Abstract
We hypothesized that prior colonization with antibiotic-resistant Gram-negative bacteria is associated with increased risk of subsequent antibiotic-resistant Gram-negative bacteremia among cancer patients. We performed a matched case-control study. Cases were cancer patients with a blood culture positive for antibiotic-resistant Gram-negative bacteria. Controls were cancer patients with a blood culture not positive for antibiotic-resistant Gram-negative bacteria. Prior colonization was defined as any antibiotic-resistant Gram-negative bacteria in surveillance or non-sterile-site cultures obtained 2–365 days before the bacteremia. Thirty-two (37%) of 86 cases and 27 (8%) of 323 matched controls were previously colonized by any antibiotic-resistant Gram-negative bacteria. Prior colonization was strongly associated with antibiotic-resistant Gram-negative bacteremia (odds ratio [OR] 7.2, 95% confidence interval [CI] 3.5–14.7) after controlling for recent treatment with piperacillin-tazobactam (OR 2.5, 95% CI 1.3–4.8). In these patients with suspected bacteremia, prior cultures may predict increased risk of antibiotic-resistant Gram-negative bacteremia.
Keywords: Antimicrobial resistance, Surveillance cultures, Neutropenic fever
1. Introduction
Infections with Gram-negative bacteria resistant to broad-spectrum antibiotics, such as cephalosporin, carbapenems, and piperacillins, are increasingly common in the United States (Cosgrove, 2006; Giske et al., 2008; Mauldin et al., 2010; Siegel et al., 2007; Tumbarello et al., 2009; Wisplinghoff et al., 2004). These infections are associated with higher hospital cost, length of stay, and mortality particularly among cancer patients (Cosgrove, 2006; Giske et al., 2008; Mauldin et al., 2010; Tumbarello et al., 2009). Bacteremia in cancer patients is associated with indwelling devices, e.g., intravenous lines and urinary catheters, and the disruption of mucosal surfaces following chemotherapy (Freifeld et al., 2011; Owens and Berg, 1980; Steffen and Berg, 1983; Tancrede and Andremont, 1985; Wells et al., 1987). Colonization of the skin and mucosal tissue with opportunistic pathogens such as Gram-negative bacteria frequently precedes infection. Surveillance and clinical cultures are used in many U.S. hospitals to identify patients colonized with antibiotic-resistant organisms for the purposes of infection control, but results are not routinely used to guide antimicrobial therapy choices (Hughes et al., 1990; Tacconelli et al., 2008).
Clinical studies in bone marrow transplant patients have shown that vancomycin-resistant Enterococcus colonization is associated with subsequent vancomycin-resistant Enterococcus bacteremia and that Enterococcus stool isolates resistant to trimethoprim-sulfamethoxazole are associated with subsequent trimethoprim-sulfamethoxazole– resistant bacteremia (Salgado, 2008;Weinstock et al., 2007;Wells et al., 1987). Additionally, several studies have identified prior antimicrobial exposure as a risk factor for antibiotic-resistant Gram-negative bacteremia among cancer patients (Garnica et al., 2009; Oliveira et al., 2007; Spanik et al., 1999; Vigil et al., 2009). Identifying risk factors for antibiotic-resistant Gram-negative bacteremia may guide empiric antibiotic therapy and thus improve outcomes among these patients. We hypothesized that prior colonization with antibiotic-resistant Gram-negative bacteria is associated with increased risk of subsequent antibiotic-resistant Gram-negative bacteremia among cancer patients with suspected bacteremia.
2. Methods
2.1. Study population
The study population was derived from cancer patients with a suspected infection who received inpatient care at the University of Maryland Medical Center (UMMC) Greenebaum Cancer Center between January 1, 2001, and December 31, 2010. The UMMC Greenebaum Cancer Center is a National Cancer Institute–designated cancer center in Baltimore, Maryland, with 41,000 outpatient and 1600 inpatient visits annually. Eligible patients had 1) a cancer diagnosis, 2) at least 1 blood culture during the course of their hospitalization, and 3) a non-sterile-site or surveillance culture in the 2–365 days prior to the relevant blood culture. Patient data were obtained from the UMMC Central Data Repository.
2.2. Study design
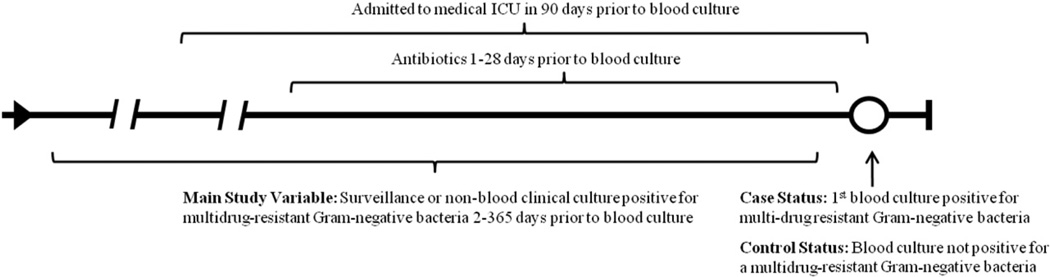
We performed a matched case-control study of cancer patients with suspected bacteremia and prior surveillance or non-sterile-site cultures (see Fig. 1). Cases were selected from patients with a blood culture that grew antibiotic-resistant Gram-negative bacteria for the first time. Controls were selected from patients with 1 or more blood cultures that did not grow antibiotic-resistant Gram-negative bacteria. Case patients could not serve as control patients during other episodes of suspected bacteremia. Antibiotic resistance was defined as full or intermediate resistance to cefepime, imipenem, or piperacillin-tazobactam as determined following the Clinical laboratory Standards Institute guidelines (CLSI, 2011). These antibiotics were selected because of their role as key antimicrobial agents used in the empiric treatment of patients with neutropenic fever (Freifeld et al., 2011). Up to 4 controls were matched to each case on: age ±10 years, type of malignancy, date of admission ±180 days, and duration of hospitalization at the time of the blood culture ±25%. These criteria were chosen to match cases and controls with respect to changes over time in surveillance and clinical culture practice, changes in microbiology laboratory practice, and time at risk for infection in the hospital. Malignancy categories used were acute lymphocytic leukemia (ALL), chronic lymphocytic leukemia (CLL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), other leukemia, Hodgkin’s lymphoma, non-Hodgkin's lymphoma myeloma, solid tumor, or other.
Fig. 1.
Diagram of the relationship of case and control status to the main study variable and other covariates of interest.
2.3. Study variables
The primary exposure variable was colonization with antibiotic-resistant Gram-negative bacteria during the 2–365 days prior to the bacteremia-defining blood culture. Patients were considered colonized if any antibiotic-resistant Gram-negative bacteria were isolated from any surveillance or non-sterile-site (e.g., wound, urine, sputum) culture. Patients were considered not colonized if no antibiotic-resistant Gram-negative bacteria were isolated from any surveillance or non-sterile-site culture.
Covariates included in the analysis were sex, race, admission to the medical intensive care unit (ICU) in the 90 days prior to the defining blood culture (yes versus no), white blood cell (WBC) count ≤1.0 × 103 cells per mm3 within ±1 day of the defining blood culture (yes vs. no), and treatment with antimicrobial agents with broad-spectrum activity against Gram-negative pathogens 1–28 days prior to the defining blood culture (yes versus no). Antimicrobials analyzed included aztreonam, cefepime, doripenem, imipenem, moxifloxacin, and piperacillin-tazobactam. Antimicrobials used in the 24 hours prior to the defining blood culture were excluded, as they may have been started in response to early clinical signs of infection, i.e., after the outcome. WBC count was used as a surrogate for neutropenia because differential counts were not always available.
2.4. Statistical analysis
All statistical analyses were performed using SAS 9.22 (SAS Institute, Cary, NC, USA). All bivariate and multivariable analyses were performed using conditional logistic regression to account for the matched case-control design. The initial multivariable conditional logistic regression was adjusted for variables previously shown to be associated with bacteremia caused by multidrug-resistant organisms and potential confounders. The significance of individual covariates and interaction between covariates was assessed using the likelihood ratio test. All associations are reported as odds ratios (ORs) with 95% confidence intervals (CIs), and a 2-tailed P < 0.05 was considered statistically significant.
This research was conducted in accordance with the Declaration of Helsinki and national and institutional standards. The University of Maryland Baltimore Institution Review Board approved the project with a waiver of informed consent (HP-42197).
3. Results
We identified 86 cases and matched 323 controls. On average, 3.8 controls were matched to each case. Cases and controls did not differ significantly with regard to age, days from admission to culture, year of admission, malignancy type, and sex (Table 1). Controls were more likely to be of white race than cases. There were 13,147 eligible prior cultures. A total of 6277 (48%) were surveillance cultures, and 6870 (52%) were non-sterile site clinical cultures. There were no differences in the distribution of surveillance versus clinical cultures or body site sources among cases and controls (data not shown). The number of cases ranged from 5 to 13 per year, and no significant long-term trends or major outbreaks emerged during the study period. Thirty-two (37%) cases and 27 (8%) controls were found to be colonized with any antibiotic-resistant Gram-negative bacteria in the prior year.
Table 1.
Demographic and hospital stay characteristics of cancer patients with antibiotic resistant Gram-negative bacteremia (cases) and matched controls from the University of Maryland Greenebaum Cancer Center, 2001–2010.
| Variable | Cases | Controls | Pa |
|---|---|---|---|
| N = 86 | N = 323 | ||
| n (%) | n (%) | ||
| Main study variable | |||
| Colonization with antibiotic-resistant Gram-negative in prior year | 32 (37) | 27 (8) | <.001 |
| Matching criteria | |||
| Age (years) | 50 ± 14b | 51 ± 13b | - |
| Malignancy type | - | ||
| ALL | 13 (15) | 40 (12) | |
| CLL | 2 (2) | 2 (1) | |
| AML | 26 (30) | 106 (33) | |
| CML | 0 | 0 | |
| Other leukemia | 0 | 1 (1) | |
| Hodgkin's lymphoma | 0 | 0 | |
| Non-Hodgkin’ s lymphoma | 14 (16) | 59 (18) | |
| Myeloma | 10 (12) | 40 (12) | |
| Solid tumor | 23 (27) | 90 (28) | |
| Duration of hospitalization at the time of blood culture (days) | 10 ± 12b | 10 ± 10b | - |
| Additional covariates | |||
| Male | 56 (65) | 196 (61) | .638 |
| Race | .177 | ||
| White | 40 (47) | 170 (53) | |
| Black | 34 (40) | 129 (40) | |
| Other | 10 (12) | 23 (7) | |
| Missing | 2 (2) | 1 (1) | |
| WBC count ≤1 × 103/mm3 in ±1 day of blood culture | 37 (43) | 122 (38) | .292 |
| Admitted to medical ICU in 90 days prior to blood culture | 12 (14) | 27 (8) | .113 |
| Treatment with piperacillin-tazobactam 1– 28 days prior to blood culture | 36 (42) | 66 (20) | <.001 |
| Treatment with imipenem 1– 28 days prior to blood culture | 13 (15) | 39 (12) | .339 |
| Treatment with cefepime 1– 28 days prior to blood culture | 23 (27) | 78 (24) | .484 |
| Treatment with any antibiotic 1– 28 days prior to blood culture | 56 (65) | 157 (41) | .004 |
| Bone marrow transplant during current admission | 24 (28) | 120 (37) | .098 |
The distributions of the matching criteria were not tested.
Due to rounding, some percentages may not add to 100.
Conditional logistic regression.
Mean ± SD.
In 17 of the 32 cases (53%) previously colonized with antibiotic-resistant Gram-negative bacteria, the bacteria detected from blood culture were the same as the colonizing bacteria, and the bacteria from the blood culture and colonization had the same antimicrobial resistance pattern (e.g., Escherichia coli resistant to imipenem, susceptible to piperacillin-tazobactam and cefepime). Additionally, in 7 of the 32 cases (22%), the bacteria from the blood culture and prior cultures were different bacterial species; however, the 2 bacteria had the same antibiotic resistance (e.g., resistance to imipenem). Thus, overall, the same antibiotic resistance was detected in 75% of the cases with prior colonization with any antibiotic-resistant Gram-negative bacteria. In the remaining 8 of 32 cases (25%), there was no match between the bacteria from the blood culture and the prior cultures.
In bivariate analyses, bacteremia with any antibiotic-resistant Gram-negative bacteria was significantly associated with prior colonization by any antibiotic-resistant Gram-negative bacteria (OR 8.0, 95% CI 4.0–16.1). In a multivariable conditional logistic regression model, antibiotic-resistant Gram-negative bacteremia was independently and strongly associated with prior colonization with any antibiotic-resistant Gram-negative bacteria (OR 7.2, 95% CI 3.5–14.7) and treatment with piperacillin-tazobactam in the 1–28 days prior to drawing the blood culture (OR 2.5, 95% CI 1.3–4.8). If only cases and controls with solid tumors were included in the model, the OR was similar (OR 7.1, 95% CI 1.8–27.6). If only cases and controls with hematologic malignancies were included in the model, the OR was also similar (OR 8.4, 95% CI 3.7–18.9).
Genera represented among the antibiotic-resistant Gram-negative blood culture isolates from cases included Stenotrophomonas (31% of cases), Pseudomonas (16%), Klebsiella (13%), Escherichia (13%), Acinetobacter (6%), Sphingomonas (6%), Enterobacter (5%), Chryseobacterium (3%), Ochrobactrum (3%), Achromobacter (2%), Pantoea (1%), Roseomonas (1%), and Serratia (1%). Among these isolates, 23 (26%) had resistance to cefepime, 15 (21%) had full or intermediate resistance to imipenem, and 44 (51%) had full or intermediate resistance to piperacillin-tazobactam. Of note, if we excluded cases and associated controls with bacteria with intrinsic resistance (e.g., Stenotrophomonas maltophilia), and those for which a CLSI breakpoint interpretation does not exist, the results were similar (OR 9.7, 95% CI 4.1–23.1).
4. Discussion
We found that prior colonization with antibiotic-resistant Gram-negative bacteria was strongly (OR 7.2) associated with subsequent antibiotic-resistant Gram-negative bacteremia among adult cancer patients. This association was independent of recent exposure to antimicrobials. Three quarters of cases with prior colonization with resistant Gram-negative bacteria had the same antibiotic resistance pattern identified in prior cultures as was identified in the infecting isolate. This finding suggests that prior detection of antibiotic-resistant Gram-negative bacteria is a significant risk factor for subsequent antibiotic-resistant Gram-negative bacteremia and that prior cultures may predict antibiotic resistance in bacteremic cancer patients. Review of prior cultures could be used to select empiric antibiotic therapy in cancer patients with suspected infection.
A recent cohort study of pediatric cancer patients reported a finding of similar magnitude in patients with Gram-negative bacteremia. Isolation of antibiotic resistant Gram-negative bacteria from any site within the preceding 12 months was strongly (OR 9.9) associated with the risk of antibiotic-resistant Gram-negative bacteria among pediatric cancer patients with Gram-negative bacteremia (Haeusler et al., 2013). Two prior studies found an association between colonization with Gram-negative bacteria and subsequent Gram-negative bacteremia in cancer patients. Cohen et al. (1983) assessed hematopoietic stem-cell transplant patients colonized with antimicrobial-sensitive Gram-negative bacteria and found a 17 to 174-fold increased risk, depending on species. Wells et al. (1987) reported a 3-fold increase in trimethoprim-sulfamethoxazole– resistant Gram-negative bacteremia among bone marrow transplant patients with prior stool cultures positive for trimethoprim-sulfamethoxazole–resistant Gram-negative bacteria compared to those without positive prior cultures. Our study adds to this literature by showing that this association holds true for cefepime, imipenem, and piperacillin-tazobactam in adult cancer patients with suspected bacteremia.
Our study is subject to the limitations of retrospective data collected from a single institution. We also restricted our study population to those cancer patients with prior surveillance or nonsterile-site cultures in the last year, and this may introduce a selection bias if prior culture testing is associated with colonization and the development of infection.
Our results suggest that a review of prior cultures might reduce inappropriate empiric antibiotic therapy in cancer patients with suspected infection. Studies of critically ill patients including cancer patients have found that inappropriate empiric therapy for Gram-positive or Gram-negative infections is associated with increased mortality and length of stay (Harbarth et al., 2003; Ibrahim et al., 2000; Kang et al., 2005; Kollef et al., 1999; Leibovici et al., 1998; Siegel et al., 2007). Using prior cultures results to guide empiric antibiotic therapy, e.g., provide additional or alternate Gram-negative coverage, may reduce the risk of inappropriate therapy and reduce mortality or length of stay in settings with high rates of antibiotic-resistant Gram-negative bacteremia.
In conclusion, prior colonization with any antibiotic-resistant Gram-negative is associated with an increased risk of subsequent bacteremia with antibiotic-resistant Gram-negative among cancer patients with suspected infection. Future studies should include multiple clinical centers and molecular typing to determine in what proportion of patients the infecting and colonizing isolates are related. If studies indicate that prior antibiotic-resistant Gram-negative colonization predict subsequent antibiotic-resistant Gram-negative bacteremia in cancer patients with suspected infection, surveillance cultures to identify colonization might increase appropriate empiric antibiotic therapy and improve clinical outcomes.
Acknowledgments
We thank Jingkun Zhu for her data abstraction work and Sania Amr for manuscript advice. This work was supported in part by National Institutes of Health grant 1K24AI079040 (ADH) and 1K23AI082450-03 (KAT).
Footnotes
Portions of the data in this manuscript were presented at the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy in September of 2010.
All work described in this manuscript was performed at the University of Maryland School of Medicine, 685 W. Baltimore Street, Baltimore, MD, 21201.
Contributor Information
Aaron S. Hess, Email: ahess@epi.umaryland.edu.
Mary-Claire Roghmann, Email: mroghman@epi.umaryland.edu.
References
- Clinical Laboratory Standard Institute (CLSI) Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement (M100-S21) (ISBN 1-56238-742-1) Wayne, PA: CLSI; 2011. [Google Scholar]
- Cohen ML, Murphy MT, Counts GW, Buckner CD, Clift RA, Meyers JD. Prediction by surveillance cultures of bacteremia among neutropenic patients treated in a protective environment. J Infect Dis. 1983;147:789–793. doi: 10.1093/infdis/147.5.789. [DOI] [PubMed] [Google Scholar]
- Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(Suppl 2):S82–S89. doi: 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56-3. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- Garnica M, Maiolino A, Nucci M. Factors associated with bacteremia due to multidrug-resistant Gram-negative bacilli in hematopoietic stem cell transplant recipients. Braz J Med Biol Res. 2009;42:289–293. doi: 10.1590/s0100-879x2009000300010. [DOI] [PubMed] [Google Scholar]
- Giske CG, Monnet DL, Cars O, Carmeli Y ReAct-Action on Antibiotic Resistance. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother. 2008;52:813–821. doi: 10.1128/AAC.01169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler GM, Mechinaud F, Daley A, Starr M, Shann F, Connell TG, et al. Antibiotic resistant gram-negative bacteremia in pediatric oncology patients – risk factors and outcome. Pediatr Infect Dis J. 2013;32:723–726. doi: 10.1097/INF.0b013e31828aebc8. [DOI] [PubMed] [Google Scholar]
- Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003;115:529–535. doi: 10.1016/j.amjmed.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Hughes WT, Armstrong D, Bodey GP, Feld R, Mandell GL, Meyers JD, et al. From the Infectious Diseases Society of America. Guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. J Infect Dis. 1990;161:381–396. doi: 10.1093/infdis/161.3.381. [DOI] [PubMed] [Google Scholar]
- Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, et al. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49:760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462–474. doi: 10.1378/chest.115.2.462. [DOI] [PubMed] [Google Scholar]
- Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med. 1998;244:379–386. doi: 10.1046/j.1365-2796.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents Chemother. 2010;54:109–115. doi: 10.1128/AAC.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AL, de Souza M, Carvalho-Dias VM, Ruiz MA, Silla L, Tanaka PY, et al. Epidemiology of bacteremia and factors associated with multi-drug-resistant gram-negative bacteremia in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2007;39:775–781. doi: 10.1038/sj.bmt.1705677. [DOI] [PubMed] [Google Scholar]
- Owens WE, Berg RD. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect Immun. 1980;27:461–467. doi: 10.1128/iai.27.2.461-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado CD. The risk of developing a vancomycin-resistant Enterococcus bloodstream infection for colonized patients. Am J Infect Control. 2008;36:S175.e5–S175.e8. doi: 10.1016/j.ajic.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Siegel JD, Rhinehart E, Siegel JD, Rhinehart E, Jackson M, Chiarello L, et al. Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control. 2007;35:S165–S193. doi: 10.1016/j.ajic.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Spanik S, Krupova I, Trupl J, Kunova A, Novotny J, Mateicka F, et al. Bacteremia due to multiresistant gram-negative bacilli in neutropenic cancer patients: a case-controlled study. J Infect Chemother. 1999;5:180–184. doi: 10.1007/s101560050031. [DOI] [PubMed] [Google Scholar]
- Steffen EK, Berg RD. Relationship between cecal population levels of indigenous bacteria and translocation to the mesenteric lymph nodes. Infect Immun. 1983;39:1252–1259. doi: 10.1128/iai.39.3.1252-1259.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconelli E, Cataldo MA, De Pascale G, Manno D, Spanu T, Cambieri A, et al. Prediction models to identify hospitalized patients at risk of being colonized or infected with multidrug-resistant Acinetobacter baumannii calcoaceticus complex. J Antimicrob Chemother. 2008;62:1130–1137. doi: 10.1093/jac/dkn289. [DOI] [PubMed] [Google Scholar]
- Tancrede CH, Andremont AO. Bacterial translocation and gram-negative bacteremia in patients with hematological malignancies. J Infect Dis. 1985;152:99–103. doi: 10.1093/infdis/152.1.99. [DOI] [PubMed] [Google Scholar]
- Tumbarello M, Spanu T, Caira M, Trecarichi EM, Laurenti L, Montuori E, et al. Factors associated with mortality in bacteremic patients with hematologic malignancies. Diagn Microbiol Infect Dis. 2009;64:320–326. doi: 10.1016/j.diagmicrobio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Vigil KJ, Adachi JA, Aboufaycal H, Hachem RY, Reitzel RA, Jiang Y, et al. Multidrug-resistant Escherichia coli bacteremia in cancer patients. Am J Infect Control. 2009;37:741–745. doi: 10.1016/j.ajic.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Weinstock DM, Conlon M, Iovino C, Aubrey T, Gudiol C, Riedel E, et al. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant Enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2007;13:615–621. doi: 10.1016/j.bbmt.2007.01.078. [DOI] [PubMed] [Google Scholar]
- Wells CL, Ferrieri P, Weisdorf DJ, Rhame FS. The importance of surveillance stool cultures during periods of severe neutropenia. Infect Control. 1987;8:317–319. doi: 10.1017/s0195941700066406. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]