Abstract
Introduction
Hypothalamic-pituitary-adrenal (HPA) deregulation is commonly observed in cancer patients, but its clinical significance is not well understood. We prospectively examined the association between HPA activity, tumor-associated inflammation, and survival in ovarian cancer patients prior to treatment.
Materials and Methods
Participants were 113 women with ovarian cancer who provided salivary cortisol for three days prior to treatment for calculation of cortisol slope, variability, and night cortisol. Cox proportional hazard regression analyses were used to examine associations between cortisol and survival in models adjusting for disease stage, tumor grade, cytoreduction and age. On a subsample of 41 patients with advanced disease ascites fluid was assayed for levels of interleukin-6 (IL-6) and correlated with cortisol variables.
Results
Each cortisol measure was associated with decreased survival time, adjusting for covariates (all p<.041). A one standard deviation increase in night cortisol was associated with a 46% greater likelihood of death. Patients in the high night cortisol group survived an estimated average of 3.3 years compared to 7.3 years for those in the low night cortisol group. Elevated ascites IL-6 was associated with each cortisol measure (all r >.36, all p<.017).
Discussion
Abnormal cortisol rhythms assessed prior to treatment are associated with decreased survival in ovarian cancer and increased inflammation in the vicinity of the tumor. HPA abnormalities may reflect poor endogenous control of inflammation, dysregulation caused by tumor-associated inflammation, broad circadian disruption, or some combination of these factors. Nocturnal cortisol may have utility as a non-invasive measure of HPA function and/or disease severity.
Keywords: Ovarian Neoplasms, Inflammation, Hydrocortisone, Chronobiology Disorders, Biological Markers
1. INTRODUCTION
Ovarian cancer is the second most commonly diagnosed gynecologic malignancy and the most deadly, with five year survival rates of 44% for all patients, and 27% for patients with metastatic disease (American Cancer Society, 2014). Recent research has demonstrated effects of neuroendocrine signaling on a variety of pathways implicated in ovarian tumor growth including angiogenesis, invasion, anoikis, and promotion of inflammation in the tumor microenvironment (Cole & Sood, 2012). To date, mechanisms that have been characterized predominantly involve beta-adrenergic signaling. Neuroendocrine signals from the hypothalamic-pituitary-adrenal (HPA) axis regulate endogenous inflammation and metabolic activity, via the release of glucocorticoids (Tsigos & Chrousos, 2002). Although glucocorticoids are known to play a regulatory role in other cancers, little is known about the role of the HPA axis in the context of ovarian cancer.
Cortisol, a glucocorticoid, is released into circulation by the adrenal cortex following upstream signaling from the pituitary and hypothalamus (Rhen & Cidlowski, 2005). Cortisol follows a diurnal cycle; levels typically peak in the morning and reach a nadir during the second half of the night, though significant inter-individual differences are common even in apparently healthy people (Chrousos, 1995; Stone et al., 2001; Clow et al., 2010). Excess circulating cortisol provides negative feedback to the hippocampus and hypothalamus, however, under conditions of chronic inflammation the feedback system can become unresponsive, resulting in abnormal diurnal cortisol rhythms (Silverman & Sternberg, 2012).
Glucocorticoids have a variety of effects in cancer; they have been shown to inhibit apoptosis in breast, cervical and ovarian cancer cell lines and in animal models of breast cancer (Volden & Conzen, 2013). Incubation of ovarian cancer cells with cortisol has been shown to reduce expression of tumor-suppressor genes SLIT2 and ROBO1 (Dickinson et al., 2011), as well as to suppress the cytotoxic effects of paclitaxel, a drug commonly used in ovarian cancer chemotherapy (Flint et al., 2009). Moreover, flattened diurnal cortisol rhythms have been linked to decreased survival in breast, lung, and renal cell carcinoma patients, adjusting for clinical and demographic characteristics (Sephton et al., 2000; Cohen et al., 2012; Sephton et al., 2013). For example, in a recent study of renal cell carcinoma patients, flatter cortisol slope was associated with an approximately two-fold greater likelihood of death (HR=1.9, 95% CI 1.3, 3.0) adjusting for a variety of disease characteristics (Cohen et al., 2012).
We have previously observed an altered cortisol diurnal rhythm in ovarian cancer patients prior to surgery compared to that of pre-surgical patients with benign disease and healthy controls not facing surgery. Ovarian cancer patients show elevated afternoon and nocturnal cortisol, resulting in blunted diurnal cortisol variability (Weinrib et al., 2010). These patterns of dysregulation have been associated with elevated measures of systemic inflammation in ovarian cancer patients (Lutgendorf et al., 2008; Schrepf et al., 2013). This inflammation is hypothesized to be tumor-derived (Edgell et al., 2010). Furthermore, inflammation in the tumor microenvironment is strongly linked to ovarian cancer progression (Kulbe et al. 2012). Animal models have demonstrated that tumor growth can activate the HPA axis and induce systemic inflammatory signaling (Lamkin et al., 2011; Yang et al., 2014). However, HPA activity has not been investigated in the context of survival in ovarian cancer. Moreover, the relationship between HPA activity and inflammation measured in the vicinity of the tumor has not been previously characterized in the clinical setting of ovarian cancer.
2. MATERIALS AND METHODS
2.1 Participants
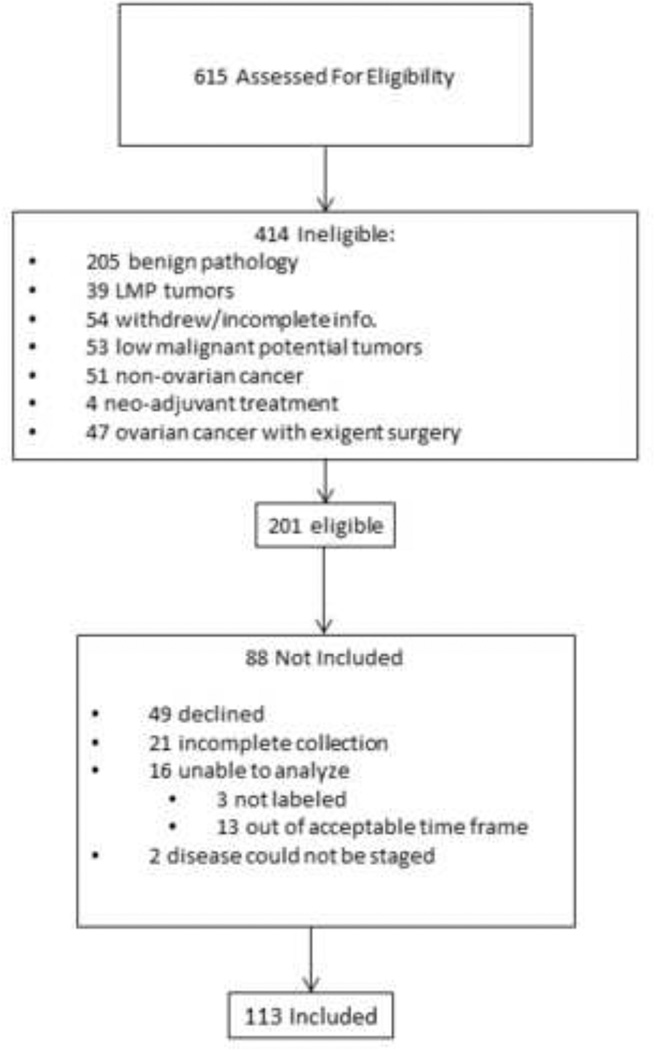
Participants were 113 women at least 18 years of age, with no auto-immune disease or systemic steroid use (i.e. prednisone) and no previous history of cancer. Participants were eligible if histology confirmed primary invasive epithelial ovarian, primary peritoneal, or fallopian tube carcinoma. Patients were excluded if diagnosis was a primary cancer of another organ, an ovarian tumor of non-epithelial origin, a tumor of low malignant potential, or benign disease. Included patients had surgery at least one year prior to the date of censoring. (See Figure 1 for Consort diagram showing included patients). All procedures were approved by the Institutional Review Boards of the University of Iowa, the University of Miami and Mercy Medical Center in Miami, FL. The earliest date of surgery was in January of 2004 and all survival information was censored on June 15th, 2013, or on the last date of the last recorded follow-up.
Figure 1.
Flow chart of patient recruitment.
2.2 Procedure
Participants were recruited sequentially from participating institutions during pre-surgical visits for pelvic/abdominal masses suspected for ovarian cancer. Participants completed demographic and psychosocial questionnaires and collected salivary cortisol three times daily for three days prior to surgery. Disease was staged as I-IV, grade as low or high, and histology as serous or non-serous tumor. Advanced stage disease (i.e. III-IV) refers to disease that has spread from the ovaries/fallopian tubes to the lining of the abdomen, adjacent organs, lymph nodes, or more distant sites. High grade tumor refers to tumor that is poorly differentiated and no longer resembles normal ovarian cells. Both advanced stage disease and high grade tumor are associated with poorer survival (American Cancer Society, 2014). Tumors of serous histology are thought to primarily arise from the fallopian tubes and are the most common and one of the most aggressive forms of epithelial ovarian cancer (Kurman, 2013). Extent of cytoreduction was classified as optimal if no visible tumor remained following surgery or if the largest remaining nodule was less than 1 cm, or suboptimal if greater than 1 cm of tumor remained. Completeness of cytoreduction has been associated with increased survival in epithelial ovarian cancer (Bristow et al., 2002). All samples were examined by a gynecologic pathologist. Most patients went on to receive at least six cycles of platinum and taxane combination chemotherapy. Demographic information was extracted from participant responses and included marital status (single, divorced, widowed, separated; or married/living with partner), and level of education attained. Sleep was assessed by the Pittsburgh Sleep Quality Inventory (PSQI; Buysse et al., 1989). Symptoms of depression were assessed by the Center for Epidemiologic Studies – Depression (CES-D) scale (Radloff, 1977). Disease symptoms, such as fatigue, nausea, and loss of appetite were measured using the Physical Well-Being (PWB) subscale of the Functional Assessment of Cancer Therapy (FACT) scale (Cella et al., 1993). Tobacco use was categorized as never a tobacco user or current/former tobacco user. Alcoholic beverages were coded as average weekly consumption. Clinical disease characteristics, histological information, and date/cause of death were extracted from medical records or state/national death indices where needed. Survival time was calculated as the number of days between date of tumor resection and date of death, censoring, or last recorded follow-up.
2.3 Cortisol Collection
Salivary cortisol was collected by participants in provided salivettes three times daily for three days immediately prior to surgery. Participants were instructed to collect the first sample immediately upon waking, the second sample at 5pm (allowing at least thirty minutes after a meal), and the final sample at bedtime. Participants were instructed not to eat, consume caffeine, or exercise for thirty minutes prior to sample collection, and to place salivettes in the refrigerator at the end of the day. Participants wrote the time of collection on tubes and returned them prior to surgery. Self-report of collection has previously been shown to be a reliable method of assessment (Kraemer et al., 2006). Salivary cortisol is stable at room temperature and correlates well with serum cortisol levels (Kirschbaum & Hellhammer, 1994). Samples were assayed by chemiluminescence immunoassay (IBL, Hamburg, Germany) at the Technical University of Dresden. The lower detection limit is 0.41 nmol/L, and inter-assay and intra-assay coefficients of variance are <10%.
2.4 Cortisol Analyses
All cortisol values were transformed by a natural logarithm to normalize their distribution. Cortisol slope, a measure of the hourly decrement in salivary cortisol concentrations over the course of the day, was calculated by regressing each of the 9 individual cortisol values on their time of collection. Cortisol variability was calculated as the magnitude of decrease from the earliest collection time point (approximating peak concentrations) to the night time point (closest to the nadir) or: ((Morning-Night)/Morning)*100, as described previously (Jehn et al., 2006). Night cortisol was calculated as a simple average of the night cortisol values for each patient. Greater cortisol variability and steeper (more negative) cortisol slopes are considered to be more normal cortisol profiles. Following recommended best practices for cortisol analyses, only values represented by at least two samples per time point (e.g. two morning, two afternoon, two night for calculation of slope) were included in the analyses, as a single day of collection is not considered reliable for salivary cortisol (Kraemer et al., 2006). The most frequently cited reason for incomplete collection was that patients were too preoccupied in preparation for surgery. Twenty one participants were excluded from cortisol analyses because of incomplete collection. However, as a conservative measure, models were also tested using all available cortisol data from patients who had incomplete collection. Samples collected outside an acceptable time frame were excluded, for purpose of homogeneity (waking sample: 0400h-0900h-, afternoon sample: 1600h–1830h, night time sample:2000h–2400h). Three participants did not label their salivettes properly and were excluded, and 13 collected too many samples outside the acceptable time frames, resulting in too few collections to calculate cortisol values, and were excluded as well.
2.5 Cytokines
Ascites fluid was obtained during surgery from patients with advanced stage disease and high grade tumor during surgery (n=41). Plasma was drawn from all eligible patients willing and able to provide blood the morning of surgery (n=93). Blood and ascites were centrifuged at 1,126× g at 4°C for 15 minutes and frozen at −80°C until testing. Interleukin-6 (IL-6) was assayed in duplicate in ascites and plasma by ELISA (R & Diagnostics, Minneapolis, MN) with results interpolated from the standard curve provided with the kits. The minimum detectable level is less than 0.7 pg/mL and inter-assay variability ranges from 3.3% to 6.4%. IL-6 levels in ascites are correlated with tumor levels and are thought to represent the amount produced by tumor (Burger et al., 1995). IL-6 values were not correlated with sampling time (r=0.12, p=.48).
2.6 Statistical Analyses
Analyses were performed with SPSS, version 21.0 (Armonk, NY). Cytokine values were log10 transformed to correct for non-normal distributions. To determine if cortisol values varied according to clinical and demographic characteristics, and to inform covariate selection, oneway Analysis of Variance (ANOVA)s and Pearson correlations were used. We also conducted multivariate General Linear Model (GLM) analyses with significant disease and demographic characteristics to determine which if any were most strongly associated with cortisol variables. GLMs allow for both continuous and categorical covariates to be tested simultaneously. ANOVA and Chi-square tests conducted on these variables to compare disease characteristics between participants included in the analysis and those who declined to participate, stopped collecting, or had data that could not be analyzed, to ensure representativeness. Stage was dichotomized into early (I-II) and late (III-IV) disease. Cox proportional hazard regression analyses were conducted to determine the univariate association of each clinical disease/treatment characteristic (stage, grade, optimal/suboptimal cytoreduction, serous/non-serous tumor, presence of ascites fluid, # of cycles of chemotherapy initially received) and potential confounding demographic/psychosocial characteristics (BMI category, tobacco use, weekly alcohol consumption, age, sleep quality, physical well-being, and depression score) with survival time. Variables that were significant or approaching significance (p <.10) were retained in multivariate analyses that included the cortisol variables of interest. Median splits on cortisol variables were used in Kaplan-Meier curves for illustrative purposes. All models were evaluated for fit and adequacy (Singer & Willett, 2003). On a subsample of 41 patients with advanced stage-disease and high-grade tumors (characteristics typical of a majority of our sample and most ovarian cancer patients) who had available ascites fluid, we conducted multiple regression controlling for age and BMI to test the association between IL-6 in ascites and the cortisol variables for the purpose of determining if dysregulated cortisol patterns were associated with inflammation in the vicinity of the tumor. Similarly, the association between circulating IL-6 and cortisol values was tested by General Linear Models controlling for age, disease stage (early vs. late), and tumor grade (low vs. high-grade tumor). The level of significance for all analyses was p < .05 and all relevant tests were two-sided.
3. RESULTS
3.1 Sample Characteristics
Patients were approximately 58 years old (on average) at the time of study entry, married/living with a partner, and college educated. Demographic and clinical information is presented in Table 1. Patients who participated in the study did not differ significantly from those who declined to supply cortisol (n=49) on demographics (all p<.19) or disease characteristics including disease stage (p = .60), tumor grade (p = .84), cytoreduction (p = .50) tumor histology (p = .10), initial cycles of chemotherapy (p = .61), or survival (p = .66). When comparing all patients eligible but not included in the study (patients with exigent surgery, patients that declined to collect cortisol and patients with incomplete or unusable cortisol collection, n =135) there were no differences on any demographic variable (all p>.12) except ethnicity, as a slightly higher proportion of patients not included were Hispanic (10% for patients not included vs. 3% of included patients, p = .022). Similarly there were no differences between included and not included patients on disease stage (p = .68), tumor grade (p = .42), cytoreduction (p = .92), initial cycles of chemotherapy (p = .99), or survival (p = .93). A slightly higher proportion of patients not included had serous vs. non-serous disease (81% of not included patients vs. 67% of included patients, p =.012). Of the 113 ovarian cancer patients who participated, 53 died prior to the end of the study and 60 were censored on June 15th, 2013 or on the date of their most recent clinic visit if lost to follow-up. Cause of death for the 53 patients who had died was persistent or recurrent ovarian cancer or complications associated with cancer disease and treatment. Median follow-up time was 2 years, 11 months (range: 1 day - 9 years, 4 months).
Table 1.
Demographic characteristics.
| Participants N=113 | Mean, Median (S.D.) |
|---|---|
| Age | 57.99, 59, (11.73) |
| CES-Depression Score | 14.01,13, (8.76) |
| Pittsburgh Sleep Quality Inventory Score | 7.18, 7, (3.56) |
| Physical Well Being Score | 21.26, 22, (5.48) |
| Frequency (%) | |
| Education | |
| High school or less | 35(31) |
| Some college/trade school | 38(34) |
| College graduate | 30(27) |
| Advanced degree | 9(8) |
| Not answered | 1(1) |
| Race | |
| American Indian/Alaska Native | 1(1) |
| Asian | 1(1) |
| Black/African American | 2(2) |
| White | 108(96) |
| Not answered | 1(1) |
| Ethnicity | |
| Hispanic | 3(3) |
| Non-Hispanic | 109(97) |
| Not answered | 1(1) |
| Relationship Status | |
| Single | 12(11) |
| Divorced | 12(11) |
| Married | 72(64) |
| Widowed | 14(12) |
| Living with partner | 3(3) |
| Body Mass Index category | |
| <20 underweight | 2(2) |
| 20–25 normal | 29(26) |
| 25–30 overweight | 44(39) |
| 30–40 obese | 32(28) |
| >40 morbidly obese | 6(5) |
| Disease Stage | |
| Early | 32(28) |
| Late | 81(72) |
| Tumor Grade | |
| Low | 13(12) |
| High | 100(89) |
| Cytoreduction | |
| Optimal | 82(73) |
| Suboptimal | 31(27) |
| Histology | |
| Serous | 76(67) |
| Non-serous | 37(33) |
CES= Center for Epidemiologic Studies
3.2 Demographics, Disease Characteristics, and Cortisol
Among the 113 patients for whom complete data was available, advanced age was associated with higher night cortisol (p=.004) but not variability (p=.14) or slope (p=.10). Late stage disease was associated with higher night cortisol (p=.005) and marginally associated with flattened slope (p=.07) and reduced variability (p=.09). High grade disease was associated with higher night cortisol, flattened slope and reduced variability (all p < .046) but cortisol variables were not associated with histology (all p >.33; see Table 2). In multivariate GLMs including stage, grade, and serous/non-serous tumors only the association between high tumor grade and elevated night cortisol remained significant (p=.013). Moreover, there were no significant associations between night cortisol, cortisol variability, or cortisol slope and demographic characteristics, BMI category, race, ethnicity, relationship status, education level, smoking status, alcohol consumption or sleep quality, or depression score (all p values >.11). Poorer physical well-being was associated with elevated night cortisol (p = .022), flattened cortisol slope (p = .042), and reduced cortisol variability (p = .005), similar to our previous investigation of somatic symptoms and salivary cortisol in ovarian cancer patients prior to treatment (Weinrib et al., 2010). Night cortisol was highly correlated with cortisol variability (r = −.727, p < .001) and slope (r = .758, p < .001) , suggesting that night cortisol may be a reliable proxy for cortisol assessments that require more complex collection procedures. Diurnal slope and cortisol variability were also strongly associated (r = .880, p < .001).
Table 2.
Cortisol variables and disease characteristics.
| Disease Characteristics |
Night Cortisol nmol/L (ln) mean, (SD) |
p | Cortisol Slope |
p | Cortisol Variability % |
p |
|---|---|---|---|---|---|---|
| Disease Stage | ||||||
| Early (n=32) | 1.110(.703) | .005 | −.117(.046) | .07 | 75.2(19.9) | .09 |
| Advanced (n=81) | 1.566(.784) | −.099(.049) | 67.5(21.7) | |||
| Tumor Grade | ||||||
| Low (n=13) | .711(.761) | <.001 | −.129 (.041) | .045 | 81.2(13.1) | .038 |
| High (n=100) | 1.534(.736) | −.100(.049) | 68.2(21.9) | |||
| Histology | ||||||
| Serous (n=76) | 1.466(.761) | .62 | −.100(.047) | .33 | 69.5(20.4) | .91 |
| Non-serous (n=37) | 1.388(.829) | −.110(.053) | 70.0(23.7) | |||
3.3 Cortisol and survival
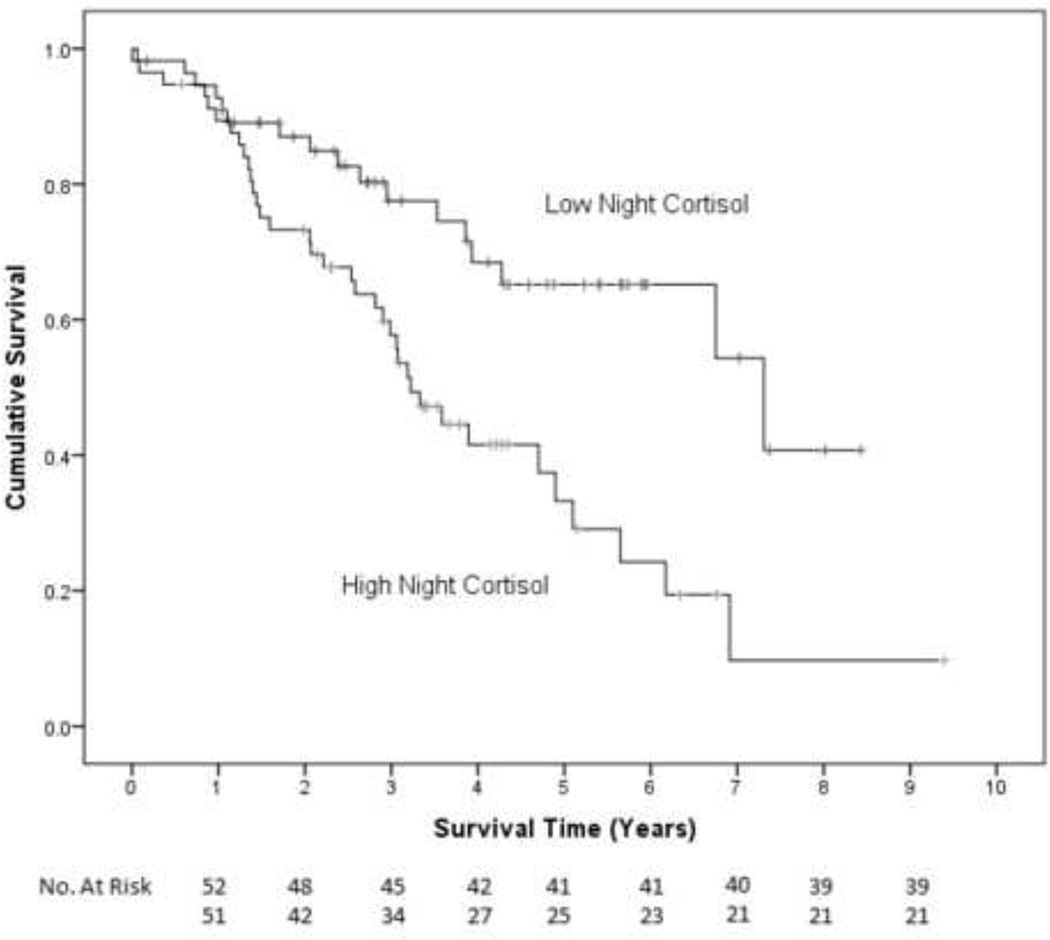
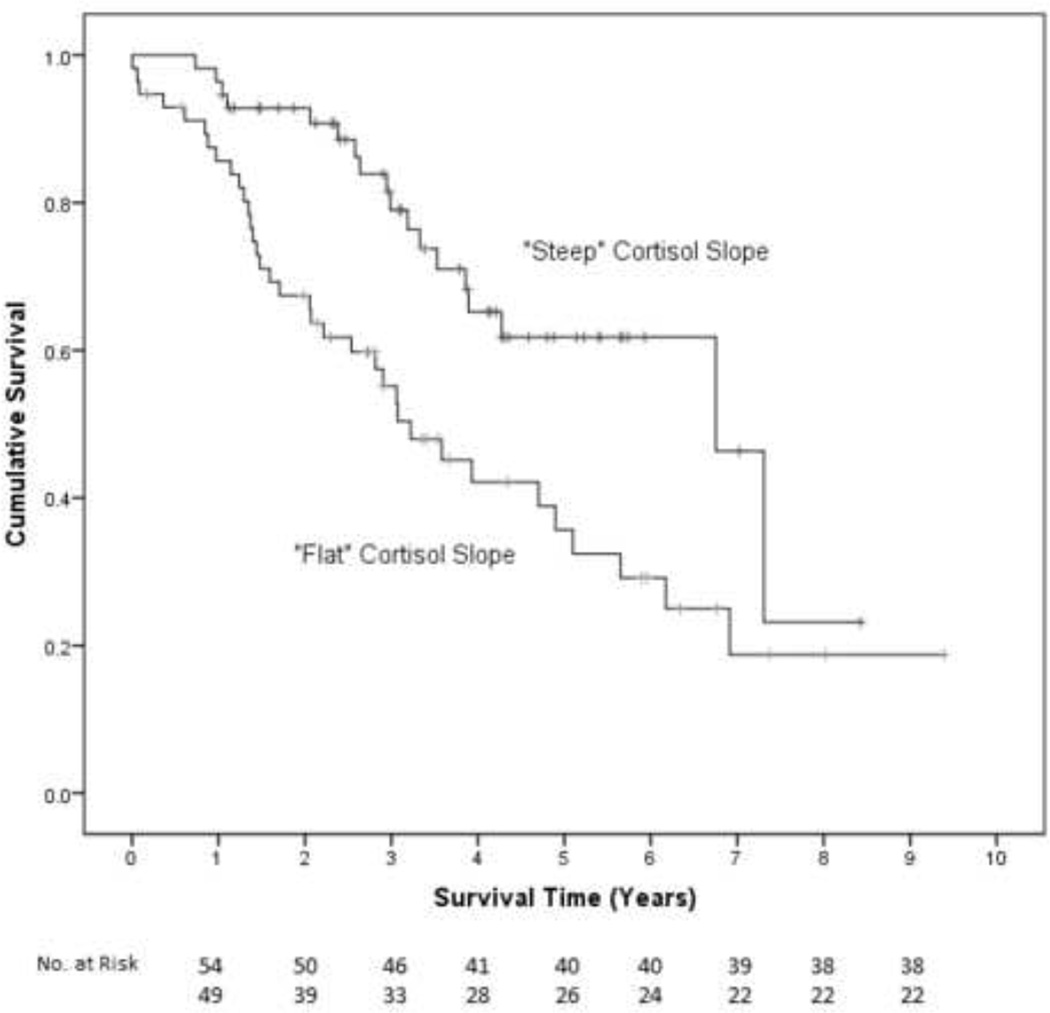
In univariate Cox proportional hazard regression analyses, advanced stage disease (p=.003), high grade tumor (p=.073), suboptimal cytoreduction (p=.037), and older age (p=0.001) were associated with shortened survival time, while serous histology and number of cycles of chemotherapy were not (all p>.13). BMI category, sleep quality, CES-D depression score, physical well-being, lifetime tobacco use and weekly alcoholic beverages were not significant predictors of survival (all p>.19) and thus were not retained in the final models. See Table 3 for univariate analyses. In the first multivariate Cox model controlling for disease stage, tumor grade, cytoreduction, and age, elevated night cortisol levels prior to surgery were independently associated with shorter survival time (β=.375, H.R.=1.455, 95% CI=1.059, 1.998, p=.021). Thus, a patient whose nocturnal cortisol level was 2.22 (ln) nmol/L (one standard deviation above the mean) would have a 46% greater risk of death compared to a patient whose nocturnal cortisol was 1.44 nmol/L (the mean for in this sample). Kaplan-Meier curves based on a median split of night cortisol are shown for illustrative purposes (see Figure 2). These plots indicate that patients in the low night cortisol group survived an estimated median 7.3 years (95% CI =3.8, 10.8 years) compared to 3.3 years (95% CI=2.6, 3.8 years) for those in the high night cortisol group. Results for multivariate Cox regression models for cortisol variability and cortisol slope were very similar (cortisol variability: β= −.314, HR=.731, 95% CI=.570, .937, p=.013; cortisol slope: β=.325, HR=1.384, 95% CI=1.015, 1.886, p=.040). For example, a patient whose cortisol declined 91% (one standard deviation above the mean) throughout the day would have 29% lower risk of death compared to a patient whose salivary cortisol declined 69% throughout the day (the mean decline in this sample). See Figure 3. See Table 4 for all parameters in each model.1
Table 3.
Hazard ratios for survival for selected risk factors in univariate Cox regression analyses.
| Risk Factor | B. | S.E. | Hazard Ratio |
95% CI | p |
|---|---|---|---|---|---|
| Late Stage | 1.305 | .435 | 3.689 | 1.574, 8.648 | .003 |
| High Grade Tumor | 1.297 | .723 | 3.659 | .888, 15.082 | .073 |
| Suboptimal Cytoreduction | .301 | .144 | 1.351 | 1.018, 1.791 | .037 |
| Serous Histology | .131 | .162 | 1.139 | .830, 1.564 | .419 |
| # Chemotherapy Cycles | .028 | .059 | 1.029 | .917,1.154 | .631 |
| Night Cortisola | .589 | .149 | 1.802 | 1.347, 2.412 | <.001 |
| Diurnal Cortisol Slopeb | .490 | .151 | 1.633 | 1.214, 2.195 | .001 |
| Cortisol Variabilityc | −.440 | .116 | .644 | .513, .809 | <.001 |
| Body Mass Index Category (reference category=normal) |
.893 | ||||
| Underweight | −.333 | .821 | .717 | .144, 3.581 | |
| Overweight | .180 | .307 | 1.197 | .656, 2.186 | |
| Obese | −.114 | .351 | .892 | .448, 1.777 | |
| Morbidly obese | .049 | .609 | 1.051 | .318, 3.467 | |
| Age (one year change) | .043 | .013 | 1.044 | 11.018, 1.070 | .001 |
| Pittsburgh Sleep Quality Index score (one unit change) |
.010 | .042 | 1.016 | .939, 1.100 | .689 |
| CES-Depression score (one unit change) |
.020 | .015 | 1.020 | .990,1.051 | .196 |
| Physical Well Being score (one unit change) |
−.026 | .023 | .974 | .930, 1.020 | .262 |
| Former/Current Tobacco Use | .013 | .147 | 1.013 | .759, 1.353 | .928 |
| Weekly Alcoholic Beverages (one unit change) |
.316 | .305 | 1.372 | .754, 2.496 | .301 |
CES= Center for Epidemiologic Studies
Greater hazard reflects 1 SD increase night cortisol (.787 nmol/L, (ln))
Greater hazard reflects 1 SD smaller hourly decline in salivary cortisol (.049 nmol/L, (ln)).
Smaller hazard reflects 1 SD greater decline in salivary cortisol over course of the day (21.4%)
Figure 2.
Kaplan Meier survival curve for patients with high nocturnal cortisol (median 3.3 years, 95% CI=2.6, 3.8 years) vs. patients with low nocturnal cortisol (7.3 years, 95% CI =3.8, 10.8 years). Cox regression adjusted for covariates indicates that patients with lower nocturnal cortisol had longer survival times (p=.021).
Figure 3.
Kaplan Meier survival curve for patients with flat diurnal cortisol slope (median 3.2 years, 95% CI=2.1, 4.3 years) vs. patients with steep diurnal cortisol slope (6.8 years, 95% CI =4.5, 9.0 years). Cox regression adjusted for covariates indicates that patients with steeper cortisol slopes had longer survival times (p=.040).
Table 4.
Parameters for Cox proportional-hazard regression models for overall survival in patients with ovarian cancer, (regression coefficients, standard errors, p values, Hazard Ratios, and 95% confidence intervals for Hazard Ratios).
| B | S.E. |
P- Value |
Hazard Ratio |
95% CI | |
|---|---|---|---|---|---|
| Stage (early vs advanced) | −0.338 | 0.241 | 0.161 | 0.713 | 0.445–1.143 |
| Grade (low vs high) | −0.192 | 0.378 | 0.612 | 0.825 | 0.393–1.732 |
| Cytoreduction , (optimal vs suboptimal) | 0.105 | 0.157 | 0.506 | 1.110 | 0.816–1.512 |
| Age | 0.026 | 0.014 | 0.055 | 1.027 | 0.999–1.055 |
| Nocturnal Cortisola | 0.375 | 0.162 | 0.021 | 1.455 | 1.059–1.998 |
| B | S.E. |
P- Value |
Hazard Ratio |
95% CI | |
| Stage (early vs advanced | −0.341 | 0.241 | 0.158 | 0.711 | 0.443–1.141 |
| Grade (low vs high) | −0.241 | 0.381 | 0.527 | 0.786 | 0.373–1.657 |
| Cytoreduction , (optimal vs suboptimal) | 0.119 | 0.157 | 0.451 | 1.126 | 0.827–1.533 |
| Age | 0.030 | 0.013 | 0.022 | 1.031 | 1.004–1.058 |
| Cortisol Slopeb | 0.325 | 0.158 | 0.040 | 1.384 | 1.015–1.886 |
| B | S.E. |
P- Value |
Hazard Ratio |
95% CI | |
| Stage (early vs advanced) | −0.350 | 0.238 | 0.140 | 0.704 | 0.442–1.122 |
| Grade (low vs high) | −0.260 | 0.377 | 0.491 | 0.771 | 0.368–1.615 |
| Cytoreduction, (optimal vs suboptimal) | 0.110 | 0.158 | 0.485 | 1.116 | 0.820–1.520 |
| Age | 0.030 | 0.014 | 0.025 | 1.031 | 1.004–1.059 |
| Cortisol Variabilityc | −0.314 | 0.127 | 0.013 | 0.731 | 0.570–0.937 |
Greater hazard reflects 1 SD increase night cortisol (.787 nmol/L, (ln))
Greater hazard reflects 1 SD smaller hourly decline in salivary cortisol (.049 nmol/L, (ln)).
Smaller hazard reflects 1 SD greater decline in salivary cortisol over course of the day (21.4%)
3.4 Cortisol, Tumor-Associated Inflammation, and Circulating Levels Inflammation
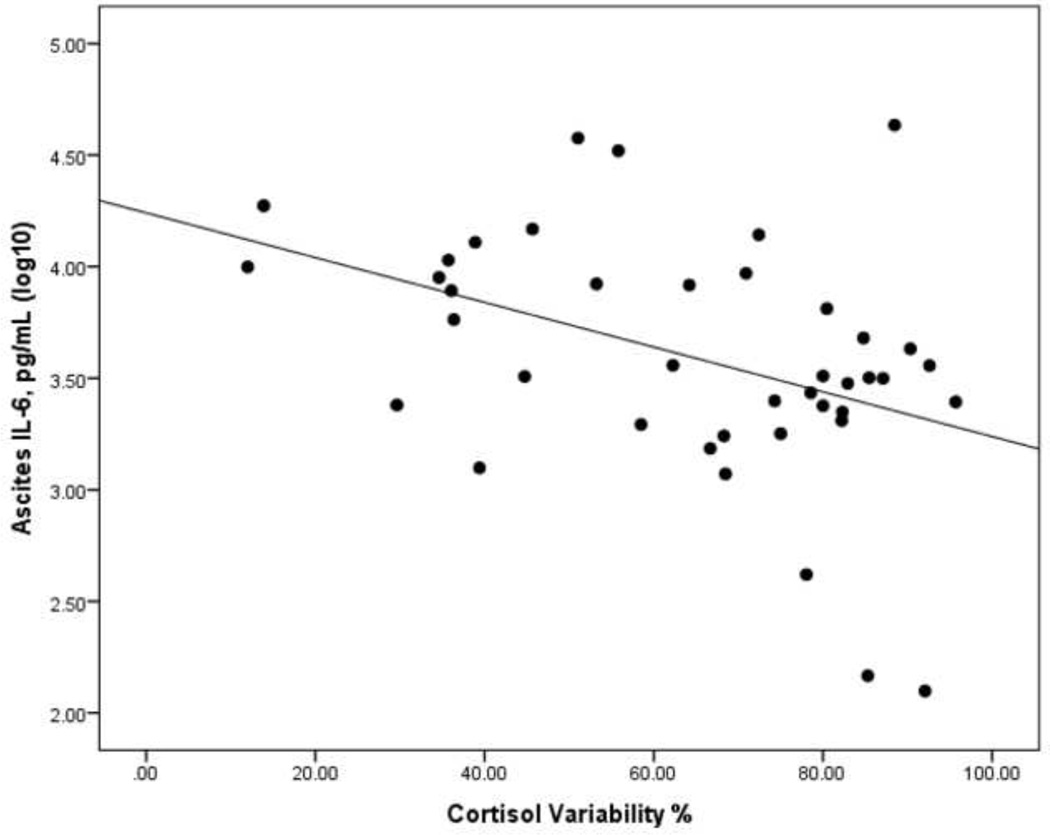
Elevated night cortisol (β=.38, p=.029), flattened cortisol slope (β=43, p=.013) and reduced cortisol variability (β=−.43, p=.013) were each associated with higher levels of inflammation as indicated by higher levels of ascites IL-6. See Figure 4. Similarly, elevated night cortisol (β=.30, p< .001), flattened cortisol slope (β=.29, p < .001) and reduced cortisol variability (β= − .30, p < .001) were each associated with higher levels of circulating inflammation indicated by higher levels of plasma IL-6.
Figure 4.
Scatterplot of Interleukin-6 assayed in ascites fluid and diurnal cortisol variability.
4. DISCUSSION
The key finding of this study is that dysregulated function of the HPA axis, evidenced by reduced diurnal cortisol variability, flattened diurnal cortisol slope, and elevated night cortisol, is associated with decreased survival time in ovarian cancer patients, controlling for relevant clinical and demographic covariates. This is the first study demonstrating that HPA function at the time of surgery is associated with significant differences in survival for ovarian cancer. It should be noted that cortisol levels were assessed prior to any treatment, indicating that HPA differences were not due to inter-individual responses to treatment or attendant aspects of treatment such as the use of glucocorticoids during chemotherapy. The relationship between cortisol dysregulation and overall survival does not appear to be mediated by sleep dysregulation, symptoms of disease, or depression, as none of these factors were associated with overall survival. These findings are consistent with prior research linking altered cortisol slope to shorter survival in breast, lung and renal cell cancer patients (Sephton et al., 2000; Cohen et al., 2012; Sephton et al., 2013). They are also consistent with previous experimental data demonstrating that invasive carcinomas can up-regulate circulating glucocorticoid levels in animal models of human cancer as well as circulating levels of IL-6 (Lamkin et al., 2011; Yang et al., 2014). As each of the clinical studies adjusted for clinical factors including disease severity, these findings suggest that HPA function may play a role in disease progression and cancer survival or, alternately, may be a marker for disease-related processes, such as those related to inflammation or broader circadian disruption.
Analysis of IL-6 in ascites of a subsample of participants demonstrated that blunted diurnal cortisol variability, flattened slope, and elevated night cortisol were each associated with higher levels of this pro-inflammatory cytokine in the vicinity of the tumor, suggesting greater HPA dysregulation accompanies higher levels of tumor-associated inflammation. Ovarian cancer cells secrete pro-inflammatory cytokines, such as IL-6, IL-1β, and TNF-α, that promote invasion of, and attachment to, surrounding tissue, as well as angiogenesis and the activation of tumor associated macrophages (TAMs) to a tumorigenic phenotype, all of which are related to poorer survival (Offner et al., 1995; Obata et al., 1997; Jeannin, Duluc, & Delneste, 2011;;; Schauer et al. 2013). Higher levels of IL-6 in ascites have recently been associated with cell surface expression of CD163, a marker of TAMs, and shortened time to recurrence (Lane et al.,, 2011; Reinartz et al., 2014). Additionally, blunted variability and diurnal slope, as well as elevated night cortisol were each associated with higher IL-6 in plasma, confirming the results of earlier studies in our lab (Lutgendorf et al., 2008). It is notable that in both ascites and plasma, higher IL-6 was associated with elevated night time cortisol during the prior three days, the opposite effect that would be expected if cortisol was acting to suppress inflammation.
The relationship between tumor-associated inflammation and HPA function may be bidirectional, as sustained inflammatory signaling is believed capable of inducing alterations in HPA activity, and altered HPA activity can lead to reduced inflammatory control (Silverman & Sternberg, 2012). Each level of the HPA axis contains cytokine receptors and pro-inflammatory cytokines are capable of crossing the blood brain barrier and signaling the brain via the vagus nerve (Dantzer & Kelley, 2007). In vitro studies have demonstrated that pro-inflammatory cytokines exert a profound influence on glucocorticoid receptors (GRs) and their function, including downregulated GR-mediated gene transcription, reduced GR binding to Glucocorticoid Response Elements, and reduced GR translocation (Pace & Miller, 2009). Therefore, it is possible that dysregulated HPA rhythms indicate impairment of inflammatory control in the tumor microenvironment and associated risk factors such as polarization of macrophages to a tumorigenic phenotype, increased tumor vascularization, and enhanced invasive potential. However, it is not possible to determine in this observational study whether HPA dysregulation is secondary to tumor biology, or if HPA dysregulation contributes to the conditions favoring the development of aggressive tumor or both. Both pathways are biologically plausible.
Most ovarian cancer patients respond favorably to front-line treatment and achieve a period of disease remission, however, the development of drug resistant disease in subsequent recurrence is a major factor in survival (Sood & Buller, 1998). Interestingly, a recent study found that breast cancer cell lines incubated with cortisol demonstrated marked resistance to the effects of paclitaxel, a common front-line chemotherapeutic agent in the treatment of ovarian cancer, and this effect was reversed by co-incubation with a glucocorticoid receptor antagonist (Flint et al., 2009). Similarly, incubation of freshly isolated ovarian cancer cells with dexamethasone, a synthetic glucocorticoid, inhibited the apoptotic effects of cisplatin, a frontline platinum based chemotherapeutic agent used in the treatment of ovarian cancer. Additionally, cisplatin reduced tumor volume in nude mice xenografted with human ovarian cancer cells, but this effect was reversed when mice were co-treated with dexamethasone (Zhang et al., 2006). These findings suggest that dysregulated cortisol rhythms resulting in higher tonic exposure of tumor tissue to cortisol may contribute to the development of drug-resistance disease.
The HPA diurnal cycle is tied to central regulatory mechanisms such as the suprachiasmatic nucleus of the hypothalamus that governs a broad range of biological functions. In ovarian cancer persistent sleep difficulties have been noted (Clevenger, et al. 2013), and significant differences in the expression of clock genes have been described in ovarian tumor tissue when compared to normal ovaries (Tokunaga et al., 2008). Cortisol has recently been shown to play a role in the synchronization of peripheral tissues with central mechanisms of diurnal regulation (Mavroudis et al., 2012). Therefore, HPA activity may be linked to broader circadian disruption, which has been linked to cancer incidence, progression and survival (Sephton, et al, 2013). In particular, inflammatory processes and circadian disruption are known to interact in ways which might influence survival outcomes. For instance, rest-activity patterns measured by actigraphy are associated with levels of serum cortisol levels and cytokines in metastatic colorectal cancer patients. This suggests that altered circadian timing systems may promote loss of suppression of tumor-inflammation by the HPA axis, or vice versa (Rich et al. 2005). Additionally, as prior studies found diurnal rhythmicity to be associated with survival when cortisol is measured post-treatment, tumor inflammation is unlikely to explain the all the variation in HPA axis function. This variation may be attributable, in part, to more persistent variability in circadian timing systems (e.g. the expression of central clock genes; Sephton et al. 2000; Sephton et al. 2013;). In fact, disruption of core circadian rhythms, which HPA axis dysregulation may indicate, is associated with down-regulation of clock genes in peripheral cells, and this down-regulation has been demonstrated to accelerate tumor growth in experimental animal models of breast cancer (Yang et al., 2009). It is therefore possible that HPA dysregulation in ovarian cancer, and the associated increase in tumor-associated inflammation, represents suppression of cellular clocks at the level of tumor tissue.
4.1 Clinical Implications
Elevations in salivary cortisol sampled prior to bedtime were associated with higher grade tumors, a more pronounced inflammatory profile in ascites, and shorter survival time controlling for relevant clinical and demographic covariates. This raises the possibility that non-invasive sampling of nocturnal salivary cortisol may have potential as a biomarker of more refractory disease and help identify patients in need of specific treatment regimens, though more research is required to replicate these findings and explore their molecular correlates.
4.2 Limitations
It should be noted that these findings are correlational and thus limit causal inferences. Ultimately, pharmacologic inhibition of inflammatory mediators and glucocorticoid levels will be required to determine whether these dynamics have clinically relevant causal influences on ovarian cancer progression rates. Our sample was disproportionately Caucasian and non-Hispanic. As a slightly higher proportion of patients not included were Hispanic it is important for these findings to be replicated in a more diverse patient sample. Although a substantial number of patients declined or did not collect enough cortisol for analysis, the final sample appears to be broadly representative of the patient population and thus findings can be generalized, with caution (while a slightly higher proportion of patients not included had serous disease, this factor was not associated with survival).
4.3 Conclusions
HPA axis dysregulation prior to surgery was linked to post-surgical ovarian cancer survival, above and beyond the correlation of HPA axis activity with tumor grade and stage. HPA axis dysregulation is also correlated with both circulating and tumor-associated levels of the pro-inflammatory cytokine IL-6. As such, night time cortisol levels may provide a non-invasive biomarker of ovarian cancer disease severity that isindependent of established pathological markers. The observation that all cortisol measures were strongly associated with one another suggests these measures are facets of a unitary construct representing cortisol dysregulation. Night cortisol may be a sufficient and parsimonious measure of HPA function for researchers interested in ovarian cancer populations.
ACKNOWLEGEDMENTS
The authors gratefully acknowledge the assistance of Lauren Clevenger, Desire Christensen, Amy Nichols, Katherine Collins, Heena Maiseri, the Gynecologic Oncology faculty and staff at all institutions, and the time and efforts of the patients who participated in this study. This research was funded in part by grants CA88293 (Lutgendorf), CA104825 (Lutgendorf), CA140933 (Lutgendorf), CA109298 (Sood), and P50CA083639 (Bast), from the National Cancer Institute.
Role of the Funding Source
This research was funded in part by grants CA88293 (Lutgendorf), CA104825 (Lutgendorf), CA140933 (Lutgendorf), CA109298 (Sood), and P50CA083639 (Bast), from the National Cancer Institute. The National Cancer Institute had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In supplemental analyses with all available cortisol data, including patients with incomplete collection, results were very similar: night cortisol, n=134, H.R.=1.339, 95% CI= 1.007, 1.779, p=.045; cortisol slope, n=122, H.R =1.313, 95% CI= .978, 1.762, p=.068; cortisol variability, n=122, H.R.=.784, 95% CI= .639, .962, p=.019. In secondary analyses including physical well-being as a covariate, the results were virtually unchanged: night cortisol, n=109, H.R.=1.542, 95% CI= 1.085, 2.191, p=.016; cortisol slope, n=109, H.R =1.436, 95% CI=1.027, 2.008, p=.034; cortisol variability, n=109, H.R.=.694, 95% CI= .526, .916, p=.010.
Financial Disclosures: The authors have no financial disclosures.
Author Contributions:
Study conception and design:
Lutgendorf, Sood
Acquisition of data:
Thaker, Goodheart, Bender, Mendez, DeGeest, Penedo
Analysis and interpretation of data:
Schrepf, Lutgendorf, Dahmoush,
Drafting of manuscript:
Schrepf, Lutgendorf
Critical revision:
All Authors
References
- American Cancer Society: Cancer Facts & Figures. Atlanta, GA: American Cancer Society; 2014. Available from URL: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. [Google Scholar]
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J. Clin. Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- Burger RA, Grosen EA, Ioli GR, Van Eden ME, Park M, Berman ML, Manetta A, Disaia PJ, Granger GA, Gatanaga T. Spontaneous release of interleukin-6 by primary cultures of lymphoid and tumor cell populations purified from human ovarian carcinoma. J. Interferon Cytokine Res. 1995;15:255–260. doi: 10.1089/jir.1995.15.255. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, Eckberg K, Lloyd S, Purl S, Blendowski C, Goodman M, Barnicle M, Stewart I, McHale M, Bonomi P, Kaplan E, Taylor SIV, Thomas CR, Jr, Harris J. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Clevenger L, Schrepf A, Degeest K, Bender D, Goodheart M, Ahmed A, Dahmoush L, Penedo F, Lucci J, 3rd, Thaker PH, Mendez L, Sood AK, Slavich GM, Lutgendorf SK. Sleep disturbance, distress, and quality of life in ovarian cancer patients during the first year after diagnosis. Cancer. 2013;119:3234–3241. doi: 10.1002/cncr.28188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neurosci. Biobehav. Rev. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Cohen L, Cole SW, Sood AK, Prinsloo S, Kirschbaum C, Arevalo JM, Jennings NB, Scott S, Vence L, Wei Q, Kentor D, Radvanyi L, Tannir N, Jonasch E, Tamboli P, Pisters L. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PLoS One. 2012;7:e42324. doi: 10.1371/journal.pone.0042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin. Cancer. Res. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson RE, Fegan KS, Ren X, Hillier SG, Duncan WC. Glucocorticoid regulation of SLIT/ROBO tumour suppressor genes in the ovarian surface epithelium and ovarian cancer cells. PLoS One. 2011;6:e27792. doi: 10.1371/journal.pone.0027792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell T, Martin-Roussety G, Barker G, Autelitano DJ, Allen D, Grant P, Rice GE. Phase II biomarker trial of a multimarker diagnostic for ovarian cancer. J. Cancer Res. Clin. Oncol. 2010;136:1079–1088. doi: 10.1007/s00432-009-0755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint MS, Kim G, Hood BL, Bateman NW, Stewart NA, Conrads TP. Stress hormones mediate drug resistance to paclitaxel in human breast cancer cells through a CDK-1-dependent pathway. Psychoneuroendocrinology. 2009;34:1533–1541. doi: 10.1016/j.psyneuen.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Innominato PF, Roche VP, Palesh OG, Ulusakarya A, Spiegel D, Lévi FA. The circadian timing system in clinical oncology. Ann. Med. 2014;46:191–207. doi: 10.3109/07853890.2014.916990. [DOI] [PubMed] [Google Scholar]
- Jeannin P, Duluc D, Delneste Y. IL-6 and leukemia-inhibitory factor are involved in the generation of tumor-associated macrophage: regulation by IFN-γ. Immunotherapy. 2011;3:23–26. doi: 10.2217/imt.11.30. [DOI] [PubMed] [Google Scholar]
- Jehn CF, Kuehnhardt D, Bartholomae A, Pfeiffer S, Krebs M, Regierer AC, Schmid P, Possinger K, Flath BC. Biomarkers of depression in cancer patients. Cancer. 2006;107:2723–2729. doi: 10.1002/cncr.22294. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Giese-Davis J, Yutsis M, O'Hara R, Neri E, Gallagher-Thompson D, Taylor CB, Spiegel D. Design decisions to optimize reliability of daytime cortisol slopes in an older population. Am. J. Geriatr. Psychiatry. 2006;14:325–33. doi: 10.1097/01.JGP.0000201816.26786.5b. [DOI] [PubMed] [Google Scholar]
- Kulbe H, Chakravarty P, Leinster DA, Charles KA, Kwong J, Thompson RG, Coward JI, Schioppa T, Robinson SC, Gallagher WM, Galletta L, Australian Ovarian Cancer Study Group. Salako MA, Smyth JF, Hagemann T, Brennan DJ, Bowtell DD, Balkwill FR. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72:66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann. Oncol. 2013;(Suppl 10):x16–x21. doi: 10.1093/annonc/mdt463. [DOI] [PubMed] [Google Scholar]
- Lamkin DM, Lutgendorf SK, Lubaroff D, Sood AK, Beltz TG, Johnson AK. Cancer induces inflammation and depressive-like behavior in the mouse: modulation by social housing. Brain Behav. Immun. 2011;25:555–564. doi: 10.1016/j.bbi.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D, Matte I, Rancourt C, Piché A. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer. 2011;11:210. doi: 10.1186/1471-2407-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Weinrib AZ, Penedo F, Russell D, DeGeest K, Costanzo ES, Henderson PJ, Sephton SE, Rohleder N, Lucci JA, 3rd, Cole SW, Sood AK, Lubaroff DM. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J. Clin. Oncol. 2008;26:4820–4827. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavroudis PD, Scheff JD, Calvano SE, Lowry SF, Androulakis IP. Entrainment of peripheral clock genes by cortisol. Physiol. Genomics. 2012;44:607–621. doi: 10.1152/physiolgenomics.00001.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata NH, Tamakoshi K, Shibata K, Kikkawa F, Tomoda Y. Effects of interleukin-6 on in vitro cell attachment, migration and invasion of human ovarian carcinoma. Anticancer Res. 1997;17:337–342. [PubMed] [Google Scholar]
- Offner FA, Obrist P, Stadlmann S, Feichtingera H, Klinglerb P, Heroldc M, Zwierzinac H, Hittmaira A, Mikuza G, Abendsteind B, Zeimete A, Marth C. IL-6 secretion by human peritoneal mesothelial and ovarian cancer cells. Cytokine. 1995;7:542–547. doi: 10.1006/cyto.1995.0073. [DOI] [PubMed] [Google Scholar]
- Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann. N. Y. Acad. Sci. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, Krause M, Schwörer AM, Wagner U, Müller-Brüsselbach S, Müller R. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int. J. Cancer. 2014;134:32–42. doi: 10.1002/ijc.28335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N. Engl. J. Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, Jasmin C, Lévi F. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin. Cancer Res. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- Schauer IG, Zhang J, Xing Z, Guo X, Mercado-Uribe I, Sood AK, Huang P, Liu J. Interleukin-1β promotes ovarian tumorigenesis through a p53/NF-κB-mediated inflammatory response in stromal fibroblasts. Neoplasia. 2013;15:409–420. doi: 10.1593/neo.121228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepf A, Clevenger L, Christensen D, DeGeest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L, Penedo F, Lucci JA, 3rd, Ganjei-Azar P, Mendez L, Markon K, Lubaroff DM, Thaker PH, Slavich GM, Sood AK, Lutgendorf SK. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain Behav. Immun. 2013;(30 Suppl):S126–S134. doi: 10.1016/j.bbi.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl. Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, Spiegel D, Salmon P. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav. Immun. 2013;(30 Suppl):S163–S70. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Pearce BD, Biron CA, Miller AH. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 2005;18:41–78. doi: 10.1089/vim.2005.18.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann. N.Y. Acad. Sci. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Sood AK, Buller RE. Drug resistance in ovarian cancer: from the laboratory to the clinic. Obstet Gynecol. 1998;92:312–319. doi: 10.1016/s0029-7844(98)00184-7. [DOI] [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Smyth J, Kirschbaum C, Cohen S, Hellhammer D, Grossman S. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26:295–306. doi: 10.1016/s0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Tokunaga H, Takebayashi Y, Utsunomiya H, Akahira J, Higashimoto M, Mashiko M, Ito K, Niikura H, Takenoshita S, Yaegashi N. Clinicopathological significance of circadian rhythm-related gene expression levels in patients with epithelial ovarian cancer. Acta. Obstet. Gynecol. Scand. 2008;87:1060–1070. doi: 10.1080/00016340802348286. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Volden PA, Conzen SD. The influence of glucocorticoid signaling on tumor progression. Brain Behav. Immun. 2013;(30 Suppl):S26–S31. doi: 10.1016/j.bbi.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrib AZ, Sephton SE, Degeest K, Penedo F, Bender D, Zimmerman B, Kirschbaum C, Sood AK, Lubaroff DM, Lutgendorf SK. Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer. 2010;116:4410–4419. doi: 10.1002/cncr.25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wood PA, Oh EY, Du-Quiton J, Ansell CM, Hrushesky WJ. Down regulation of circadian clock gene Period 2 accelerates breast cancer growth by altering its daily growth rhythm. Breast Cancer Res. Treat. 2009;117:423–431. doi: 10.1007/s10549-008-0133-z. [DOI] [PubMed] [Google Scholar]
- Yang M, Kim J, Kim JS, Kim SH, Kim JC, Kang MJ, Jung U, Shin T, Wang H, Moon C. Hippocampal dysfunctions in tumor-bearing mice. Brain Behav. Immun. 2014;36:147–55. doi: 10.1016/j.bbi.2013.10.022. [DOI] [PubMed] [Google Scholar]
- Zhang C, Marmé A, Wenger T, Gutwein P, Edler L, Rittgen W, Debatin KM, Altevogt P, Mattern J, Herr I. Glucocorticoid-mediated inhibition of chemotherapy in ovarian carcinomas. Int J Oncol. 2006;28:551–558. [PubMed] [Google Scholar]