Abstract
We conducted a systematic review and meta-analysis to estimate the potential association between LCω-3PUFAs and prostate cancer (PC). A comprehensive literature search was performed through 2013 to identify prospective studies that examined dietary intakes of long-chain omega-3 polyunsaturated fatty acids (LCω-3PUFA) or blood biomarkers of LCω-3PUFA status and risk of PC. Random-effects meta-analyses were conducted to generate summary relative risk estimates (SRREs) for LCω-3PUFAs and total PC, and by stage and grade. Subgroup analyses were also conducted for specific fatty acids and other study characteristics. Twelve self-reported dietary intake and 9 biomarker studies from independent study populations were included in the analysis, with 446,243 and 14,897 total participants, respectively. No association between LCω-3PUFAs and total PC was observed (SRRE = 1.00, 95% CI: 0.93–1.09) for the dietary intake studies (high vs. low LCω-3PUFAs category comparison) or for the biomarker studies (SRRE of 1.07, 95% CI: 0.94–1.20). In general, most summary associations for the dietary intake studies were in the inverse direction, whereas the majority of summary associations for the biomarker studies were in the positive direction, but all were weak in magnitude. The results from this meta-analysis do not support an association between LCω-3PUFAs and PC.
INTRODUCTION
Prostate cancer (PC) is the most common cancer among men in the United States, with 233,000 incident cases and 29,480 deaths estimated for 2014 (1,2). PC is the second-leading cause of malignant death in U.S. men, behind lung cancer. The etiology of PC is largely unknown, although older age, African American race, family history of PC, and genetic variations and mutations have been shown to be associated with the disease (1,2). Potential modifiable risk factors, such as obesity, lack of exercise, and smoking, have been linked with risk of PC; however, the magnitude of these factors on PC risk has yet to be established (3–6). Despite the abundance of epidemiologic studies examining the role of diet and dietary/nutrition/food supplements on PC risk, no dietary risk factor has shown any clear associations with this malignancy (6).
Animal and in vitro experimental studies have indicated that LCω-3PUFAs may inhibit carcinogenesis (15). One hypothesis for these results is that LCω-3PUFAs may reduce inflammation given that regions of proliferative inflammatory atrophy in prostate epithelial cells may be etiologically important (12,13). Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) omega-3 fatty acids, for example, may reduce inflammation via both by the displacement of arachidonic acid (ARA) in membrane phospholipids, which can reduce the production of some proinflammatory signaling molecules (7,8), and by the direct production of antiinflammatory or proresolution mediators from EPA and DHA (9–11).
Epidemiological studies of a possible relationship between LCω-3PUFA intake and PC have been mixed. Relative risks (RRs) above and below the null value have been reported across the prospective cohort studies. Most dietary intake studies reported RRs below 1.0, whereas most biomarker studies reported RRs above 1.0. However, results within these groups have been variable by exposure factors and outcome factors, such as specific LCω-3PUFA and tumor histology.
Findings from 2 recent meta-analyses (16,17) were not consistent with the hypothesis that LCω-3PUFAs had anticarcinogenic activity; indeed, both reported some positive associations between LCω-3PUFA levels and risk for high-grade PC. Although Chua et al. (17) reported nonsignificant inverse findings between individual LCω-3PUFAs and total and advanced PC, the authors found a significant positive association between DHA and EPA (combined as one exposure variable) and high-grade prostate cancer. Brasky et al. (16) reported a statistically significant positive association between total LCω-3PUFAs and high grade PC, and positive results for total, low-grade, and high-grade PC for EPA (nonsignificant) and DHA (significant) in their meta-analysis. These 2 meta-analyses only examined biomarker studies and did not evaluate studies based on dietary LCω-3PUFAs intakes. Together both types of exposure measurements would allow for a more complete understanding of the potential relationship between LCω-3PUFAs and PC.
In light of the mixed results from epidemiological studies and meta-analyses, we conducted a meta-analysis using data from both LCω-3PUFA dietary intake studies and LCω-3PUFAs biomarker studies, data from recently published prospective studies (that were not included in previous meta-analyses), and data obtained from direct correspondence with authors. The primary objective was to estimate summary associations for high LCω-3PUFAs categories compared with low categories (separately for dietary intake and biomarker studies). Secondary aims were to (a) examine potential sources of statistical heterogeneity among subgroup stratifications, such as specific fatty acids and PC stage and grade; (b) conduct sensitivity analyses based on individual study influence and other study parameters; (c) estimate the relative influence of each study to the overall estimate; (d) examine dose-response patterns on a study-by-study basis; and (e) evaluate the potential for publication bias.
METHODS
Literature Search and Study Inclusion
A systematic literature search, following guidelines discussed in the scientific literature (18,19) of the MEDLINE database through 2013, was performed using the following search algorithm: (“Fish oils” [MeSH] OR seafood [MeSH] OR EPA OR DHA OR DPA OR fish OR seafood OR “algae oil” OR docosahexaenoic OR eicosapentaenoic OR docosapentaenoic OR “fatty acids” OR “omega-3” OR “n-3”) AND (prostate OR prostatic) AND (mortality OR cancer OR neoplasms [MeSH] OR carcinoma OR neoplasm OR tumor) AND (“cohort studies” [MeSH] OR “cohort” OR “longitudinal” OR “prospective” OR “controlled trial” OR “RCT” OR “randomized controlled trial” OR “nested case-control” OR “clinical trial” [publication type] OR “meta-analysis” [publication type] OR meta-analysis OR “systematic review”) NOT (“animal experimentation” [MeSH] OR “case reports” [publication type] OR editorial [publication type] OR letter [publication type] OR “in vitro” [publication type] OR comment [publication type] OR “case-control studies” [MeSH] OR “cross-sectional studies”[MeSH]). There were no limits by year of publication and all available literature to date was searched. The literature search yielded 313 references that were subsequently screened by title, abstract, and in some cases, full text-review. A complete manual search of reference lists of original studies was conducted. Supplementary searches included reviewing the World Cancer Research Fund (WCRF)/American Institute for Cancer Research report (6), and the FAO/WHO joint expert consultation (20). However, no additional studies were identified based on the supplemental literature searches.
The review was conducted in accordance with established guidelines for systematic reviews specific to human intervention studies in nutritional science (64). Eligible study designs were prospective studies (including cohorts and randomized controlled trials) published in English language that reported quantitative data for LCω-3PUFAs (dietary intake, supplemental intake, or biomarker status). Consistent with the available literature, LCω-3PUFAs included EPA, DHA, and docosapentaenoic acid (DPA) as reported in the individual studies. The outcomes of interest were incident and fatal PC, which included specific subtypes such as nonadvanced/advanced, metastatic, and low/high grade PC. Although studies may differ slightly in diagnostic vernacular, PC is generally defined as “advanced” when it spreads outside the prostate gland to nearby tissues, and “metastatic” when it spreads beyond tissues directly adjacent to the prostate gland. PC grade refers to the tumor's appearance and indicates the rapidity of malignant growth.
Study populations included free-living adults, and studies were required to report RRs with 95% confidence intervals (CIs). We excluded editorials, abstracts, case-control studies, cross-sectional studies, case reports or series, and animal or in vitro studies. Studies that reported only total seafood or fish intake and did not provide quantitative estimates of LCω-3PUFAs were also excluded.
Of the original 313 references identified in the literature search, 254 were excluded based on initial screening. Twenty-two review articles were retained for examination of reference lists, and thirty-seven articles—all of which were observational studies—underwent full-text review. One randomized controlled clinical trial of LCω-3PUFAs and cardiovascular events after myocardial infarction was identified (21) for which PC incidence was listed in the supplementary material. This article was retrieved but not included in our quantitative assessment because PC occurrence was reported as a possible adverse event and limited data on this outcome were available. Based on full-text review and applying the above inclusion and exclusion criteria, a total of 21 prospective cohort studies published in the English-language were included in this analysis.
Data Extraction and Statistical Analysis
We extracted qualitative and quantitative information from each study, including author and year of study, geographic study location, the name of the cohort (if applicable), study size, years of follow-up, type of exposure, method of exposure assessment (i.e., dietary intake, biomarker), exposure metric units, analytical comparisons, number of exposed cases, RR estimates for overall outcome measures as well as subgroup comparisons, 95% CIs, and the variables that were statistically adjusted for or were matched on for each respective RR estimate.
Studies provided the median intake or biomarker levels for each quantile (dietary intake category or biomarker status category expressed as tertiles, quartiles, or quintiles) and an associated RR. Although all studies reported risk estimates for total PC, not all reported data for specific types of PC, such as grade. Thus, we contacted authors from the most recent publications to request estimates for these specific outcomes. We were able to obtain additional risk estimates on tumor grade and stage from Bassett et al. (14) and Park et al. (22). Statistical analyses were based on comparisons of the highest exposure category with the lowest. In addition, we reviewed dose-response patterns on a study-by-study basis to see if trends were apparent (i.e., a monotonic increase in risk based on increasing exposure). We did not combine such data in a categorical dose–response meta-analysis because of the variability of exposure categories.
Random-effects models were used to calculate summary RR estimates (SRREs), 95% CIs, and corresponding P values for heterogeneity. The primary meta-analysis models consisted of combined data from all studies (EPA, DHA, and DPA combined, and total PC). For example, if EPA and DHA were reported separately for the same study (total not reported), data for each category were combined in a fixed effects model to produce a single estimate. This estimate was then used in a random effects meta-analysis model with all other studies. Separate subgroup models by tumor stage and grade, specific fatty acid, and other study characteristics were generated. The relative influence of each study on the overall risk estimate was determined in sensitivity analyses (i.e., each individual study was removed and the summary estimate with and without the study was evaluated to determine the sensitivity of the model based on study inclusion/exclusion). If data for specific PC outcomes, such as nonadvanced and advanced but not total PC were reported, each point estimate and CI was included in the model as these were considered mutually exclusive cases. Statistical heterogeneity was assessed using the Cochran's Q test and I2 statistic. The presence of publication bias was assessed visually by examining a funnel plot measuring the standard error as a function of effect size, as well as performing Egger's regression method and the Duval and Tweedie imputation method (23). All statistical analyses were performed using the Comprehensive Meta-Analysis statistical software package (24).
RESULTS
The primary study characteristics of the 21 prospective cohorts are shown in Table 1. A total of 446,243 participants were included in the meta-analysis of dietary intake studies and 14,807 in the meta-analysis of biomarker studies; 1 cohort (14) examined both biomarkers and self-reported dietary intakes and was included in both analyses. Two studies (25,27) reported specifically on PC mortality. Follow-up ranged from 1.9 yr (22) to 20 yr (26, 27). The majority of dietary intake studies assessed diet using food-frequency questionnaires, whereas most biomarker studies examined serum or plasma phospholipid levels. Twelve cohorts were in the United States or Canada, 8 were in Europe, and 1 was in Australia. The meta-analysis results are summarized in Table 2 (based on high vs. low intake or biomarker level).
TABLE 1 .
Characteristics of the prospective cohorts and nested case-control studies included in the meta-analysis
| Study | Cohort | Age, y | Country | Follow-up, y | LCω-3PUFAs examined | Year LCω-3PUFAs assessed | LCω-3PUFAs assessment method | Sample size | Outcome(s) |
|---|---|---|---|---|---|---|---|---|---|
| Studies using self-reported dietary intakes | |||||||||
| Augustsson, 2003 | HPFS | 40–75 | U.S. | 12 | Total | 1986 | FFQ | 47,882 | Advanced |
| Basset, 2013 | MCCS | 27–80 | Australia | 8.9 | EPA, DHA, DPA | 1990 | FFQ | 1717/464 | Total |
| Chavarro, 2008 | PHS | 40–84 | U.S. | 19 | Total | 1982 | FFQ | 20,167 | Total, fatal |
| Crowe, Key 2008 | EPIC | 50–69 | 8 European Countries | 8.7 | Total | 1992 | FFQ or diet history | 142,520 | Total, nonadvanced, advanced, low-grade, high-grade |
| Epstein, 2012 | Swedish Cohort | ≤80 | Sweden | 20 | Total, EPA, DHA, DPA | 1989 | FFQ | 525 | Total, nonadvanced, advanced |
| Giovannucci, 1993 | HPFS | 40–75 | U.S. | 5 | Total | 1986 | FFQ | 47,855 | Advanced |
| Koralek, 2006 | PLCO | 55–74 | U.S. | 5.1 | Total | 1993 | FFQ | 29,592 | Total |
| Kristal, 2010 | PCPT | ≥55 | U.S. + Canada | 7 | Total | 1994 | FFQ + supplement use questionnaire | 9,559 | Low-grade, high-grade |
| Leitzmann, 2004 | HPFS | 40–75 | US | 14 | Total, EPA, DHA | 1986 | FFQ | 47,886 | Total, nonadvanced, advanced |
| Männisto, 2003e | ATBC | 50–69 | Finland | 8 | EPA, DHA | 1985 | FFQ | 198/198 | Total |
| Park, 2007 | MEC | ≥45 | U.S. | 8 | EPA, DHA | 1993 | FFQ | 82,483 | Total, advanced |
| Schuurman, 1999 | NLCS | 55–69 | The Netherlands | 6.3 | EPA, DHA | 1986 | FFQ | 1525/642 | Total, nonadvanced, advanced |
| Torfadottir, 2013 | AGES-Reykjavik | 67–96 | Iceland | 5.1 | Total | 2002 | FFQ | 2,268 | Total, nonadvanced, advanced |
| Wallstrom, 2007 | MDC | 45–73 | Sweden | 11 | Total, EPA, DHA | 1991 | Diet history | 10,564 | Total, advanced |
| Studies using biomarkers of intake | |||||||||
| Bassett, 2013 | MCCS | 27–80 | Australia | 8.9 | EPA, DHA, DPA | 1990 | Plasma PL FA | 1717/464 | Total |
| Brasky, 2011 | PCPT | 55–84 | U.S. | 7 | Total, EPA, DHA | 1994 | Serum PL FA | 1803/1658 | Total, low-grade, high-grade |
| Brasky, 2013 | SELECT | ≥50 | U.S. | 6 | Total, EPA, DHA | 2001 | Plasma PL FA | 1393/834 | Total, low-grade, high-grade |
| Chavarro, 2007 | PHS | 40–84 | U.S. | 13 | Total, EPA, DHA, DPA | 1982 | Whole blood FA | 476/476 | Total, nonadvanced, advanced, low-grade, high-grade |
| Cheng, 2013 | CARET | 45–69 | U.S. | 20 | Total, EPA, DHA, DPA | 1985 | Serum PL FA | 1398/641 | Nonadvanced, advanced |
| Crowe, Allen 2008 | EPIC | 35–70 | 8 European countries | 4.2 | EPA, DHA, DPA | 1992 | Plasma PL FA | 1061/962 | Total, nonadvanced, low-grade |
| Harvei, 1997 | Norway cohort | 50 (mean) | Norway | 11.6 | EPA, DHA, DPA | 1973 | Serum PL FA | 282/141 | Total |
| Männisto, 2003 | ATBC | 50–69 | Finland | 8 | EPA, DHA | 1985 | Serum cholesterol FA | 198/198 | Total |
| Park, 2009 | MEC | 45–75 | U.S. | 1.9 | EPA, DHA, DPA | 2001 | RBC membrane FA | 729/376 | Total, advanced |
AGES-Reykjavik = Age, Gene/Environment Susceptibility-Reykjavik Study; ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CARET = Carotene and Retinol Efficacy Trial; DHA = docosahexaenoic acid; DPA = docosapentaenoic acid; EPA = eicosapentaenoic acid; EPIC = European Prospective Investigation into Cancer and Nutrition; FA = fatty acid; FFQ = food frequency questionnaire; HPFS = Health Professionals Follow-up Study; MCCS = Melbourne Collaborative Cohort Study; MDC = Malmö Diet and Cancer Study; MEC = Multiethnic Cohort Study; NLCS = Netherlands Cohort Study; PCPT = Prostate Cancer Prevention Trial; PHS = Physician's Health Study; PL = phospholipid; PLCO = Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; RBC = red blood cell; SELECT = Selenium and Vitamin E Cancer Prevention Trial.
Mean provided when age range was not reported.
Sample size for nested case-control studies are shown as controls/cases.
Basset et al. (2013) investigated associations between fatty acids assessed in plasma phospholipids or diet and prostate cancer risk.
Kristal et al. (2010) assessed EPA and DHA intake using an FFQ and structured supplement-use questionnaire.
Männisto et al. (2003) evaluated both serum and dietary fatty acids.
Torfadottir et al. (2013) evaluated fish liver oil supplements in liquid or capsules.
Wallstrom et al. (2007) evaluated EPA and DHA from both diet and supplement use.
TABLE 2 .
Summary of meta-analysis results for long chain omega-3 polyunsaturated fatty acids and prostate cancer (high vs. low exposure)
| Model (number of data points) | SRRE | 95% CI | P value for Heterogeneity and I2 |
|---|---|---|---|
| Studies using self-reported dietary intakes | |||
| All studies (n = 13) | 1.00 | 0.93–1.09 | 0.019, I2 = 50.4 |
| Studies conducted in North America (n = 7) | 1.02 | 0.96–1.09 | 0.298, I2 = 17.3 |
| Studies conducted in Europe and Australia (n = 6) | 0.94 | 0.76–1.16 | 0.006, I2 = 69.5 |
| Initial dietary assessment period <1990 (n = 6) | 0.96 | 0.82–1.12 | 0.033, I2 = 58.7 |
| Initial dietary assessment period 1990+ (n = 7) | 1.03 | 0.93–1.14 | 0.072, I2 = 48.2 |
| Follow-up period <10 years (n = 9) | 1.01 | 0.94–1.08 | 0.334, I2 = 12.1 |
| Follow-up period 10+ years (n = 4) | 0.96 | 0.77–1.21 | 0.002, I2 = 80.1 |
| Nonadvanced prostate cancer (n = 3) | 0.91 | 0.96–1.09 | 0.533 |
| Advanced prostate cancer (n = 6) | 0.83 | 0.67–1.04 | 0.078 |
| EPA and total prostate cancer (n = 7) | 0.96 | 0.84–1.10 | 0.033, I2 = 56.3 |
| DHA and total prostate cancer (n = 7) | 0.97 | 0.84–1.12 | 0.023, I2 = 59.2 |
| DPA and total prostate cancer (n = 2) | 0.92 | 0.71–1.19 | 0.487, I2 = 0.0 |
| Studies using biomarkers of intake | |||
| All studies (n = 10) | 1.07 | 0.94–1.20 | 0.065, I2= 44.0 |
| Studies conducted in North America (n = 6) | 1.08 | 0.89–1.30 | 0.043, I2 = 56.4 |
| Studies conducted in Europe and Australia (n = 4) | 1.05 | 0.89–1.23 | 0.228, I2 = 30.8 |
| Initial dietary assessment period <1990 (n = 5) | 0.93 | 0.75–1.14 | 0.222, I2 = 30.0 |
| Initial dietary assessment period 1990+ (n = 5) | 1.14 | 1.01–1.30 | 0.159, I2 = 39.3 |
| Follow-up period <10 years (n = 6) | 1.12 | 0.99–1.27 | 0.157, I2 = 37.4 |
| Follow-up period 10+ years (n = 4) | 0.93 | 0.71–1.22 | 0.131, I2 = 46.7 |
| Non-advanced prostate cancer (n = 3) | 0.96 | 0.65–1.41 | 0.054, I2 = 65.7 |
| Advanced prostate cancer (n = 3) | 0.98 | 0.68–1.42 | 0.524, I2 = 0.0 |
| Low-grade prostate cancer (n = 6), | 1.12 | 0.96–1.32 | 0.139, I2 = 40.0 |
| High-grade prostate cancer (n = 6), | 1.21 | 0.83–1.75 | 0.037, I2 = 57.7 |
| EPA and total prostate cancer (n = 8) | 1.07 | 0.93–1.23 | 0.230 |
| DHA and total prostate cancer (n = 8) | 1.06 | 0.87–1.29 | 0.018 |
| DPA and total prostate cancer (n = 7) | 0.85 | 0.72–0.99 | 0.764, I2 = 0.0 |
SRRE = summary relative risk estimates; CI = confidence interval; EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid; DPA = docosapentaenoic acid.
Park et al. (22) was contacted to obtain risk estimates for advanced prostate cancer.
Park et al. (22) was contacted to obtain risk estimates for low-grade and high-grade prostate cancer.
Risk estimates from correspondence with Bassett et al. for low-grade and high-grade prostate cancer.
Dietary Intake of LCω-3PUFAs
No association was observed in the meta-analysis of 12 studies that reported data for dietary intake of LCω-3PUFAs and total PC (SRRE = 1.00, 95% CI: 0.93–1.09) (Table 2, Fig. 1). Significant heterogeneity was evident in this model (I2 = 50.4%, p-H = 0.019). Visual inspection of the funnel plot, Egger's regression method, and the Duval and Tweedie imputation method showed no evidence of publication bias. Subgroup meta-analyses stratified by study country were similar. The 6 studies conducted in North America resulted in an SRRE = 1.02 (95% CI: 0.96–1.09), whereas the 5 studies conducted in Europe and the one Australian study resulted in an SRRE of 0.94 (95% CI: 0.76–1.16). Significant heterogeneity was apparent in the studies conducted in Europe and Australia (I2= 69.5, P = 0.006). No appreciable differences were observed when studies were stratified by year of initial dietary assessment period or by duration of follow-up (Table 2, Fig. 1).
FIG. 1.
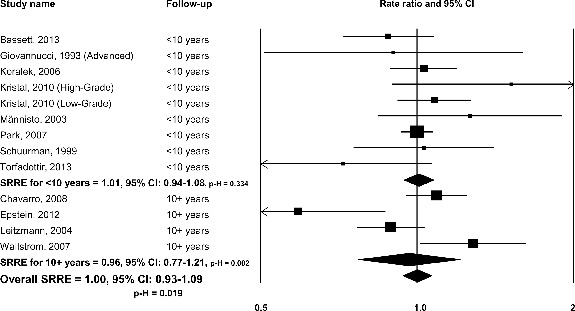
Omega-3 long chain-polyunsaturated fatty acids and total prostate cancer: dietary intake studies, overall and by follow-up duration.
Six studies reported RRs between LCω-3PUFA intake and advanced PC, resulting in a decreased risk of 0.83 (95% CI: 0.67–1.04). Five of the 6 studies reported inverse results. The SRRE for nonadvanced PC was 0.91 (95% CI: 0.96–1.09), based on data from 3 studies. Limited data from dietary intake studies on associations between LCω-3PUFA and tumor grade precluded a formal meta-analysis. Kristal et al. (28) reported a positive result between dietary EPA + DHA and high-grade PC (RR = 1.46, 95% CI: 0.86–2.50), but Wallstrom et al. (29) observed an inverse result between EPA + DHA and high-grade/advanced PC combined (RR = 0.86, 95% CI: 0.58–1.28).
No significant result was found in the meta-analysis of seven studies that reported data for dietary EPA and total PC (SRRE = 0.96, 95% CI: 0.84–1.10) (Table 2). Significant heterogeneity was observed in this model (p-H = 0.033), largely due to the influence of Wallstrom et al. (29). Removal of this study in a sensitivity analysis resulted in an SRRE of 0.92 (95% CI: 0.82–1.04), with less between-study statistical variability (p-H = 0.182). Similar results were found for dietary EPA and total PC, but the removal of the Wallstrom study (29) reduced heterogeneity (p-H = 0.123). Only 2 dietary intake studies investigated DPA and PC, resulting in an SRRE of 0.92 (95% CI: 0.71–1.19).
No evidence of a dose-response relationship between increasing quantiles of LCω-3PUFA intake and PC was found. Augustsson et al. (30) reported a significant reduced risk of metastatic PC (RR = 0.76 per 0.5 g/day increase of marine fatty acids, 95% CI: 0.58–0.98) based on their analysis of continuous data. Leitzman et al. (31) observed a nonsignificant RR of 0.53 for each 0.5 g/day increase of EPA + DHA and advanced PC. Using continuous data, Bassett et al. (14) observed nonsignificant decreased risks for total PC and EPA, DHA, and DPA. Analyzing a 1% unit increase in energy from fish fat, Crowe et al. (32) reported no associations for total, nonadvanced, advanced, low-grade, and high-grade PC. Epstein et al. (27) reported no significant associations for fatty acids and total, nonadvanced, or advanced PC, although most RRs were below unity. Schuurman et al. (33) reported no significant associations for EPA or DHA and PC.
Some studies did not analyze continuous data to generate risk estimates based on dose-response units. For these studies, we reviewed the RRs based on each increasing category of exposure to determine if a dose-response trend was apparent (i.e., a monotonic increase in risk based on increasing exposure). Chavarro et al. (25) reported decreasing RRs of 0.98, 0.78, 0.65, and 0.65 (significant) for fatal PC based on increasing exposure categories of seafood LCω-3PUFA intake. Koralek et al. (34) (total PC), Park et al. (35) (total PC, advanced PC), Torfadottir et al. (36) (total, nonadvanced, advanced PC), and Wallstrom et al. (29) (advanced/high grade PC, total PC) observed no evidence of a dose-response trend for LCω-3PUFA intake. Giovannucci et al. (37) found nonsignificant positive RRs in their first 2 exposure categories of fish LCω-3PUFA intake and advanced PC but inverse RRs were observed in their 2 highest exposure categories. Kristal et al. (28) reported nonsignificant RRs of 1.09, 1.05, and 1.08 based on increasing categories of EPA + DHA and low-grade PC, and nonsignificant RRs of 1.25, 1.20, and 1.52 for high-grade PC. Mannisto et al. (38) reported a RR of 1.69 in their lowest exposure category for EPA and total PC, but the associations attenuated (1.56 and 1.22) in their highest categories of exposure. Nonsignificant RRs of 1.46, 1.18, and 1.31 were reported for DHA and total PC.
Biomarkers of LCω-3PUFAs
A nonsignificant SRRE was observed in the meta-analysis of 9 biomarker studies of LCω-3PUFAs (SRRE = 1.07, 95% CI: 0.94–1.20; p-H = 0.065) (Table 2, Fig. 2). A 1-study-removed meta-analysis was conducted to evaluate the relative influence that each study had on the overall model. Removal of each study had a negligible impact on the overall summary effect; however, removal of Chavarro et al. (39) resulted in a statistically significant SRRE of 1.11 (95% CI: 1.01–1.21). Visual inspection of funnel plots and Egger's regression method and the Duval and Tweedie imputation method did not indicate the presence of publication bias. No differences in summary associations were observed after stratifying by study country (Table 2).
FIG. 2.
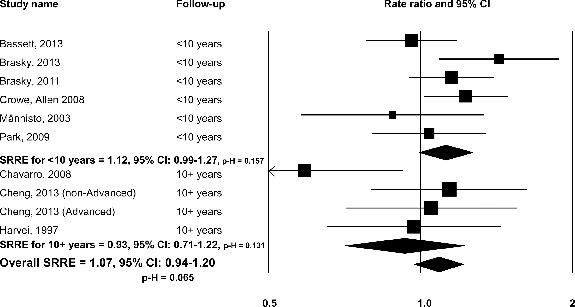
Omega-3 long chain-polyunsaturated fatty acids and total prostate cancer: biomarker studies, overall and by follow-up duration.
There was some evidence of effect modification by biomarker status period and duration of follow-up, with nonsignificant inverse risk estimates among studies with initial assessments before 1990 and among studies with 10 or more years of follow-up compared with a significant positive association among studies with initial assessment after 1990 (SRRE = 1.14, 95% CI: 1.01–1.30), and a nonsignificant positive association among studies with less than 10 years of follow-up (SRRE of 1.12, 95% CI: 0.99–1.27) (Table 2, Fig. 2).
Nonsignificant inverse SRREs were observed for LCω-3PUFAs biomarkers and nonadvanced and advanced PC, although only 3 data points were analyzed for each model whereas nonsignificant positive SRREs were found for both low and high-grade PC (Table 2, Fig. 3).
FIG. 3.
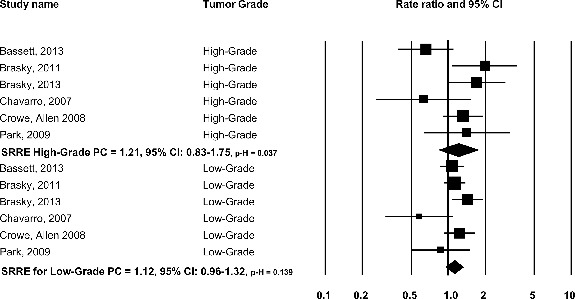
Omega-3 long chain-polyunsaturated fatty acids and total prostate cancer by grade: biomarker studies.
Analyses of EPA and DHA separately and total PC, produced similar nonsignificant positive SRREs. Half of the point estimates from the individual studies for DHA were in the inverse direction, whereas individual point estimates for EPA, except for 1, were in the positive direction. However, removal of Chavarro et al. (39) (the single study showing a decreased risk for EPA) did not affect the summary association. A significant inverse summary association between DPA and total PC was observed (SRRE = 0.85, 95% CI: 0.72–0.99) with no statistical heterogeneity.
Similar to the dietary intake studies, no consistent dose-response relationships were evident in the biomarker studies. Two studies analyzed dose-response relationships using continuous data analysis. Bassett et al. (2013) reported no dose-response associations based on %phospholipid (PPL) LCω-3PUFAs and total PC in their continuous data analysis. In contrast, Brasky et al. (16) reported significant positive dose-response associations for total (RR = 1.23, 95% CI: 1.07–1.40), low-grade (RR = 1.24, 95% CI: 1.07–1.43), and high-grade (RR = 1.24, 95% CI: 1.00–1.54) PC based on %PPL LCω-3PUFAs.
The remainder of the biomarker studies reported RRs for exposure categories, thus, we reviewed the associations based on each increasing category of exposure. Brasky et al. (40) reported significant positive associations for LCω-3PUFAs and high-grade PC (RR = 1.99), DHA and high-grade PC (RR = 2.50), and DHA and total PC (RR = 1.22, data available for highest category only) in their highest exposure categories. However, RRs were stronger in magnitude for the lowest categories of exposure (RR for LCω-3PUFAs and high-grade PC = 2.15; RR for DHA and high-grade PC = 2.65), and thus, clear dose-response relationships are not supported. There was no consistent evidence for a dose-response relationship in the remaining six biomarker studies (22,26,38,39,41,42), with most RRs close to the null value and not statistically significant at the upper end of exposure, and many RRs in the lower categories of exposure were stronger in magnitude than the RRs in the higher categories of exposure.
DISCUSSION
This meta-analysis does not support an association between LCω-3PUFAs and PC risk. This is the first quantitative assessment to examine dietary intakes of LCω-3PUFAs as well as biomarkers of LCω-3PUFAs in relation to PC. For both analyses, summary results were weak in magnitude and generally close to null. Very few meta-analysis models produced statistically significant associations, and heterogeneity was present in many models. Such statistical heterogeneity would be expected in the absence of a clear and independent relationship between LCω-3PUFAs and PC risk, because RRs from individual studies would likely be in the vicinity of 1.0, on both sides of the null value. This is indeed the case in the dietary intake studies, with 6 point estimates below the null value for total PC, and 7 point estimates above it (Fig. 1). Similarly, statistical heterogeneity was apparent in many of the biomarker studies, and this variation was partially explained by differences in the exposure ascertainment period and the duration of follow-up. For an outcome such as PC, which has a relatively long latency period (43), a longer follow-up time would be more appropriate to capture a true association, if the exposure was causally related to risk of disease. However, summary associations were in the inverse direction for the studies that followed participants for more than 10 yr and/or among studies with an initial dietary assessment period prior to 1990. In contrast, summary associations were in the positive direction for studies with a follow-up period less than 10 yr and/or among studies with initial dietary assessment periods in 1990 or later.
An overall lack of consistency is apparent in the summary results across the various models (Table 2). The most striking observation is that most summary associations are in the inverse direction in the analyses of the dietary intake studies of LCω-3PUFAs, and most, but not all, summary associations are in the positive direction for the biomarker studies. Each method of estimating exposure to LCω-3PUFAs is informative, with important advantages and limitations; thus, both types of studies should be appraised critically when reviewing evidence on LCω-3PUFAs and PC risk. The most frequently used self-report diet assessment tool among the individual studies included in this meta-analysis was the food frequency questionnaire. This tool captures intakes of foods, beverages, and, in some cases, dietary supplements, consumed by individuals over a specified reference period (often 1 yr). However, diet is measured with error, which can be random or systematic (44). Such exposure misclassification could bias results in either direction. This measurement error has led some researchers to investigate theoretically more objective measures of LCω-3PUFA intake, namely, biomarkers, including fatty acid levels in plasma/serum phospholipids, whole blood, cholesterol esters, or red blood cell (RBC) membranes. Compared with intakes estimated from self-report methods, these biochemical markers could provide a more accurate estimate of exposure without the potential for self-reporting bias (45). Indeed, the LCω-3PUFA content of RBCs (46–51) and of phospholipids (52,53) are both validated biomarkers of intake. However, such measurement methods are not without limitations. Many of the serum/plasma-based biomarkers used in the individual studies included in this meta-analysis reflect relatively recent intake (past 1 to 2 days) and display fairly high intraindividual variability (55). RBC membranes, on the other hand, reflect intakes of approximately one month (54) and are less variable day to day (55). Another key consideration favoring the use of biomarkers as measures of exposure is that LCω-3PUFA blood concentrations are not solely from marine sources, as a small proportion is derived from the conversion of plant-derived alpha-linolenic acid to LCω-3PUFA, which can vary from person to person (49).
Recent meta-analyses of epidemiologic studies have examined the relationship between LCω-3PUFAs and PC (16,17,56,57), with mixed conclusions. Differences in these meta-analyses likely contributed to the discrepant findings. These differences include the use of fixed-effects models (accounts for within-study variation) (16) vs. the more appropriate random-effects models (accounts for both, within, and between-study variation) (17,56,57), and differences in the types of study designs included. Chua et al. (56) reported associations close to the null value between higher self-reported dietary intakes of total LCω-3PUFAs, as well as EPA and DHA separately, and total PC incidence. In a separate meta-analysis of biomarker studies, Chua et al. (17) reported nonsignificant associations between higher vs. lower blood levels of individual EPA, DHA, and DPA and total PC incidence. In a subgroup analysis of studies that examined high-grade PC, nonstatistically significant positive findings were observed (17). Summary associations between total LCω-3PUFAs levels, as well as individual EPA and DHA levels, and total PC from a meta-analysis of biomarker studies (57) were close to the null value, but a statistically significant reduced risk for total PC was reported for higher DPA levels. This latter finding remained significant in a meta-analysis that removed the retrospective case-control studies (57). Brasky et al. (16), using a fixed effects meta-analysis model, examined prospective nested case-control and case-cohort studies. Among the 9 summary associations from stratified analyses by fatty acid (EPA, DHA, and total LCω-3PUFAs) and grade of PC (total, low-grade, and high-grade), 4 were significantly positive. Brasky et al. (40) reported inverse associations with plasma phospholipid trans-fatty acid levels and high-grade PC risk, which is inconsistent given that trans-fatty acids have been linked with higher inflammatory status (58).
Our methodological protocol for the biomarker studies analysis is similar to the one used by Brasky et al. (16) in that we included only studies with prospective designs. However, we also included dietary intake studies, as was done by Chua et al. (56). Our summary associations in the analysis of biomarker studies are closer to the null value compared with those reported by Brasky et al. (16) [Summary RR comparing highest to lowest level of blood LCn3PUFA: 1.51 (95% CI: 1.08, 2.11)]. This discrepancy might be attributable to the inclusion of data from 2 recently published studies (14,26) in this meta-analysis but not included by Brasky et al. (16), a larger number of subgroup and sensitivity analyses, and utilization of random-effects meta-analysis models that take into account both within- and between-study variability. As indicated, we conducted numerous subgroup and sensitivity analyses to examine consistency of findings across different models with the expectation that summary associations would be consistently elevated if LCω-3PUFAs were associated with increasing the risk of PC. If a true independent association were to exist, it would be expected that associations would be relatively strong in magnitude and that there would be a dose-response relationship. Moreover, it would be expected that summary associations by various methodological designs would substantiate each other. None of these situations have been satisfied: Summary associations are close to the null value and above and below 1.0, evidence of a dose-response relationship across the individual studies is lacking, and findings are inconsistent.
There is biological evidence supporting a role for LCω-3PUFAs in the inhibition of prostate carcinogenesis (15,26). LCω-3PUFAs may inhibit prostate cell growth, and they may also have antiinflammatory, antiproliferative, and proapoptotic effects on PC cells (15,26,59). In contrast, there are few hypothesized mechanisms for which LCω-3PUFAs may contribute to prostate carcinogenesis. In the aforementioned analysis by Brasky et al. (16), the authors note the beneficial physiological effects of LCω-3PUFAs, and they stated that it was unclear as to why LCω-3PUFAs could increase PC risk. Chua et al. (17) suggested that modifications in the androgen milieu from dietary fat intake may contribute to PC, and that the positive association observed for high-grade tumors may be due to a biochemical process in the prostate tissue. He et al. (60) and Azordegan et al. (61) both provide evidence that carcinogenesis (breast, lung) itself alters tissue fatty acid metabolism (e.g., increases in the activity of delta-6-desaturase). This could increase tissue (and possibly plasma) levels of LCω-3PUFAs. This would be an example of reverse causation, where the disease caused the change in biomarker. Because in Brasky et al. (16) a much larger proportion of men who ultimately developed PC (30–40%) had PSA levels >3 at baseline (compared to 7% of the controls), it is possible that subclinical PC was already developing in the higher risk men, and differences in tissue biology, not fish intake or fish oil supplement use were responsible for the observed associations between incident PC and plasma phospholipid LCω-3PUFA levels.
Cheng et al. (26) indicated that the multiple double bonds in LCω-3PUFAs may attract reactive oxygen species or free radicals, which may lead to membrane and DNA damage that foster cancer development (62). Further, Chua et al. (17) opined that an association may be apparent for high-grade tumors rather than for total or indolent PC because PSA screening may detect these tumors at an earlier stage (63). Despite these hypotheses, findings from both the dietary intake and the biomarker status studies do not support a clear or consistent association between LCω-3PUFA biomarker levels and incident PC.
In summary, our meta-analysis of the published studies to date does not support an association between LCω-3PUFAs and the risk of PC. Analysis of dietary intake studies shows no overall association, and some models indicate small inverse associations. The majority of the biomarker studies showed slight positive and nonstatistically significant findings, but some summary associations were in the inverse direction. Importantly, weakly positive associations were produced by studies that used shorter follow-up periods, whereas analyses of the studies with follow-up periods more representative of the long latency period for PC tended to exhibit no or slightly inverse associations, an effect that was consistent in both dietary intake and biomarker studies. Finally, a plausible biological mechanism by which LCω-3PUFAs could facilitate prostate carcinogenesis has not been identified whereas antiinflammatory properties of these fatty acids would suggest anticarcinogenic effects. For all these reasons, any posited association between LCω-3PUFAs and PC, either positive or negative, is not supported by current epidemiologic evidence.
Funding
This work was supported by the Global Organization for EPA and DHA Omega-3s (GOED). Drs. Harris, Weed, Bassett and Barrett did not receive funding from GOED.
REFERENCES
- American Cancer Society . Cancer Facts & Figures. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- National Cancer Institute SEER Stat Fact Sheets: Prostate Cancer: Surveillance, Epidemiology, and End Results Program. National Cancer Institute, National Institutes of Health. 2014 [Google Scholar]
- International Agency for Research on Cancer . Weight control and physical activity. In: Vainio H, Bianchini F, editors. IARC Handbook of Cancer Prevention. Lyon, France: IARC Press; 2002. http://www.iarc.fr/en/publications/pdfs-online/prev/handbook6/Handbook6.pdf [Google Scholar]
- Morote J, Celma A, Planas J, Placer J, Konstantinidis C.Sedentarism and overweight as risk factors for the detection of prostate cancer and its aggressivenes Actas Urol Esp 382013 [DOI] [PubMed] [Google Scholar]
- Murphy AB, Akereyeni F, Nyame YA, Guy MC, Martin IK. Smoking and prostate cancer in a multi-ethnic cohort. Prostate. 2013:1518–1528. doi: 10.1002/pros.22699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology. Br J Clin Pharmacol. 2013:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Zirpoli H, Qi K. n-3 fatty acids modulate adipose tissue inflammation and oxidative stress. Curr Opin Clin Nutr Metab Care. 2013;(2):124–132. doi: 10.1097/MCO.0b013e32835c02c8. [DOI] [PubMed] [Google Scholar]
- Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. 2010:781–792. doi: 10.1016/j.jnutbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009:187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Systems approach with inflammatory exudates uncovers novel anti-inflammatory and pro-resolving mediators. Prostaglandins Leukot Essent Fatty Acids. 2008:157–163. doi: 10.1016/j.plefa.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012:199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Bergh A, Damber JE. Morphological transition of proliferative inflammatory atrophy to high-grade intraepithelial neoplasia and cancer in human prostate. Prostate. 2009:1378–1386. doi: 10.1002/pros.20992. [DOI] [PubMed] [Google Scholar]
- Bassett JK, Severi G, Hodge AM, MacInnis RJ, Gibson RA. Plasma phospholipid fatty acids, dietary fatty acids and prostate cancer risk. Int J Cancer. 2013:1882–1891. doi: 10.1002/ijc.28203. [DOI] [PubMed] [Google Scholar]
- Rose DP. Effects of dietary fatty acids on breast and prostate cancers: evidence from in vitro experiments and animal studies. Am J Clin Nutr. 1997;(6, Suppl):1513S–1522S. doi: 10.1093/ajcn/66.6.1513S. [DOI] [PubMed] [Google Scholar]
- Brasky TM, Darke AK, Song X, Tangen CM, Goodman PJ. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2013:1132–1141. doi: 10.1093/jnci/djt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua ME, Sio MC, Sorongon MC, Morales ML. The relevance of serum levels of long chain omega-3 polyunsaturated fatty acids and prostate cancer risk: A meta-analysis. Can Urol Assoc J. 2013;(5–6):E333–E343. doi: 10.5489/cuaj.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein AH, Yetley EA, Lau J. Application of systematic review methodology to the field of nutrition. J Nutr. 2008:2297–2306. doi: 10.3945/jn.108.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Tricco AC. Issues related to the conduct of systematic reviews: a focus on the nutrition field. Am J Clin Nutr. 2008:1191–1199. doi: 10.3945/ajcn.2008.26255. [DOI] [PubMed] [Google Scholar]
- Nishida C, Uauy R, Kumanyika S, Shetty P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutr. 2004;(1A):245–250. doi: 10.1079/phn2003592. [DOI] [PubMed] [Google Scholar]
- Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- Park SY, Wilkens LR, Henning SM, Le Marchand L, Gao K. Circulating fatty acids and prostate cancer risk in a nested case-control study: the Multiethnic Cohort. Cancer Causes Control. 2009:211–223. doi: 10.1007/s10552-008-9236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein H, Sutton A, Borenstein M. Publication Bias in Meta-Analysis: Prevention, Assessment, and Adjustments. Chichester, England: John Wiley and Sons, Ltd; 2005. [Google Scholar]
- Comprehensive Meta-Analysis . Englewood, NJ: Biostat; Version 2.2.046. [Google Scholar]
- Chavarro JE, Stampfer MJ, Hall MN, Sesso HD, Ma J. A 22-y prospective study of fish intake in relation to prostate cancer incidence and mortality. Am J Clin Nutr. 2008:1297–1303. doi: 10.3945/ajcn.2008.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TY, King IB, Barnett MJ, Ambrosone CB, Thornquist MD. Serum phospholipid fatty acids, genetic variation in myeloperoxidase, and prostate cancer risk in heavy smokers: a gene-nutrient interaction in the carotene and retinol efficacy trial. Am J Epidemiol. 2013:1106–1117. doi: 10.1093/aje/kws356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein MM, Kasperzyk JL, Mucci LA, Giovannucci E, Price A. Dietary fatty acid intake and prostate cancer survival in Orebro County, Sweden. Am J Epidemiol. 2012:240–252. doi: 10.1093/aje/kwr520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal AR, Arnold KB, Neuhouser ML, Goodman P, Platz EA. Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2010:566–577. doi: 10.1093/aje/kwq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallstrom P, Bjartell A, Gullberg B, Olsson H, Wirfalt E. A prospective study on dietary fat and incidence of prostate cancer (Malmo, Sweden) Cancer Causes Control. 2007:1107–1121. doi: 10.1007/s10552-007-9050-4. [DOI] [PubMed] [Google Scholar]
- Augustsson K, Michaud DS, Rimm EB, Leitzmann MF, Stampfer MJ. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003:64–67. [PubMed] [Google Scholar]
- Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;(1):204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- Crowe FL, Key TJ, Appleby PN, Travis RC, Overvad K. Dietary fat intake and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008:1405–1413. doi: 10.1093/ajcn/87.5.1405. [DOI] [PubMed] [Google Scholar]
- Schuurman AG, van den Brandt PA, Dorant E, Brants HA, Goldbohm RA. Association of energy and fat intake with prostate carcinoma risk: results from The Netherlands Cohort Study. Cancer. 1999:1019–1027. [PubMed] [Google Scholar]
- Koralek DO, Peters U, Andriole G, Reding D, Kirsh V. A prospective study of dietary alpha-linolenic acid and the risk of prostate cancer (United States) Cancer Causes Control. 2006:783–791. doi: 10.1007/s10552-006-0014-x. [DOI] [PubMed] [Google Scholar]
- Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Fat and meat intake and prostate cancer risk: the multiethnic cohort study. Int J Cancer. 2007:1339–1345. doi: 10.1002/ijc.22805. [DOI] [PubMed] [Google Scholar]
- Torfadottir JE, Valdimarsdottir UA, Mucci LA, Kasperzyk JL, Fall K. Consumption of fish products across the lifespan and prostate cancer risk. PLoS One. 2013 doi: 10.1371/journal.pone.0059799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Ascherio A. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993:1571–1579. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- Mannisto S, Pietinen P, Virtanen MJ, Salminen I, Albanes D. Fatty acids and risk of prostate cancer in a nested case-control study in male smokers. Cancer Epidemiol Biomarkers Prev. 2003:1422–1428. [PubMed] [Google Scholar]
- Chavarro JE, Stampfer MJ, Li H, Campos H, Kurth T. A prospective study of polyunsaturated fatty acid levels in blood and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2007:1364–1370. doi: 10.1158/1055-9965.EPI-06-1033. [DOI] [PubMed] [Google Scholar]
- Brasky TM, Till C, White E, Neuhouser ML, Song X. Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2011:1429–1439. doi: 10.1093/aje/kwr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe FL, Allen NE, Appleby PN, Overvad K, Aardestrup IV. Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008:1353–1363. doi: 10.3945/ajcn.2008.26369. [DOI] [PubMed] [Google Scholar]
- Harvei S, Bjerve KS, Tretli S, Jellum E, Robsahm TE. Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. Int J Cancer. 1997:545–551. doi: 10.1002/(sici)1097-0215(19970516)71:4<545::aid-ijc7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- DePrimo SE, Shinghal R, Vidanes G, Brooks JD. Prevention of prostate cancer. Hematol Oncol Clin North Am. 2001:445–457. doi: 10.1016/s0889-8588(05)70225-2. [DOI] [PubMed] [Google Scholar]
- Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol. 2003:22–26. doi: 10.1093/aje/kwg091. [DOI] [PubMed] [Google Scholar]
- Hedrick VE, Dietrich AM, Estabrooks PA, Savla J, Serrano E. Dietary biomarkers: advances, limitations and future directions. Nutr J. 2012:109. doi: 10.1186/1475-2891-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block RC, Harris WS, Pottala JV. Determinants of blood cell omega-3 fatty acid content. Open Biomark J. 2008:1–6. doi: 10.2174/1875318300801010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning LM, Walker CG, Mander AP, West AL, Madden J. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr. 2012:748–758. doi: 10.3945/ajcn.112.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA. Determinants of erythrocyte omega-3 Fatty Acid content in response to fish oil supplementation: a dose-response randomized controlled trial. J Am Heart Assoc. 2013;(6):e000513. doi: 10.1161/JAHA.113.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WS, Pottala JV, Lacey SM, Vasan RS, Larson MG. Clinical correlates and heritability of erythrocyte eicosapentaenoic and docosahexaenoic acid content in the Framingham Heart Study. Atherosclerosis. 2012:425–431. doi: 10.1016/j.atherosclerosis.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury AC, Amin AP, Harris WS, Chan PS, Gosch KL. Predictors of omega-3 index in patients with acute myocardial infarction. Mayo Clin Proc. 2011:626–632. doi: 10.4065/mcp.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005:343–347. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- Garneau V, Rudkowska I, Paradis AM, Godin G, Julien P. Omega-3 fatty acids status in human subjects estimated using a food frequency questionnaire and plasma phospholipids levels. Nutr J. 2012 doi: 10.1186/1475-2891-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DI, Reis GJ, Sacks FM, Boucher TM, Pasternak RC. Usefulness of plasma phospholipid N-3 fatty acid levels in predicting dietary fish intake in patients with coronary artery disease. Am J Cardiol. 1990:860–862. doi: 10.1016/0002-9149(90)90367-a. [DOI] [PubMed] [Google Scholar]
- Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997:2012–2022. [PubMed] [Google Scholar]
- Harris WS, Thomas RM. Biological variability of blood omega-3 biomarkers. Clin Biochem. 2010:338–340. doi: 10.1016/j.clinbiochem.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Chua ME, Sio MC, Sorongon MC, Dy JS. Relationship of dietary intake of omega-3 and omega-6 Fatty acids with risk of prostate cancer development: a meta-analysis of prospective studies and review of literature. Prostate Cancer. 2012:826254. doi: 10.1155/2012/826254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorongon-Legaspi MK, Chua M, Sio MC, Morales M. Blood level omega-3 Fatty acids as risk determinant molecular biomarker for prostate cancer. Prostate Cancer. 2013:875615. doi: 10.1155/2013/875615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- Astorg P. Dietary N-6 and N-3 polyunsaturated fatty acids and prostate cancer risk: a review of epidemiological and experimental evidence. Cancer Causes Control. 2004:367–386. doi: 10.1023/B:CACO.0000027498.94238.a3. [DOI] [PubMed] [Google Scholar]
- He C, Qu X, Wan J, Rong R, Huang L. Inhibiting delta-6 desaturase activity suppresses tumor growth in mice. PLoS One. 2012;(10):e47567. doi: 10.1371/journal.pone.0047567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azordegan N, Fraser V, Le K, Hillyer LM, Ma DW. Carcinogenesis alters fatty acid profile in breast tissue. Mol Cell Biochem. 2013:223–232. doi: 10.1007/s11010-012-1523-4. [DOI] [PubMed] [Google Scholar]
- Catala A. A synopsis of the process of lipid peroxidation since the discovery of the essential fatty acids. Biochem Biophys Res Commun. 2010:318–323. doi: 10.1016/j.bbrc.2010.07.087. [DOI] [PubMed] [Google Scholar]
- Legler JM, Feuer EJ, Potosky AL, Merrill RM, Kramer BS. The role of prostate-specific antigen (PSA) testing patterns in the recent prostate cancer incidence decline in the United States. Cancer Causes Control. 1998:519–527. doi: 10.1023/a:1008805718310. [DOI] [PubMed] [Google Scholar]
- Welch RW, Antoine JM, Berta JL, Bub A, de Vries J. Guidelines for the design, conduct and reporting of human intervention studies to evaluate the health benefits of foods. Br J Nutr. 2011;(Suppl 2):S3–S15. doi: 10.1017/S0007114511003606. [DOI] [PubMed] [Google Scholar]


