ± 196 |
| AMIC I/III | 645 ± 308 | 408 ± 148 |
| AMIC II | 393 ± 144 | 310 ± 125 |
Note: The mean tissue thickness was calculated from 3 repeated measurements of the distance from tidemark to surface. The values in micrometers are depicted as means and standard deviations. Significant more repair tissue was induced when using a collagen I/III matrix was used compared with controls and MF (P < 0.05).
Biomechanical Results
The indentation data were evaluated using start deformation and 25-s creep indentation, considering cartilage thickness to determine the 25-s creeping index and the Elastic Modulus E (Young’s modulus), a measure of the stiffness of a given material, as parameters. Because of the exclusion criteria, for groups MACT II and MF with regard to condylar defects, only 1 or 2 measured values could be accounted.
The 25-s creeping index was lower in remote area cartilage at the femoral condyle compared with the trochlea. At the treated defect sites, values were higher compared with remote area cartilage at both locations—the femoral condyle and the trochlea. However, comparing data from treated groups with data from control groups, no striking differences were measurable in general. After analyzing the data in detail, it must be assessed that repair tissues at the femoral condyle of the MF group were more viscous than in the other groups, including the use of a scaffold (Fig. 3 A). Results of the treatment groups at the trochlea did also not differ to a large extent (Fig. 3 B). A bias can be declared for the results of groups MACT I/III and AMIC I/III (Chondro-Gide matrix) showing a slightly higher deformity of repair tissue compared with groups MACT II and AMIC II (Chondrocell matrix). Both groups, using the AMIC technique, lead to softer repair tissue compared with groups using the MACT procedure.
Figure 3.

Box and Whisker plot of the creep index at the condylus medialis (A) and at the trochlea (B). Values are depicted for the treatment groups (1-5), controls, and remote area cartilage. Scores are presented as medians; the ends of the boxes define the 25th and 75th percentiles.
Note: MACT I/III = matrix-associated autologous chondrocyte transplantation + Chondro-Gide scaffold; MACT II = matrix-associated autologous chondrocyte transplantation + Chondrocell scaffold; MF = microfracture; AMIC I/III = autologous membrane-induced chondrogenesis + Chondro-Gide scaffold; AMIC II = autologous membrane induced chondrogenesis + Chondrocell scaffold; RAC = remote area cartilage.
The instantaneous Young’s module was lowest in remote area cartilage, intermediate in treated areas, and highest in control groups at the femoral condyle (Fig. 4 A). Values at the trochlea were lowest in the treated groups, intermediate in control groups, and highest in remote area cartilage. As shown for the 25-s creeping index, data strengthen the fact that intact condylar cartilage is softer than at the trochlear site, represented by lower values of the instantaneous Young’s module. Treated and control defects at the trochlea showed softer repair tissue than remote area cartilage. No differences can be constituted comparing treated and control groups in general at the trochlear site. Analyzing the data in detail, values of groups MACT I/III and AMIC I/III using a collagen I/III matrix showed higher values compared with groups using a collagen II membrane (Fig. 4 B). Focusing on the femoral condyle, results of control, treated, and remote area cartilage do not differ in a great measure. A bias can be declared for the results of groups, including the AMIC technique showing lower values compared with the groups using MACT.
Figure 4.

Box and Whisker plot of the Young’s modulus at the condylus medialis (A) and at the trochlea (B). Values are depicted for the treatment groups (1-5), controls, and remote area cartilage. Scores are presented as medians; the ends of the boxes define the 25th and 75th percentiles.
Note: MACT I/III = matrix-associated autologous chondrocyte transplantation + Chondro-Gide scaffold; MACT II = matrix-associated autologous chondrocyte transplantation + Chondrocell scaffold; MF = microfracture; AMIC I/III = autologous membrane-induced chondrogenesis + Chondro-Gide scaffold; AMIC II = autologous membrane-induced chondrogenesis + Chondrocell scaffold; RAC = remote area cartilage.
Histological Testing
Synovial tissue analysis
Specimens were evaluated according to the above-mentioned protocol. Synovial biopsies of both knees (treatment groups and controls) were investigated. Synovial biopsies at the time of primary surgery showed no significant differences in pathodiagnostic results. At the time of sacrifice, a minimal to moderate villous synovitis characterized by lymphoplasmacellular infiltrates was observed in almost all samples (Fig. 5). Minimal to moderate subsynovial edema was also noted in these joints as well as mild hemarthrosis. No obvious differences were visible comparing the treatment groups and controls in general. No difference was seen comparing cell-based cartilage repair procedures (MACT, AMIC) and MF, pinpointing the fact that neither a collagen I/III nor a collagen II scaffold evokes inflammatory response (e.g., synovitis or foreign body reactions).
Figure 5.
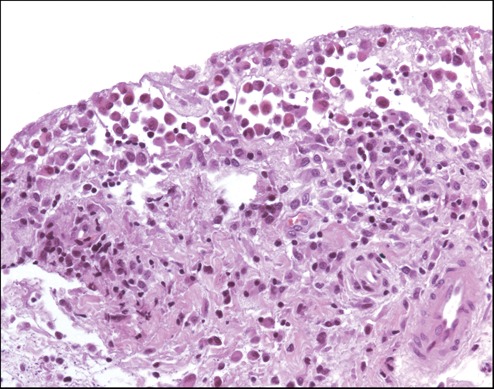
Synovial biopsy at the time of sacrifice of sheep no. 30 (AMIC II group); hematoxylin-eosin staining showing lymphoplasmacellular infiltrates (original magnification ×100).
Cartilage tissue analysis
In general, repair tissue analysis confirmed the macroscopic results, in which most of the controls showed empty defects with no or very limited repair tissue. Figure 6 shows for all treatment and control groups histological tissue sections after hematoxylin-eosin and Alcian blue staining. In some areas, a columnar and chondron-like distribution of cells with some clustering was found. In general, repair tissue was mostly neocartilage of partly hyline-like characteristics. There were no signs of abnormal calcification, infiltration of immunological cells, apoptosis of cells, or necrosis. Only partial defect filling in the control groups was observed in 30% of untreated defects in the trochlea and in 10% at the medial condyle defects, whereas treatment induced repair tissue formation in most of the defects. Interestingly, most of the spontaneous repair occurred in animals with less than 41 kg of body weight.
Figure 6.

Examples of hematoxylin-eosin stained sections from defects in the condylar and Alcian blue–stained defects in the trochlear region. Arrows mark the borders of the defects; stars indicate more or less intact cartilage tissue surrounding the defect area. Because of the high variance in staining and structure of the tissue, these examples do not represent a mean histological outcome of the corresponding experimental group but give an impression of the quality of histological staining and structure in general.
Note: MACT I/III = matrix-associated autologous chondrocyte transplantation + Chondro-Gide scaffold; MACT II = matrix-associated autologous chondrocyte transplantation + Chondrocell scaffold; MF = microfracture; AMIC I/III = autologous membrane-induced chondrogenesis + Chondro-Gide scaffold; AMIC II = autologous membrane-induced chondrogenesis + Chondrocell scaffold.
Histological assessment was made according to a slightly modified O’Driscoll score. Repair tissue in treated defects displayed superior histological results when compared with the spontaneously formed tissue that was found in some of the control defects (Fig. 7). Mean values for treated defects ranged between 10 and 13 at the condyle and 11.5 and 14.2 at the trochlear site, whereas untreated defects with spontaneous repair showed mean values of 8 and 10.1, respectively. Therefore, scores from defects at the trochlea site were slightly higher than those at the condyles, independent on the kind of treatment. Together with the fact that more spontaneous repair was found in trochlear defects, it can be concluded that in the ovine model, trochlear cartilage defects show a better repair response than defects at the medial condyle. However, the Kruskal-Wallis test indicated that there was no significant difference in the structure of the repair tissue and the remote cartilage dependent on the different kinds of treatment.
Figure 7.
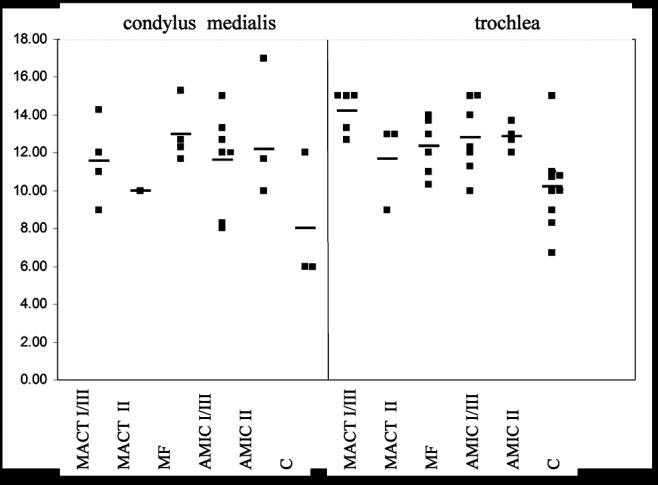
The overall values of the O’Driscoll score are depicted in the graphs as a function of the index procedure and defect localization. Each dot represents the outcome of an individual cartilage defect. In some cases, untreated defects showed a spontaneous repair response; this repair tissue was also evaluated, and values are presented as controls. Dropouts are due to insufficient repair tissue formation.
Note: MACT I/III = matrix-associated autologous chondrocyte transplantation + Chondro-Gide scaffold; MACT II = matrix-associated autologous chondrocyte transplantation + Chondrocell scaffold; MF = microfracture; AMIC I/III = autologous membrane-induced chondrogenesis + Chondro-Gide scaffold; AMIC II = autologous membrane-induced chondrogenesis + Chondrocell scaffold; C = control.
Collagen type II and I had been stained by immunohistochemistry (see examples in Fig. 8) and scored for no (0), moderate (1), or strong (2) staining in the repair tissue. To show relative levels of staining, the data from collagen type I was subtracted from the values of collagen type II staining, and the results are given in Figure 9. Looking at all defects, there was a significantly stronger staining of collagen type II than type I in condylar defects compared with defects in the trochlear region (shown by the positive values; P < 0.02). In addition, there was a trend showing that collagen type II staining was stronger than collagen type I staining in defects treated with I/III membranes. However, because of the limited number of defects, this trend slightly failed to be significant (P < 0.06).
Figure 8.
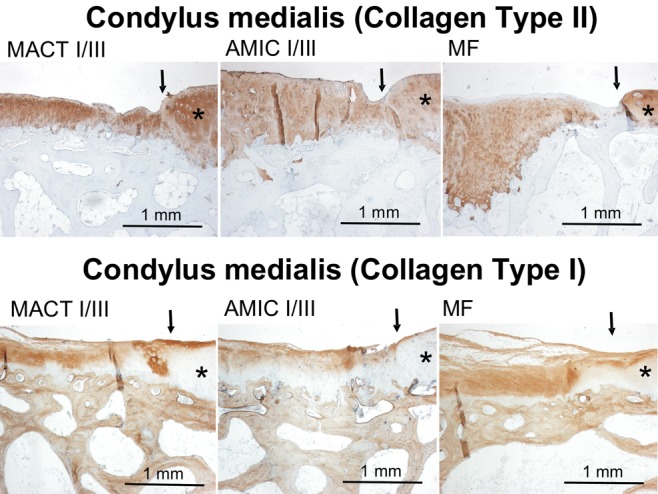
Examples of immunohistochemical collagen type II and type I staining in condylar defects of different experimental groups. Collagen expression is indicated by brown staining. There is no collagen type II staining in subchondral bone, while bone tissue is positively stained for collagen type I, giving an internal positive control. Arrows mark the borders of the defects; stars indicate more or less intact cartilage tissue surrounding the defect area. Because of the high variance in staining, these examples do not represent a mean intensity of staining of the corresponding experimental group but give an impression of the quality of staining in general.
Note: MACT I/III = matrix-associated autologous chondrocyte transplantation + Chondro-Gide scaffold; MF = microfracture; AMIC I/III = autologous membrane-induced chondrogenesis + Chondro-Gide scaffold.
Figure 9.

Repair tissues had been evaluated for immunohistochemical staining of collagen types II and I. The intensity of staining at the edges and the center of each defect had been scored for no (0), moderate (1), or strong (2) staining. To show relative expression levels of collagen, the values for collagen type I had been subtracted from corresponding collagen type II values, giving positive values if type II expression is higher and negative values if type II staining is less intensive than collagen type I staining. Each dot in the chart represents the relative collagen staining of a single defect: black dots in condylar and white squares in trochlear defects.
Note: MACT I/III = matrix-associated autologous chondrocyte transplantation + Chondro-Gide scaffold; MACT II = matrix-associated autologous chondrocyte transplantation + Chondrocell scaffold; MF = microfracture; AMIC I/III = autologous membrane-induced chondrogenesis + Chondro-Gide scaffold; AMIC II = autologous membrane-induced chondrogenesis + Chondrocell scaffold.
Discussion
In this study, we compared the biomechanical and histological properties of lesion tissue, remote articular cartilage, and control specimens harvested from stifle joints 12 mo after different cartilage repair techniques (microfracture, MACT, and AMIC).
Clinically, the MF technique is a frequently used, first-line cartilage repair option and induces the formation of cartilage repair tissue by perforating the subchondral bone.28 In former studies, the hypothesis was verified that perforation of the subchondral bone plate gives rise to the stem cell pool of the bone marrow and leads to release of further marrow elements as growth factors and cytokines.29,30 However, the repair tissue that is induced by MF may appear unstructured and shows predominantly fibrocartilage.28
The implantation of first-generation tissue-engineering grafts such as the ACI has been shown to be suitable for the regeneration of posttraumatic defects.31 The elements of ACI have been improved continually to regenerate cartilage of better quality and to establish procedures that are technically more attractive. To overcome the intrinsic technical disadvantages of ACI, cartilage tissue-engineering grafts were developed that use the regenerative potential of autologous chondrocytes with 3-dimensional scaffolds to stabilize the graft. Biodegradable polymers serve as scaffolds in which cells are able to proliferate and differentiate.14,32 In a former study, we presented promising midterm results following 3rd-generation chondrocytes transplantation (MACT) in a clinical trial.33
The attempt to overcome the inadequate supply of autogenous cartilage by laboratory-expanded chondrocytes, which is part of the MACT technique, is associated with several disadvantages (e.g., 2nd surgery). In contrast, no damage to healthy cartilage is carried out in performing AMIC, and this procedure can be done in a 1-step surgery. Moreover, in vitro cultivation and differentiation of cells can be avoided using this enhanced MF technique.34 We have presented strong evidence that bone marrow cells can be guided directly to a cartilage defect by a collagenous matrix and that mesenchymal stem cells (MSCs) can be isolated regularly from the matrix.29 In general, this technique is less expensive, less time intensive, and available to all patients.34
To the best of our knowledge, this is the first description of a randomized trial comparing different matrix-based cartilage repair techniques in an ovine model. We tested 2 different concepts in matrix-associated cartilage regeneration: The matrix can be seeded in vitro before implantation (tissue engineering12) or the scaffold is implanted as an acellular matrix for intrinsic seeding in vivo (guided tissue regeneration35). Despite this, we performed the MF procedure as described previously.16
The sheep model was chosen for our experiments because its limbs bear great weight and is considered to model the human more closely than a rabbit model.36 Even if many questions on tissue regeneration can be answered in relatively simple animals (such as in small animals), the load could not be accounted for as a critical factor in the success of a tissue-engineered procedure.37 Final preclinical tests in large animals present relevant loading conditions and allow the adoption of a similar surgical technique that may be used in the final procedure in humans.37 Our data endorse the fact that cartilage repair depends on loading conditions, because repair tissue was primarily observed in untreated control defects of light-weight sheep. This is in accordance with clinical observations that cartilage degeneration is linked to obesity and body fat mass.38
There were no significant differences in the pathodiagnostic results of the synovial tissue analysis between treatment and control groups. At 1 y postoperatively, a minimal to moderate villous synovitis characterized by lymphoplasmacellular infiltrates was observed. This is in accordance with the literature, in which no adverse cellular response to collagen matrices of the synovial tissue was observed in an ovine model.39
In the present study, we used 2 different collagen membranes (Chondro-Gide, Chondrocell) because of their biodegradable nature, artificial origin (no donor site morbidity), and lack of inflammatory reaction.10,35 Good results have been reported for these scaffolds, but comparative studies are missing, and possible differences need to be addressed in randomized trials.6 In the present study, no significant differences were measurable between the 2 collagen sponges in general, but results point to the fact that a collagen I/III scaffold is associated with stronger induction of repair tissue formation compared with a collagen II membrane, especially at the femoral condyle. In a canine model, a collagen II scaffold showed better biochemical attributes on the basis of a higher percentage of chondrocytes retaining spherical morphology and greater biosynthetic activity that was reflected in a greater increase of GAG content, compared with a collagen I matrix.40 In contrast, no notable histochemical and immunohistochemical differences between the type I and II collagen scaffolds were obvious after 4 wk of culture.41 Although detrimental effects of type I collagen compared with type II collagen on chondrocytes was demonstrated in previous studies,42 the effect of type I collagen in our 3-dimensional matrix remains vague.
Even though collagen staining varied from defect to defect, even within experimental groups, there were some clear trends showing that collagen type II expression was higher than collagen type I expression in most cases, and that especially in condylar defects, collagen type II staining was significantly stronger. Independent on the kind of treatment (MACT or AMIC), the addition of a collagen I/III membrane gave better values of collagen staining and supports the notion that use of collagen I/III membranes might give better results than the use of collagen II membranes in the treatment of cartilage defects. However, these results slightly failed to be significant and need further investigation.
Mechanical testing of articular cartilage and repair tissue enables judgment of their capacity in withstanding mechanical loading. In the past, different methods have been developed requiring a complex technical setup and extensive data analysis.22 Therefore, a simple measuring device for laboratory indentation tests on cartilage was developed, as previously published.24 In the present study, biomechanical properties showed significant differences among lesion tissue, remote articular cartilage, and control area specimens. Our findings are in agreement with other studies using matrix-based strategies for cartilage repair that have also found that the biomechanical properties of repair tissue were inferior to those of normal articular cartilage.24 In a porcine full-thickness defect model, Liu et al.43 reported that at 8 wk after surgery, repair tissue had approximately 50% the biomechanical properties of normal articular cartilage. Lee et al. reported similar results in a study examining the transplantation of autologous chondocyte-seeded type II collagen scaffold into canine cartilage defects.44 At 15 wk, confined compression testing of repair tissue resulted in an instantaneous Young’s modulus that was 20-fold lower than that of normal articular cartilage controls. Our data show that lesion samples had a mean instantaneous Young’s modulus that was approximately 2-fold less than their normal remote area counterparts. Although the instantaneous Young’s modulus of repair tissue was higher in our study than in that of Lee et al., results of Strauss et al. are similar to ours. Strauss et al. report long-term results as we do, with a follow-up time of 12 mo.45 In a study of intra-articular step-off fractures, Trumble et al.46 reported evidence of a tendency for articular congruency to improve after fracture, reflecting structural adaption. This is of interest because a remodeling process might also occur in adjacent articular cartilage around a defect. Taking this into account, the difference of indentation testing in short- and long-term follow-up, as mentioned above, could be argued.
In the acute implant model, defects revealed in situ matrices throughout the entire operation. We did not observe transplant loosening, debonding of the graft, or ablation and in turn clinical complications and reoperations. Yet dislocation of the implant could have occurred within the 12-mo follow-up period. This is in accordance with the literature, in which one explanation for small quantity of repair tissue in an ovine model is early dislocation of matrices in some defects.47 Bleeding due to MF, forming a so-called “super clot,” might have lifted off the matrix at the bone-matrix interface, leading to dislocation of the matrix. In contrast to former studies, matrices were fixed with fibrin glue, making the fixation more reliable. One can suggest that matrix dislocation is therefore less frequent, because in a comparison of 4 techniques for fixation of a collagen scaffold in human cadaveric knees, fibrin glue ensured satisfactory scaffold stability.48 Besides fibrin glue, the graft could have been secured to the surrounding native cartilage by sutures, which is a technically demanding and time-consuming procedure and may further damage the native tissue.6 In a goat model, suturing of articular cartilage induced severe local damage, which was progressive and reminiscent of that associated with the early stages of osteoarthritis.20 Behind the background of this fact, scaffolds were glued and not sutured into the defect.
No superiority of AMIC or MACT could be determined from a general biomechanical and histological point of view. But a bias can be declared for defects at the femoral condyle, with the MACT leading to slightly better results compared with the AMIC. We hypothesize that cell implantation may be beneficial and that host influx should also be taken into account, as was published previously.14 But it is still unclear which cell type is optimal for articular cartilage tissue engineering. The chondrocyte is the predominant cell type but has limited potential for intrinsic repair. Adult MSCs, on the other hand, are readily available and possess the ability to differentiate into a number of different cell types.49 The use of embryonic stem cells and induced pluripotent stem cells in articular repair is very much in its infancy.50 Controlling the differentiation of either of these cell types may be the key to producing quality repair tissue.
In the present study, we aimed to remove the calcified layer without damaging the subchondral bone. Histological analysis of the defect revealed that it is difficult to consistently remove the entire zone of calcified cartilage, even when the procedure is carefully carried out. This is in accordance with the literature, which shows that an average of 48% to 60% of calcified cartilage still remained after creation of the defects.26
An important finding of this experiment was the rare spontaneous healing in accurately created chondral defects without penetrating the subchondral plate in the sheep model. This is in accordance with previous published data,47 showing a total defect fill of 22% in the untreated control group. Dorotka et al.47 stated that defect filling with a larger amount of reparative tissue may be based on some communication between the defect and the marrow spaces of the subchondral region, facilitated by the thin subchondral bone plate of the sheep.47
There are 2 limitations that need to be acknowledged and addressed regarding the present study. The first limitation concerns the animal model, because no animal model exists that is directly applicable to the human, as discussed above. The second limitation has to do with the extent to which the findings can be generalized beyond the cases studied. The number of cases is too limited for broad generalization. However, these limitations can be seen as fruitful avenues for future research under the same theme.
Conclusion
In conclusion, the repair tissue’s origin and the role and fate of the implanted cells remain unanswered. However, we successfully demonstrated the principle of cartilage restoration with a cell-laden or cell-free scaffold.
Covering of defects with suitable matrices promotes repair tissue formation and is suggested to be a promising treatment option for cartilage defects. However, it failed to improve the biomechanical and histological properties of regenerated articular cartilage compared with MF alone in an ovine model under the given circumstances.
Acknowledgments
The authors gratefully acknowledge the skilled technical assistance of Ms. Petra Tiede, Ms. Rita Kirsch, and Dr. Sasha Sappan, MD.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
Funding: This study was funded by Geistlich Biomaterials, Wolhusen, Switzerland. None of the authors or coauthors has a financial interest in products relevant to the article.
References
- 1. Johnson L. Arthroscopic abrasion arthroplasty. New York: Raven Press; 1991. [Google Scholar]
- 2. Brittberg M, Lindahl A, Nilsson A. Treatment of full thickness cartilage defects in the human knee with cultured autologous chondrocytes. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 3. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994. October 6;331(14):889-95. [DOI] [PubMed] [Google Scholar]
- 4. Ossendorf C, Kaps C, Kreuz PC, Burmester GR, Sittinger M, Erggelet C. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther. 2007;9(2):R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trattnig S, Ba-Ssalamah A, Pinker K, Plank C, Vecsei V, Marlovits S. Matrix-based autologous chondrocyte implantation for cartilage repair: noninvasive monitoring by high-resolution magnetic resonance imaging. Magn Reson Imaging. 2005. September;23(7):779-87. [DOI] [PubMed] [Google Scholar]
- 6. Chiang H, Jiang CC. Repair of articular cartilage defects: review and perspectives. J Formos Med Assoc. 2009. February;108(2):87-101. [DOI] [PubMed] [Google Scholar]
- 7. Frenkel S, Di Cesare P. Scaffolds for articular cartilage repair. Ann Biomed Eng. 2004;32(1):26-34. [DOI] [PubMed] [Google Scholar]
- 8. Paletta G, Arnocczky S, Warren R. The repair of osteochondral defects using an exogenous fibrin clot: an experimental study in dogs. Am J Sports Med. 1992;20:725-31. [DOI] [PubMed] [Google Scholar]
- 9. Vacanti C, Kim W, Schloo B, Upton J, Vacanti J. Joint resurfacing with cartilage grown in situ from cell-polymer structures. Am J Sports Med. 1994;22:485-8. [DOI] [PubMed] [Google Scholar]
- 10. Ehlers E, Fuß M, Rohwedel J, Russlies M, Kühnel W, Behrens P. Development of a biocomposite to fill out articular cartilage lesions. Light, scanning and transmission electron microscopy of sheep chondrocytes cultured on a collagen I/III sponge. Ann Anat. 1999;181:513-8. [DOI] [PubMed] [Google Scholar]
- 11. Gille J, Ehlers E, Okroi M, Russlies M, Behrens P. Apoptotic chondrocyte death in cell-matrix biocomposites used in autologous chondrocyte transplantation. Ann Anat. 2002;184(4):325-32. [DOI] [PubMed] [Google Scholar]
- 12. Russlies M, Behrens P, Wünsch L, Gille J, Ehlers E. A cell-seeded biocomposite for cartilage repair. Ann Anat. 2002;184(4):317-23. [DOI] [PubMed] [Google Scholar]
- 13. Nehrer S, Breinan H, Ramappa A, Young G, Shortkroff S, Louie L, et al. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials. 1997;18:769-76. [DOI] [PubMed] [Google Scholar]
- 14. Schagemann JC, Erggelet C, Chung HW, Lahm A, Kurz H, Mrosek EH. Cell-laden and cell-free biopolymer hydrogel for the treatment of osteochondral defects in a sheep model. Tissue Eng Part A. 2009. January;15(1):75-82. [DOI] [PubMed] [Google Scholar]
- 15. Russlies M, Behrens P, Ehlers E, Broehl C, Vindigni C, Spector M, et al. Periosteum stimulates subchondral bone densification in autologous chondrocyte transplantation in a sheep model. Cell Tissue Res. 2005;319:133-42. [DOI] [PubMed] [Google Scholar]
- 16. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003. May-Jun;19(5):477-84. [DOI] [PubMed] [Google Scholar]
- 17. Gill TJ, McCulloch PC, Glasson SS, Blanchet T, Morris EA. Chondral defect repair after the microfracture procedure: a nonhuman primate model. Am J Sports Med. 2005. May;33(5):680-5. [DOI] [PubMed] [Google Scholar]
- 18. Allen M, Houlton J, Adams S. The surgical anatomy of the stifle joint in sheep. Vet Surg. 1998;27:596-605. [DOI] [PubMed] [Google Scholar]
- 19. Russlies M, Rüther P, Köller W, Stromberg P, Behrens P. Biomechanische Eigenschaften von Knorpelersatzgewebe nach verschiedenen Methoden der Knorpeldefektbehandlung beim Schaf. Z Orthop. 2003;141:465-71. [DOI] [PubMed] [Google Scholar]
- 20. Hunziker EB, Stahli A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthr Cartil. 2008. September;16(9):1067-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gille J, Meisner U, Ehlers EM, Muller A, Russlies M, Behrens P. Migration pattern, morphology and viability of cells suspended in or sealed with fibrin glue: a histomorphologic study. Tissue Cell. 2005. October;37(5):339-48. [DOI] [PubMed] [Google Scholar]
- 22. Koeller W, Kunow J, Ostermeyer O, Stomberg P, Boos C, Russlies M. A simple measuring device for laboratory indentation tests on cartilage. Biomed Tech (Berl). 2008;53(2):59-64. [DOI] [PubMed] [Google Scholar]
- 23. Lyyra-Laitinen T, Niinimaki M, Toyras J, Lindgren R, Kiviranta I, Jurvelin JS. Optimization of the arthroscopic indentation instrument for the measurement of thin cartilage stiffness. Phys Med Biol. 1999. October;44(10):2511-24. [DOI] [PubMed] [Google Scholar]
- 24. Russlies M, Ruther P, Koller W, Stomberg P, Behrens P. [Biomechanical properties of cartilage repair tissue after different cartilage repair procedures in sheep]. Z Orthop Ihre Grenzgeb. 2003. Jul-Aug;141(4):465-71. [DOI] [PubMed] [Google Scholar]
- 25. Moojen DJ, Saris DB, Auw Yang G, Dhert WJ, Verbout AJ. The correlation and reproducibility of histological scoring systems in cartilage repair. Tissue Eng. 2002. August;8(4):627-34. [DOI] [PubMed] [Google Scholar]
- 26. Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005. December;87(12):2671-86. [DOI] [PubMed] [Google Scholar]
- 27. O’Driscoll SW, Keeley FW, Salter RB. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion: a follow-up report at one year. J Bone Joint Surg Am. 1988. April;70(4):595-606. [PubMed] [Google Scholar]
- 28. Erggelet C, Endres M, Neumann K, Morawietz L, Ringe J, Haberstroh K, et al. Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J Orthop Res. 2009. October;27(10):1353-60. [DOI] [PubMed] [Google Scholar]
- 29. Kramer J, Bohrnsen F, Lindner U, Behrens P, Schlenke P, Rohwedel J. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci. 2006. March;63(5):616-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002 summer;15(3):170-6. [PubMed] [Google Scholar]
- 31. Browne JE, Anderson AF, Arciero R, Mandelbaum B, Moseley JB, Jr, Micheli LJ, et al. Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res. 2005. July;(436):237-45. [DOI] [PubMed] [Google Scholar]
- 32. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cart. 2002. June;10(6):432-63. [DOI] [PubMed] [Google Scholar]
- 33. Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee. 2006. June;13(3):194-202. [DOI] [PubMed] [Google Scholar]
- 34. Behrens P. Matrixgekoppelte Mikrofrakturierung. Arthroskopie. 2005;18:193-7. [Google Scholar]
- 35. Anders S, Schaumburger J, Schubert T, Grifka J, Behrens P. [Matrix-associated autologous chondrocyte transplantation (MACT): minimally invasive technique in the knee]. Oper Orthop Traumatol. 2008. September;20(3):208-19. [DOI] [PubMed] [Google Scholar]
- 36. Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009. January;37(1):33-41. [DOI] [PubMed] [Google Scholar]
- 37. Buma P, Schreurs W, Verdonschot N. Skeletal tissue engineering-from in vitro studies to large animal models. Biomaterials. 2004. April;25(9):1487-95. [DOI] [PubMed] [Google Scholar]
- 38. Masuko K, Murata M, Suematsu N, Okamoto K, Yudoh K, Nakamura H, et al. A metabolic aspect of osteoarthritis: lipid as a possible contributor to the pathogenesis of cartilage degradation. Clin Exp Rheumatol. 2009. Mar-Apr;27(2):347-53. [PubMed] [Google Scholar]
- 39. Tortelli F, Cancedda R. Three-dimensional cultures of osteogenic and chondrogenic cells: a tissue engineering approach to mimic bone and cartilage in vitro. Eur Cell Mater. 2009;17:1-14. [DOI] [PubMed] [Google Scholar]
- 40. Nehrer S, Breinan HA, Ramappa A, Shortkroff S, Young G, Minas T, et al. Canine chondrocytes seeded in type I and type II collagen implants investigated in vitro. J Biomed Mater Res. 1997 summer;38(2):95-104. [DOI] [PubMed] [Google Scholar]
- 41. Zhang L, Spector M. Comparison of three types of chondrocytes in collagen scaffolds for cartilage tissue engineering. Biomed Mater. 2009. July 28;4(4):45012. [DOI] [PubMed] [Google Scholar]
- 42. Dorotka R, Toma CD, Bindreiter U, Zehetmayer S, Nehrer S. Characteristics of ovine articular chondrocytes in a three-dimensional matrix consisting of different crosslinked collagen. J Biomed Mater Res B Appl Biomater. 2005. January 15;72(1):27-36. [DOI] [PubMed] [Google Scholar]
- 43. Liu Y, Chen F, Liu W, Cui L, Shang Q, Xia W, et al. Repairing large porcine full-thickness defects of articular cartilage using autologous chondrocyte-engineered cartilage. Tissue Eng. 2002. August;8(4):709-21. [DOI] [PubMed] [Google Scholar]
- 44. Lee CR, Grodzinsky AJ, Hsu HP, Spector M. Effects of a cultured autologous chondrocyte-seeded type II collagen scaffold on the healing of a chondral defect in a canine model. J Orthop Res. 2003. March;21(2):272-81. [DOI] [PubMed] [Google Scholar]
- 45. Strauss EJ, Goodrich LR, Chen CT, Hidaka C, Nixon AJ. Biochemical and biomechanical properties of lesion and adjacent articular cartilage after chondral defect repair in an equine model. Am J Sports Med. 2005. November;33(11):1647-53. [DOI] [PubMed] [Google Scholar]
- 46. Trumble T, Allan CH, Miyano J, Clark JM, Ott S, Jones DE, et al. A preliminary study of joint surface changes after an intraarticular fracture: a sheep model of a tibia fracture with weight bearing after internal fixation. J Orthop Trauma. 2001. Jun-Jul;15(5):326-32. [DOI] [PubMed] [Google Scholar]
- 47. Dorotka R, Bindreiter U, Macfelda K, Windberger U, Nehrer S. Marrow stimulation and chondrocyte transplantation using a collagen matrix for cartilage repair. Osteoarthr Cartil. 2005. August;13(8):655-64. [DOI] [PubMed] [Google Scholar]
- 48. Drobnic M, Radosavljevic D, Ravnik D, Pavlovcic V, Hribernik M. Comparison of four techniques for the fixation of a collagen scaffold in the human cadaveric knee. Osteoarthr Cart. 2006. April;14(4):337-44. [DOI] [PubMed] [Google Scholar]
- 49. Lee EH, Hui JH. The potential of stem cells in orthopaedic surgery. J Bone Joint Surg Br. 2006. July;88(7):841-51. [DOI] [PubMed] [Google Scholar]
- 50. Getgood A, Brooks R, Fortier L, Rushton N. Articular cartilage tissue engineering: today’s research, tomorrow’s practice? J Bone Joint Surg Br. 2009. May;91(5):565-76. [DOI] [PubMed] [Google Scholar]


