Abstract
Significant public health problems associated with methamphetamine (MA) production and use in the United States have emerged over the past 25 years. Although the popular press (Newsweek, Aug 8, 2008), has called MA “America’s Most Dangerous Drug” there has been considerable controversy about the size of the problem. Epidemiological indicators have given a mixed picture. National surveys of the adult U.S. population and school-based populations have consistently been used to support the position that MA use is a relatively minor concern (NSDUH, 2006; Johnston & O’Malley, 2007). However, many other data sources, including law-enforcement groups, welfare agencies, substance abuse treatment program admission data, data on criminal justice populations, and state/county executives indicate that MA is a very significant public health problem for many communities throughout much of the country (NDIC, 2007b; NACO, 2005, 2006; NIDA CEWG, 2007). In this article, we describe (1) the historical underpinnings of the MA problem, (2) trends in the epidemiological nature of the MA problem, (3) key subgroups at risk for MA problems, (4) the health and social factors associated with MA use, (5) interventions available for addressing the MA problem, and (5) lessons learned related to the MA problem.
Critical Summary of the MA Problem in the U.S
Methamphetamine (MA) is a potent stimulant with high abuse potential that can be smoked, snorted, injected, or taken orally. The desirable short-term effects of MA or initial “rush” is characterized by increased energy and alertness, an elevated positive mood state, and decreased appetite (Rawson, Gonzales, & Brethen, 2002). Compared with other stimulants (e.g., cocaine and nicotine) the half life of MA is quite long, ranging from 8 to 12 hours. Access and availability are major contributors to the problem as MA is manufactured using readily available retail products (e.g., pseudoephedrine, hydrochloric acid, red phosphorus, ether, etc.) and numerous “recipes” on how to produce MA are widely available on the internet (Gonzales and Rawson, 2006). Although there are increasing reports of the growing misuse of pharmaceutical amphetamines (methylphenidate, Adderall and Concerta) (Low & Gendaszek, 2002; McCabe et al, 2005) particularly among college students, the vast majority of amphetamine that is abused illicitly is manufactured MA.
Use of amphetamine-type stimulants (ATS), including MA, in the U.S. predates World War II. Amphetamine tablets were extensively used by soldiers on all sides of the conflict during World War II to reduce fatigue and suppress appetite (Anglin et al., 2000). During the late 1960s, use of ATS pills became problematic among young adults, especially college students during this era of extensive drug experimentation. Increased rates of amphetamine injection brought with it serious medical and psychiatric consequences, prompting the drug prevention slogan “speed kills.” The Comprehensive Drug Abuse Prevention and Control Act of 1970 sharply limited the accepted medical uses for prescribed amphetamines, which served to greatly reduce the ATS problem in the U.S. during the 1970s. By the late 1970’s, use of ATS was limited to a few circumscribed geographic areas in California and Oregon, where MA continued to be manufactured illicitly mostly by motorcycle gangs. Their practice of carrying MA in the crankcases of their motorcycles, led to the slang term “crank” for MA.
Increased problems associated with MA resurfaced in the 1980s as motorcycle gangs began to produce much larger amounts and expanded their customer base in Southern California and Oregon. Through the 80’s MA use was fairly limited to Caucasian men, many of whom were truck drivers, construction workers and other blue collar workers. The typical route of administration was intranasal. During this same period, MA use in Hawaii began to increase dramatically, particularly on the island of Oahu, as a new form of MA called “Ice” began to be imported into the state from the Philippines and Southeast Asia. This crystallized form of MA was heated and inhaled as a vapor and as users discovered the increased intensity of euphoria produced by the smoked drug, smoking became the dominant route of administration for MA in Hawaii and subsequently throughout the U.S.
During the 1990s, the manufacture and distribution of MA expanded in two major ways. MA cooking in “mom and pop home” laboratories became widespread throughout California and other West Coast States, as well as Oklahoma, Missouri, and the Rocky Mountain States. Because these labs are easily detectable by the strong odor produced in MA production, the expansion of MA production and use became concentrated in rural areas where labs were less detectable. At the same time, large laboratories called “superlabs” that produced much larger quantities of MA developed in Southern California and Northern Mexico. MA from these labs was distributed by Mexican drug trafficking groups to key distribution points in the West and Midwest, including Salt Lake City, Oklahoma City and Des Moines. The expansion of the MA supply by these two sources resulted in the availability of massive quantities of MA throughout the West and Midwest at very low prices.
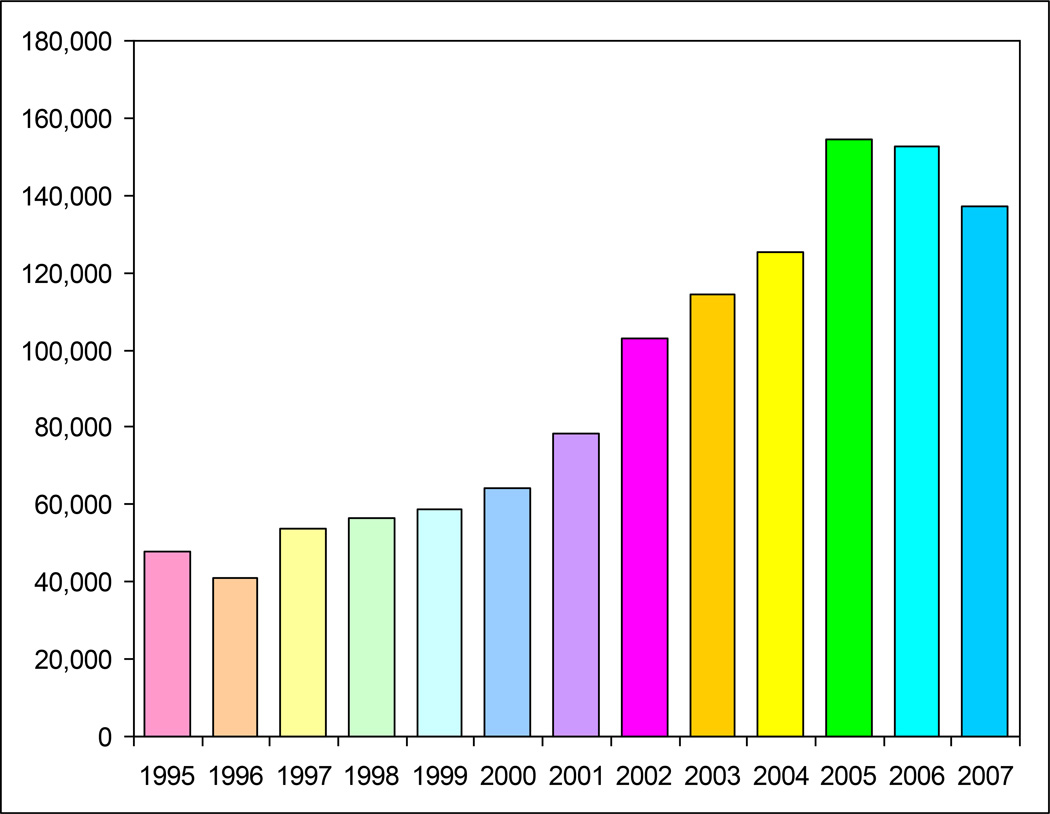
As inexpensive MA availability increased through the Western half of the U.S., the demographic profile of MA users expanded to include Latinos, Asian/Pacific Islanders, Native Americans, gay and bisexual men, offender populations, women, and adolescents (Shoptaw et al., 2002; Freese et al., 2002; Rawson et al., 2002; Brecht et al., 2004; Cretzmeyer et al., 2003; Rawson et al., 2005; Gonzales et al., 2008). In addition, the increased availability of MA became apparent in the Southeastern U.S. after 2000 and by 2005, high rates of MA use were reported in almost all parts of the U.S. expect for the Northeastern corridor. Treatment admission data, clearly a lagging indicator of the extent MA usage in communities, rose gradually through the 1990s well into the 21st Century. Treatment admission data, presented in Figure 1, reflects the escalating numbers of MA admissions across this period.
Figure 1.
U.S. Treatment Admissions for Primary Methamphetamine Abuse
Source: SAMHSA, Treatment Episode Data Set, 2009
In 2004, many state governments began to pass laws restricting the sale of the primary MA precursor, pseudoephedrine, in an effort to reduce the domestic production of MA and prevent the ongoing spread across populations and communities. The purchase of over the counter cold and sinus preparations containing pseudoephedrine became limited in a number of manners. In most states, the number of packages of tablets of these medications that could be purchased at one time was restricted, some states took these medications completely off the open counter and put them behind the counter, while other states required purchasers to show identification and sign for their pseudoephedrine products. In 2005, the Federal government passed the Combat Methamphetamine Epidemic Act, which federally regulated the sale of products containing pseudoephedrine and further reduced its availability for use in MA manufacture. These precursor efforts have produced a significant decrease in the availability of MA in many parts of the U.S. and have resulted in dramatic price increases for the supply of MA. Indicators reflecting these changes include the reduction in the number of MA labs seized by law enforcement, decreased primary MA treatment admissions, and reduced emergency room visits associated with MA (Maxwell & Rutowski, 2008).
Key Trends: Populations Disproportionately at Risk for MA Use
Men who have sex with men (MSM)
Elevated rates of MA use have been reported in many MSM communities throughout the U.S. with associations to high risk sexual behaviors that increase the transmission and infection of communicable diseases, including increased number of causal and anonymous sexual partners, decreased condom use, sex trading, group sex, more frequent and longer sexual episodes, and anal intercourse (Frosch et al, 1996; Halkitis et al. 2005; Patterson et al. 2005; Shoptaw et al. 2005). The relationship between MSM, MA use, and infectious disease infection is an important public health issue in the U.S., as MSM populations are the only behavioral risk group that is experiencing an increase in the incidence of HIV and other sexual transmitted infections (Shoptaw et al., 2005). HIV incidence in MSM averages 1.6% per year and differs geographically (CDC, 2004; Reback, 2007). Incidence rates of HIV are tripled for MSM who use MA compared to non-drug using MSM (Buchacz, et al 2005). Within this group, MA use is also associated with elevated levels of sexually transmitted infections, like syphilis (4%) (Wong et al, 2005). A recent report by the CDC on the connection between MA-use, high risk sexual behavior, and infectious disease transmission in MSM communities suggests that this combination of factors poses a major threat of a renewed increase in rates of HIV infection among MSM (CDC 2007), which is exacerbated among MA users with a history of infection with other sexually transmitted infections, like genital gonorrhea, genital herpes, and hepatitis B (Shoptaw et al., 2003).
Women
Unlike many other illegal drugs, MA is a drug that appeals equally to men and women. Among treatment samples of MA users, data indicate that nearly as many women enter treatment for MA dependence than men (Hser et al., 2005; Brecht et al., 2004; Rawson et al 2004) and female youth are more likely to use MA than their male youth counterparts (Rawson et al., 2005; Gonzales et al., 2008). Unlike men, women tend to report MA use initiation being a result of “the desire to lose weight” or “cope with depression.” MA use by pregnant women can jeopardize the health of their born and unborn children (Winslow et al., 2007). One study found that pregnant MA using women had a 3.5 times greater likelihood of having a lower birth weight child and pre-term births than non-MA using pregnant women (Smith et al., 2006).
MA-using women are more likely to report previous exposures to trauma, including physical and sexual abuse (Messina et al., 2008) especially MA women offenders (Messina et al., 2007) as well as suffer psychological distress, including depression mood disorders and suicidality (Glasner-Edwards et al., 2008) than MA-using men. MA use also plays a major role in domestic violence (Brown et al., 2005). MA use is involved in almost 90 percent of the domestic dispute cases against women investigated across the U.S (DEA, 2000). Although both men and women MA users report increased sexual desire while under the influence of MA (Rawson et al., in 2005), men tend to experience greater sexual enhancements and desires that have been linked to more unusual and riskier sexual acts (Brown et al., 2005). In qualitative interviews with MA using women, Brown et al., 2005 uncovered that violence and coercion tend to occur in MA users’ relationships, particularly because MA-using males may demand riskier sexual acts of their female partners and may be unwilling to take no for an answer.
Criminal Offenders
MA use among offender populations has been a significant problem in the criminal justice system. MA abuse is highly associated with participation in illegal behaviors such as crime and violence (Farabee et al., 2002), that tend to result in increased incarcerations and other problems within the criminal justice system (Evans & Longshore, 2004). In fact, the majority of county law-enforcement agencies report MA as their primary drug problem (NACO, 2005), with fairly equal rates between men and women. This is further demonstrated by the share of MA-related treatment admissions referred by the criminal justice system which is approximately 50% higher than for other substances of abuse (SAMHSA, 2008b).
Health Consequences of MA Use
As a central nervous system stimulant, MA facilitates the release of norepinephrine and dopamine from nerve terminals and, to a lesser extent, blocks their synaptic reuptake (King & Ellinwood, 2005). The resulting catecholamine surge mediates many of the acute symptoms and physiological changes associated with MA intoxication, including elevated heart rate and blood pressure (Newton et al., 2004). By constricting blood vessels and depriving tissues of oxygen, excess circulating norepinephrine may contribute to organ damage. Likewise, the oxidation of accumulated catecholamines may lead to the formation of reactive oxygen species and subsequent cellular toxicity (Karch, 2002; Kaye et al., 2007).
Chronic MA use is associated with serious medical conditions affecting multiple organ systems (Rawson et al., 2006; Mooney et al., in press [a&b]). Cardiovascular risks include chest pain, arrhythmias, hypertension, cardiomyopathy, and acute myocardial infarction, even in relatively young users (Turnipseed et al., 2002; Westover et al., 2008; Kaye et al., 2007). Autopsy studies have demonstrated increased frequencies of accelerated coronary artery disease and cardiac hypertrophy in MA users relative to controls (Karch, 2002). Elevated rates of electrocardiogram abnormalities, including prolonged QTc interval, have also been documented in studies of MA users (Haning and Goebert, 2007; Mooney et al., in press[b]).
In a recent investigation of MA-related emergency department visits (n=353), the most common presentations associated with MA use were mental health problems (18.7%), trauma (18.4%), skin infections (11.1%), and dental pathology (9.6%) (Hendrickson et al., 2008). Seizures and stroke have also been reported in the context of MA intoxication in both emergency department and hospital settings (Westover et al., 2007; Perez et al., 1998; Richards et al., 1999). Other MA-related neurological complications include movement disorders; during intoxication, users may exhibit hyperkinetic movements, repetitive or stereotyped behaviors, (Mattson and Calvery, 1968; Sperling and Horowitz, 1994) or choreoathetoid movement disorders (Lundh and Tunving, 1981; Rhee et al., 1998)..
An association between MA use and severe dental disease, known as “meth mouth”, has been highlighted in recent literature (Curtis, 2006; Mooney et al., in press[b]). Putative mechanisms of MA-related tooth decay include xerostomia (i.e., dry mouth), excessive soft drink consumption, poor oral hygiene, and the acidic composition of the drug (Klasser & Epstein, 2005). In addition, dermatologic manifestations of MA use have been found to result from self-inflicted injury during intoxication, infection from repeated injection, or accidental burns during the process of MA manufacture. MA users may repeatedly scratch or pick at their skin in the context of drug-induced perceptual disturbances such as formication, or the sensation of insects crawling on or underneath the skin (MacKenzie and Heischober, 1997; Bostwick and Lineberry, 2006).
Prior studies have demonstrated MA-induced neurotoxicty to dopaminergic pathways in the brain, particularly after high doses or chronic use (Scott et al., 2007). Reductions in striatal dopamine transporter activity (DAT) have been observed in MA abusers that may be clinically associated with cognitive deficits and slowed motor function (Volkow et al., 2001). According to recent estimates, up to 40% of MA users exhibit global neuropsychological impairment (Rippeth et al., 2004). Significant neurocognitive deficits related to frontostriatal and limbic circuits in the brain have been observed, including executive functions, memory, attention, language, psychomotor functions, and visuoconstruction (Scott et al., 2007; Monterosso et al, 2005; Simon et al., 2000). Importantly, DAT activity and cognitive functioning have been shown to partially recover with sustained abstinence from MA (Volkow et al., 2001; Wang et al., 2004).
In addition to physical health consequences, MA use is associated with a range of psychiatric manifestations. The most common psychiatric symptoms experienced by MA users include anxiety, depression, and psychosis (Zweben et al., 2004; Glasner-Edwards et al., in press), and the severity of these symptoms may be related to the quantity and/or frequency of MA use, the route of administration, and individual differences in sensitivity to the drug (Harris and Batki, 2000). MA-induced psychotic symptoms, which mimic those of schizophrenia, include paranoid ideation, delusions, and auditory and visual hallucinations. Psychotic symptoms occur transiently in a substantial proportion of MA users (McKetin et al., 2006) and, along with other psychiatric symptoms, typically subside within a week of abstinence (Newton et al., 2004). However, in a subset of users, psychosis may persist for several months or longer even after sustained abstinence (Iwanami et al., 1994; Ujike & Sato, 2004). When intoxicated, users may become agitated or violent (Richards et al., 1999; Zweben et al., 2004) and nearly one-third of treatment-seeking users have reported a lifetime history of suicide attempts (Zweben et al., 2004; Glasner-Edwards et al., 2008). A recent study examined differences in health related quality of life between a normative U.S. population and a MA-dependent treatment seeking sample and found that the MA sample reported substantially lower mental health status perceptions than the normative U.S. population (Gonzales et al., in press). Table 1 presents a summary of health factors associated with MA use.
Table 1.
Health Consequences of MA Use
| Cardiac Effects | Psychiatric Effects | Neurologic Effects | Other Physiological Effects |
|---|---|---|---|
| –Chest pain | -Paranoia | –Headache | –Skin ulcerations |
| –Tachycardia | -Hallucinations | –Seizures | –Dermatological infections |
| –Hypertension | -Depression | –Stroke | –Dental caries |
| –Arrhythmias | -Anxiety | –Cerebral vasculitis | –Anorexia |
| –Myocardial Infarction | -Insomina | –Hyperkinetic movements | –Pulmonary hypertension |
| –Coronary artery disease | -Suicidality | –Neurocognitive impairment | –Plumonary edema |
| –Cardiomyopathy | -Aggression | -Hyperthermia | |
| -Poor quality of life | -Fetal growth restriction | ||
| -Hepatitis C and HIV |
Social Consequences associated with MA Use
MA use poses significant public health challenges to health care professionals, social service providers, policymakers, and the law enforcement community. In a recent economic assessment, MA use cost the U.S. $23.4 billion in 2005 alone. This cost estimate included costs associated with morbidity and mortality, criminal justice and social welfare services, environmental clean up from MA chemical production, and most significantly lost productivity and quality of life burden of MA dependence (Rand Corporation, 2008). The social harms experienced by neighborhoods from systemic violence (trafficking and dealing) and MA production are tremendous. The manufacture and production of MA uses chemicals that are explosive, corrosive, and flammable, which can result in burn injuries, respiratory ailments, poisoning, and fires. In one California County, 15% of labs identified by law enforcement were due to fires (Holton, 2001). Further, because manufacturing of MA also occurs in automobiles (including trunks), there is an increased opportunity for distribution and spills of toxic wastes and chemicals into the environment, which can produce pollution to ground water sources (Holton, 2001). Given the nexus that exists between MA use and criminal involvement (Farabee et al., 2002), criminal records and jail sentences results in the loss of access to social welfare benefits (Iguchi et al., 2002) and lost quality of life, as well as substantially affects families and children via economic hardships and foster care exposure (Hiller et al., 2005).
Furthermore, because MA use in particular has been linked to many psychiatric difficulties, such as depression, irritability, insomnia, and paranoia, and aggressive behaviors, it presents a particularly serious risk for neglect and abuse to the children in these environments. According to a survey by the National Association of Counties related to the “Impact of Meth on Children” conducted in 300 counties in 13 states, MA is a major cause of child abuse and neglect: 40% of child welfare officials reported an increase in out-of-home placements because of MA in 2005. Moreover, MA use results in increased child abuse crimes and child abuse homicides (Petit & Curtis, 1997; National Survey of Child and Adolescent Well-Being Research Group, 2002). This is concerning since neglected and abused children are at risk of social, emotional, developmental, and behavioral problems during childhood and adolescence, as well as cognitive, psychological, and permanent brain damage or physical impairments (Dunn et al., 2002; Hildyard & Wolfe, 2002), and even death (Ireland, 2002).
These young children are further exposed to the highly psychoactive stimulant and the toxic precursor chemicals associated with MA production, which undermines their health and well-being (Santos et al., 2005). In almost 10,000 seizures of MA labs documented by the Drug Enforcement Agency, over one-third had children under the age of 15 years old present (Santos et al., 2005).
Interventions for MA Use
Acute agitation with paranoid ideation is the most common presentation of MA intoxication in emergency departments. In many cases, treatment does not require the use of medications, but rather a brief treatment intervention or referral to specialized treatment. Where medications are employed, the choices are usually between a benzodiazepine and an antipsychotic. Traditionally, haloperidol in 5mg parenteral repeated doses are used, often in combination with 1–2mg of lorazepam and 1mg of the anticholinergic benztropine. Patients receiving haldoperidol alone tend to experience more extrapyramidal reactions (Battaglia, et al., 1997). It remains unclear whether benzodiazepine or neuroleptic medications should be preferred in the treatment of MA-induced agitation. No specific treatments for MA psychosis have been established, and protocols similar to those used for MA acute intoxication described above are the most commonly used approach.
Research collected over the past two decades has demonstrated, to a large extent, that treatment for MA dependence is effective, resulting in measurable and desirable changes in drug use and other social behaviors compared to no treatment (Rawson et al., 2006). Psychosocial interventions that have generated the most empirical evidence include cognitive behavioral interventions that focus on self-efficacy skill building and relapse prevention (Cheng-Fang et al., 2004; Rawson, et al., 2004), contingency management approaches that provide motivational incentives for demonstration of desired non-drug use behaviors (Roll et al., 2006), stepped care for transitioning MA users through continued treatment levels based on need (Kay-Lambkin, 2008), and drug court for MA-dependent offenders (Huddleston, 2005; Marinelli-Casey et al., 2008). To date, there are no approved pharmacological treatments for MA dependence (Montoya & Vocci, 2008), with several showing potential, including bupropion (Elkashef et al., 2008; Shoptaw et al., 2008; Newton et al., 2006) and methylphenidate (Tiihonen et al., 2007).
Attempts to break the cycle of drug use and crime have included providing drug treatment to offenders while in prison. The most common treatment modality used in prisons is the therapeutic community (TC). In California specifically, MA using offenders are supported by the Substance Abuse Crime and Prevention Act (SACPA), also known as Proposition 36 that was voted into law in 2000 (Longshore et al., 2006). SACPA requires that nonviolent adult drug offenders be offered treatment in lieu of incarceration. Since the law’s implementation, more than 30,000 offenders have received SACPA treatment each year, the majority of whom are MA users (Urada et al., 2007).
MA users are seen as some of the most difficult drug treatment patients, due to protracted physiological and psychological problems caused by the drug’s impact on neural pathways. Factors related to successful treatment outcomes among MA users include lower levels of MA use at treatment admission (fewer than 15 days of MA use out of the previous 30), shorter histories of MA use (two years or less), retention in treatment for at least 90 days, and periods of abstinence during treatment at least three consecutive weeks or greater (Hillhouse et al., 2007; Rawson et al., 2004). Studies examining risk factors for poor treatment outcomes among MA users have identified continued MA use during treatment, injection use, having less than a high school education, young age at treatment admission, having a disability (Brecht et al., 2005; Hillhouse et al., 2007), polydrug use (Brecht et al., 2008), childhood trauma and abuse (Messina et al., 2008), and having an underlying psychiatric disorder (Glasner-Edwards et al., 2008a,b). Continued treatment participation and active recovery efforts, including frequent 12-step program participation, have been shown to be associated with successful treatment outcomes among MA users over time (Hser et al., 2008; Gonzales et al., in press).
Implications
The serious MA problem that evolved in the U.S. over the past 20 years has significantly impacted the public health, social welfare, and criminal justice systems. The experience with the emergence of the MA problem has implications for public health policy in the U.S.
First, there needs to be an adequate epidemiological assessment system in place to bring emerging drug problems to the attention of public health officials and policy makers. Our current epidemiological monitoring system is not adequate to fully identify and recognize emerging drug problem as was witnessed by the slow recognition of the seriousness of the MA problem. The MA problem emerged as a small local problem and spread exponentially from west to east over a 20 year span without a coordinated federal response.
Second, an effective monitoring system should incorporate public health indicators and data from multiple systems including criminal justice agencies (both police departments and correctional facilities), the educational system, social welfare agencies, and the primary care and mental health systems to adequately identify specific subgroups and geographical communities that are impacted.
Third, a coordinated national response should include comprehensive prevention and treatment programming. Prevention efforts for MA in the U.S. have been sparse and regional (i.e., Montana, Kansas, and California). Only recently, during the last few years, have we begun to see prevention efforts for MA initiated at the national level.
Forth, the response to the needs of addicted individuals should be based upon a public health approach as opposed to a criminal justice approach. Tens of thousands of MA users filled prisons in the Western and Midwestern U.S. long before there was an organized and meaningful response from the public health system.
Conclusion
This paper has provided a critical examination of the development of the MA problem in the U.S., the patterns and trends in use, key characteristics of high-risk subgroups, as well as interventions that have been used to address the problem. It is unlikely that the MA problem will go away from the landscape of drug abuse problems challenging the U.S. systems across the federal, state and local levels. Efforts targeting the MA problem over the past two decades clearly demonstrate that a strategic program of research, prevention and treatment must be developed and continuously funded to adequately address the MA problem.
LITERAURE CITED
- 1.Anglin MD. Conversation with M. Douglas Anglin. Addiction. 2006;101(2):169–180. doi: 10.1111/j.1360-0443.2006.01318.x. [DOI] [PubMed] [Google Scholar]
- 2.Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S. History of the methamphetamine problem. J. Psychoactive Drugs. 2000;32(2):137–142. doi: 10.1080/02791072.2000.10400221. [DOI] [PubMed] [Google Scholar]
- 3.Anglin MD, Hser Y-I. Treatment of drug abuse. In: Tonry M, Wilson JQ, editors. Drugs and Crime. Chicago: University of Chicago Press; 1990. pp. 1–36. [Google Scholar]
- 4.Anglin MD, Urada D, Brecht ML, Hawken A, Rawson R, Longshore D. Criminal justice treatment admissions for methamphetamine use in California: a focus on proposition 36. J. Psychoactive Drugs. 2007;4(SARC Suppl.):367–381. doi: 10.1080/02791072.2007.10399898. [DOI] [PubMed] [Google Scholar]
- 5.Bachman JD, Johnston LD, O’Malley PM. Ann Arbor, MI: Survey Research Center, Institute for Social Research, University of Michigan; 2006. Monitoring the Future: Questionnaire Responses from the Nation’s High School Seniors 2006. http://monitoringthefuture.org/datavolumes/2006/2006dv.pdf. [Google Scholar]
- 6.Battaglia J, Moss S, Rush J, Kang J, Mendoza R. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double-blind, emergency department study. Am. J. Emerg. Med. 1997;15:335–340. doi: 10.1016/s0735-6757(97)90119-4. [DOI] [PubMed] [Google Scholar]
- 7.Bostwick MJ, Lineberry TW. The ‘meth’ epidemic: managing acute psychosis, agitation, and suicide risk. Curr. Psychiatry Online. 2006;5(11) [Google Scholar]
- 8.Brecht ML, Greenwell L, Anglin MD. Methamphetamine treatment: trends and predictors of retention and completion in a large state treatment system (1992–2002) J. Subst. Abuse Treat. 2005;29(4):295–306. doi: 10.1016/j.jsat.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Brecht ML, O’Brien A, von Mayrhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addict. Behav. 2004;29(1):89–106. doi: 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- 10.Brown AH, Domier CP, Rawson RA. Stimulants, sex & gender: sexual addiction & compulsivity. J. Treat. Prev. 2005;12(2–3):169–180. [Google Scholar]
- 11.Buchacz K, McFarland W, Kellogg TA, Loeb L, Holmberg SD, Dilley J, Klausner JD. Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS. 2005;19(13):1423–1424. doi: 10.1097/01.aids.0000180794.27896.fb. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2004. Vol. 16. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2005. [Google Scholar]
- 13.Centers for Disease Control and Prevention. HIV/AIDS among men who have sex with men. Atlanta, GA: 2007. http://www.cdc.gov/hiv/topics/msm/resources/factsheets/msm.htm. [PubMed] [Google Scholar]
- 14.Cheng-Fang Y, Hsiao-Yen W, Ju-Yu Y, Chih-Hung K. Effects of brief cognitive-behavioral interventions on confidence to resist the urges to use heroin and methamphetamine in relapse-related situations. J. Nerv. Ment. Dis. 2004;192(11):788–791. doi: 10.1097/01.nmd.0000144699.80765.7f. [DOI] [PubMed] [Google Scholar]
- 15.Cretzmeyer M, Sarrazin MV, Huber DL, Block RI, Hall JA. Treatment of methamphetamine abuse: research findings and clinical directions. J. Subst. Abuse Treat. 2003;24(3):267–277. doi: 10.1016/s0740-5472(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 16.Curtis EK. Meth mouth: a review of methamphetamine abuse and its oral manifestations. Gen. Dent. 2006;54(2):125–129. [PubMed] [Google Scholar]
- 17.U.S. Drug Enforcement Administration. Statistics. 2000 www.usdoj.gov/dea.
- 18.Dunn MG, Tarter RE, Mezzich AC, Vanyukov M, Kirisci L, Kirillova G. Origins and consequences of child neglect in substance abuse families. Clin. Psychol. Rev. 2002;22(7):1063–1090. doi: 10.1016/s0272-7358(02)00132-0. [DOI] [PubMed] [Google Scholar]
- 19.Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- 20.Evans E, Longshore D. Evaluation of the substance abuse and crime prevention act: treatment clients and program types during the first year of implementation. J. Psychoactive Drugs. 2004;(Suppl 2):165–174. doi: 10.1080/02791072.2004.10400052. [DOI] [PubMed] [Google Scholar]
- 21.Farabee D, Prendergast M, Cartier L. Methamphetamine use and HIV risk among substance-abusing offenders in California. J. Psychoactive Drugs. 2002;34:295–300. doi: 10.1080/02791072.2002.10399966. [DOI] [PubMed] [Google Scholar]
- 22.Freese TE, Miotto K, Reback CJ. The effects and consequences of selected club drugs. J. Subst. Abuse Treat. 2002;23(2):151–156. doi: 10.1016/s0740-5472(02)00267-2. [DOI] [PubMed] [Google Scholar]
- 23.Frosch D, Shoptaw S, Huber A, Rawson RA, Ling W. Sexual HIV risk among gay and bisexual male methamphetamine abusers. J. Subst. Abuse Treat. 1996;13(6):483–486. doi: 10.1016/s0740-5472(96)00098-0. [DOI] [PubMed] [Google Scholar]
- 24.Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson RA. Psychopathology in methamphetamine dependent adults 3 years after treatment. Drug Alcohol Rev. doi: 10.1111/j.1465-3362.2009.00081.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson RA. Identifying methamphetamine users at risk for major depressive disorder: findings from the Methamphetamine Treatment Project at 3-year follow-up. Am. J. Addict. 2008;17:99–102. doi: 10.1080/10550490701861110. [DOI] [PubMed] [Google Scholar]
- 26.Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson RA Methamphetamine Treatment Project Corporate Authors. Risk factors for suicide attempts in methamphetamine dependent patients. Am. J. Addict. 2008;17(1):24–27. doi: 10.1080/10550490701756070. [DOI] [PubMed] [Google Scholar]
- 27.Gonzales R, Ang A, Marinelli-Casey P, Glik D, Iguchi YM, Rawson R. Health-related quality of life trajectories of methamphetamine-dependent individuals as a function of treatment completion and continued care over a 1 year period. J. Subst. Abuse Treat. doi: 10.1016/j.jsat.2009.04.001. in press. [DOI] [PubMed] [Google Scholar]
- 28.Gonzales R, Ang A, McCann M, Rawson R. Methamphetamine use among treatment seeking youth: an emerging problem? Subst. Abus. 2008;29(2):71–80. doi: 10.1080/08897070802093312. [DOI] [PubMed] [Google Scholar]
- 29.Halkitis PN, Shrem MT, Martin FW. Sexual behavior patterns of methamphetamine-using gay and bisexual men. Subst. Use Misuse. 2005;40(5):703–719. doi: 10.1081/ja-200055393. [DOI] [PubMed] [Google Scholar]
- 30.Haning W, Goebert D. Electrocardiographic abnormalities in methamphetamine abusers. Addiction. 2007;102(Suppl. 1):70–75. doi: 10.1111/j.1360-0443.2006.01776.x. [DOI] [PubMed] [Google Scholar]
- 31.Harris D, Batki SL. Stimulant psychosis: symptom profile and acute clinical course. Am. J. Addict. 2000;9(1):28–37. doi: 10.1080/10550490050172209. [DOI] [PubMed] [Google Scholar]
- 32.Hendrickson R, Cloutier R, McConnell K. Methamphetamine-related emergency department utilization and cost. Acad. Emerg. Med. 2008;15(1):23–31. doi: 10.1111/j.1553-2712.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- 33.Hildyard KL, Wolfe DA. Child neglect: developmental issues and outcomes. Child Abuse Negl. 2002;26(6–7):679–695. doi: 10.1016/s0145-2134(02)00341-1. [DOI] [PubMed] [Google Scholar]
- 34.Hiller ML, Webster JM, Garrity TF, Leukefeld C, Narevic E, Staton M. Prisoners with substance abuse and mental health problems: use of health and health services. Am. J. Alcohol Abuse. 2005;31(1):1–20. [PubMed] [Google Scholar]
- 35.Hillhouse M, Marinelli-Casey P, Gonzales R, Ang A, Rawson RA Methamphetamine Treatment Project Corporate Authors. Predicting in-treatment performance and post-treatment outcomes in methamphetamine users. Addiction. 2007;102(Suppl 1):84–95. doi: 10.1111/j.1360-0443.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- 36.Holton WC. Unlawful lab leftovers. Environ. Health Perspect. 2001;109(12):A576. doi: 10.1289/ehp.109-a576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hser YI, Evans E, Huang D, Brecht ML, Li L. Comparing the dynamic course of heroin, cocaine, and methamphetamine use over 10 years. Addict. Behav. 2008;33(12):1581–1589. doi: 10.1016/j.addbeh.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hser Y-I, Evans E, Yu-Chuang H. Treatment outcomes among women and men methamphetamine abusers in California. J. Subst. Abuse Treat. 2005;28:77–85. doi: 10.1016/j.jsat.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Huddleston CW. Drug courts: An effective strategy for communities facing methamphetamine. U.S. Department of Justice, Office of Justice Programs. [May 1–15];Bur. Justice Assistance Bull. 2005 http://www.ojp.usdoj.gov/BJA/pdf/MethDrugCourts.pdf.
- 40.Iguchi MY, London JA, Forge NG, Hickman L, Fain T, Riehman K. Elements of well-being affected by criminalizing the drug user. Public Health Rep. 2002;117(Suppl 1):S146–S150. [PMC free article] [PubMed] [Google Scholar]
- 41.Ireland TO. Child maltreatment. In: Levinson D, editor. Encyclopedia of Crime and Punishment. Vol. 1. Great Barrington, MA: Berkshire Reference Works; 2002. pp. 185–92. [Google Scholar]
- 42.Iwanami A, Sugiyama A, Kuroki N, Toda S, Kato N, Nakatani Y, Horita N, Kaneko T. Patients with methamphetamine psychosis admitted to a psychiatric hospital in Japan. A preliminary report. Acta. Psychiatr. Scand. 1994;89(6):428–432. doi: 10.1111/j.1600-0447.1994.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 43.Jefferson DJ. America’s most dangerous drug. [August 8];Newsweek. 2005 http://www.newsweek.com/id/56372. [PubMed] [Google Scholar]
- 44.Joe GW, Rowan-Szal GA, Simpson D, Greener JM, Vance J. Methamphetamine-user inmates in prison treatment: during treatment outcomes. J Subst. Abuse Treat. doi: 10.1016/j.jsat.2009.08.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karch SB. Synthetic stimulants. In: Karch SB, editor. Karch’s Pathology of Drug Abuse. 3rd Edition. Boca Raton, FL: CRC press; 2002. pp. 233–280. [Google Scholar]
- 46.Kaye S, McKetin R, Duflou J, Darke S. Methamphetamine and cardiovascular pathology: a review of the evidence. Addiction. 2007;102:1204–1211. doi: 10.1111/j.1360-0443.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 47.Kay-Lambkin FJ. Technology and innovation in the psychosocial treatment of methamphetamine use, and risk and dependence. Drug Alcohol Rev. 2008;27(3):318–325. doi: 10.1080/09595230801914768. [DOI] [PubMed] [Google Scholar]
- 48.King GR, Ellinwood EH. Amphetamines and other stimulants. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance Abuse: A Comprehensive Textbook. 4th Edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 277–302. [Google Scholar]
- 49.Klasser GD, Epstein J. Methamphetamine and its impact on dental care. J. Can. Dent. Assoc. 2005;71(10):759–762. 2005. [PubMed] [Google Scholar]
- 50.Kyle AD, Hansell B. The Meth epidemic in America: Two surveys of US Counties. Washington, DC: National Association of Counties (NACO); 2005. [Google Scholar]
- 51.Longshore D, Hawken A, Urada D, Anglin MD. SACPA cost analysis report (first and second years) Los Angeles: UCLA Integrated Substance Abuse Programs; 2006. Prepared for the Department of Alcohol and Drug Programs, California Health and Human Services Agency. [Google Scholar]
- 52.Low KG, Gendaszek AE. Illicit use of psychostimulants among college students: a preliminary study. Psychol. Health & Med. 2002;7(3):283–287. [Google Scholar]
- 53.Lundh H, Tunving K. An extrapyramidal choreiform syndrome caused by amphetamine addiction. J. Neurol. Neurosurg. Psychiatry. 1981;44:728–730. doi: 10.1136/jnnp.44.8.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacKenzie RG, Heischober B. Methamphetamine. Pediatr. Rev. 1997;18(9):305–309. doi: 10.1542/pir.18-9-305. [DOI] [PubMed] [Google Scholar]
- 55.Marinelli-Casey P, Gonzales R, Hillhouse M, Ang A, Zweben J, Cohen J, Hora PF, Rawson RA Methamphetamine Treatment Project Corporate Authors. Drug court treatment for methamphetamine dependence: treatment response and post-treatment outcomes. J. Subst. Abuse Treat. 2008;34(2):242–248. doi: 10.1016/j.jsat.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Mattson R, Calvery JR. Dextro-amphetamine-sulfate-induced dyskinesias. JAMA. 1968;204:108–110. [PubMed] [Google Scholar]
- 57.Maxwell JC, Rutkowski BA. The prevalence of methamphetamine and amphetamine abuse in North America: a review of the indicators, 1992–2007. Drug ad Alcohol Rev. 2008;27(3):229–235. doi: 10.1080/09595230801919460. [DOI] [PubMed] [Google Scholar]
- 58.McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100(1):96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- 59.McKay JR. Is there a case for extended interventions for alcohol and drug use disorders? Addiction. 2005;100(11):1594–1610. doi: 10.1111/j.1360-0443.2005.01208.x. [DOI] [PubMed] [Google Scholar]
- 60.McKetin R, McLaren J, Lubman DI, Hides L. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101(10):1473–1478. doi: 10.1111/j.1360-0443.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- 61.McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 62.McLellan AT, McKay JR, Forman R, Cacciola J, Kemp J. Reconsidering the evaluation of addiction treatment: from retrospective follow-up to concurrent recovery monitoring. Addiction. 2005;100:447–458. doi: 10.1111/j.1360-0443.2005.01012.x. [DOI] [PubMed] [Google Scholar]
- 63.Messina N, Grella C, Burdon W, Prendergast M. Childhood adverse events and current traumatic distress: a comparison of men and women prisoners. Crim. Justice Behav. 2007;34(11):1385–1401. [Google Scholar]
- 64.Messina N, Marinelli-Casey P, Hillhouse M, Rawson R, Hunter J, Ang A. Childhood adverse events and methamphetamine use among men and women. J. Psychoactive Drugs. 2008;(Suppl 5):399–409. doi: 10.1080/02791072.2008.10400667. [DOI] [PubMed] [Google Scholar]
- 65.Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79(2):273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Montoya ID, Vocci F. Novel medications to treat addictive disorders. Curr. Psychiatry Rep. 2008;10(5):392–398. doi: 10.1007/s11920-008-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.National Drug Intelligence Center (NDIC) Methamphetamine. National Drug Threat Assessment. 2003 Document ID: 2003-Q0317-001. Available at: http://www.usdoj.gov/ndic/pubs3/3300/
- 68.National Institute on Drug Abuse. Epidemiologic Trends in Drug Abuse. Community Epidemiology Work Group (CEWG). Advanced Report: June 2005. NIH Publication No. 05-5280A. Rockville, MD: U.S. Department of Health and Human Services, National Institutes of Health; 2005. http://www.drugabuse.gov/PDF/CEWG/AdvReport605.pdf. [Google Scholar]
- 69.National Survey of Child and Adolescent Well-Being (NSCAW) Research Group. Methodological lessons from the national survey of child and adolescent well-being: the first three years of the USA’s first national probability study of children and families investigated for abuse and neglect. Children and Youth Services Rev. 2002;24(6&7):513–541. [Google Scholar]
- 70.National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Summary of findings from the 2004 NSDUH. http://www.oas.samhsa.gov/nsduh/2k4nsduh/2k4Results/2k4Results.htm#toc. [Google Scholar]
- 71.Newton TF, De La Garza R, 2nd, Kalechstein AD, Nestor L. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacol. Biochem. Behav. 2005;82:90–97. doi: 10.1016/j.pbb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 72.Newton TF, Kalechstein AD, Hardy DJ, Cook IA, Nestor L, Ling W, Leuchter AF. Association between quantitative EEG and neurocognition in methamphetamine-dependent volunteers. Clin. Neurophysiol. 2004;115:194–198. doi: 10.1016/s1388-2457(03)00314-6. [DOI] [PubMed] [Google Scholar]
- 73.Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacol. 2006;31(7):1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- 74.Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J. The Economic Cost of Methamphetamine Use in the United States, 2005. Santa Monica, CA: Drug Policy Research Center, RAND Corporation; 2009. http://www.rand.org/pubs/monographs/MG829/ [Google Scholar]
- 75.Patterson TL, Semple SJ, Zians JK, Strathdee SA. Methamphetamine-using HIV-positive men who have sex with men: correlates of polydrug use. J. Urban Health. 2005;82(1) Suppl 1:i120–i126. doi: 10.1093/jurban/jti031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perez JA, Jr, Arsura EL, Strategos S. Methamphetamine-related stroke: four cases. J. Emerg. Med. 1999;17(3):469–471. doi: 10.1016/s0736-4679(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 77.Petit MR, Curtis PA. Child Abuse and Neglect: A Look at the States. Washington, DC: Child Welfare League of America Press; 1997. [Google Scholar]
- 78.Rawson RA, Gonzales RG, Brethen P. Treatment of methamphetamine use disorders: An update. J. Subst. Abuse Treat. 2002;23:145–150. doi: 10.1016/s0740-5472(02)00256-8. [DOI] [PubMed] [Google Scholar]
- 79.Rawson RA, Gonzales RG, Ling W. Methamphetamine abuse and dependence: an update. Dir. Psychiatr. 2006;26(3):221–236. [Google Scholar]
- 80.Rawson RA, Gonzales R, McCann MJ, Obert J. Methamphetamine use among treatment-seeking adolescents in Southern California: participant characteristics and treatment response. J Subst Abuse Treat. 2005;29:67–74. doi: 10.1016/j.jsat.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 81.Rawson RA, Marinelli-Casey P, Anglin MD, Dickow A, Frazier Y, Gallagher C, Herrell J, Huber A, McCann MJ, Obert J, Pennell S, Reiber C, Vandersloot D, Zweben J Methamphetamine Treatment Project Corporate Authors. Methamphetamine Treatment Project Corporate Authors. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99:708–717. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 82.Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 83.Rawson RA, Washton A, Domier CP, Reiber C. Drugs and sexual effects: role of drug type and gender. J Subst Abuse Treat. 2002;22:103–108. doi: 10.1016/s0740-5472(01)00215-x. [DOI] [PubMed] [Google Scholar]
- 84.Reback CJ, Larkins S, Shoptaw S. Methamphetamine abuse as a barrier to HIV medication adherence among gay and bisexual men. AIDS Care. 2003;15(6):775–785. doi: 10.1080/09540120310001618621. [DOI] [PubMed] [Google Scholar]
- 85.Rhee KJ, Albertson TE, Douglas JC. Choreoathetoid disorder associated with amphetamine-like drugs. Am. J. Emerg. Med. 1988;6:131–133. doi: 10.1016/0735-6757(88)90050-2. [DOI] [PubMed] [Google Scholar]
- 86.Richards JR, Bretz SW, Johnson EB, Turnipseed SD, Brofeldt BT, Derlet RW. Methamphetamine abuse and emergency department utilization. West. J. Med. 1999;170(4):198–202. [PMC free article] [PubMed] [Google Scholar]
- 87.Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- 88.Roll JM, Huber A, Sodano R, Chudzynski J, Moynier E, Shoptaw S. A comparison of five reinforcement schedules for use in contingency management-based treatment of methamphetamine abuse. Psycholog. Rec. 2006;56(1):67–81. [Google Scholar]
- 89.Santos AP, Wilson AK, Hornung CA, Polk HC, Rodriguez JL, Franklin GA. Methamphetamine laboratory explosions: a new and emerging burn injury. J. Burn Care Rehabil. 2005;26(3):228–232. [PubMed] [Google Scholar]
- 90.Scott CK, Dennis ML, Foss MA. Utilizing recovery management checkups to shorten the cycle of relapse, treatment reentry, and recovery. Drug Alcohol Depend. 2004;78:325–338. doi: 10.1016/j.drugalcdep.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol. Rev. 2007;17(3):275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 92.Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Kao UH, Wang PC, Bholat MA, Ling W. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J. Addict. Dis. 2008;27(1):13–23. doi: 10.1300/J069v27n01_02. [DOI] [PubMed] [Google Scholar]
- 93.Shoptaw S, Peck J, Reback CJ, Rotheram-Fuller E. Psychiatric and substance dependence comorbidities, sexually transmitted diseases, and risk behaviors among methamphetamine-dependent gay and bisexual men seeking outpatient drug abuse treatment. J. Psychoactive Drugs. 2003;35(Suppl 1):161–168. doi: 10.1080/02791072.2003.10400511. [DOI] [PubMed] [Google Scholar]
- 94.Shoptaw S, Reback CJ, Freese TE. Patient characteristics, HIV serostatus, and risk behaviors among gay and bisexual males seeking treatment for methamphetamine abuse and dependence in Los Angeles. J. Addict. Dis. 2002;21(1):91–115. doi: 10.1300/j069v21n01_08. [DOI] [PubMed] [Google Scholar]
- 95.Shoptaw S, Reback CJ. Associations between methamphetamine use and HIV among men who have sex with men: a model for guiding public policy. J. Urban Health. 2006;83(6):1151–1157. doi: 10.1007/s11524-006-9119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fuller E, Larkins S, Veniegas RC, Freese TE, Hucks-Ortiz C. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug Alcohol Depend. 2005;78(2):125–134. doi: 10.1016/j.drugalcdep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 97.Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9(3):222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- 98.Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Della Grotta S, Liu J, Lester BM. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118(3):1149–1156. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- 99.Sperling LS, Horowitz JL. Methamphetamine-induced choreoathetosis and rhabdomyolysis. Ann. Int. Med. 1994;121:986. doi: 10.7326/0003-4819-121-12-199412150-00019. [DOI] [PubMed] [Google Scholar]
- 100.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: Author; 2008. NSDUH Series H-34, DHHS Publication No. SMA08-4343. [Google Scholar]
- 101.Tiihonen J, Kuoppasalmi K, Föhr J, Tuomola P, Kuikanmäki O, Vorna H, Sokero P, Haukka J, Meririnne E. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am. J. Psychiatry. 2007;164:160–162. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- 102.Turnipseed SD, Richards JR, Kirk JD, Diercks DB, Amsterdam EA. Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J. Emerg. Med. 2003;24:369–373. doi: 10.1016/s0736-4679(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 103.Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann. N.Y. Acad. Sci. 2004;1025:279–287. doi: 10.1196/annals.1316.035. [DOI] [PubMed] [Google Scholar]
- 104.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Francheschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatry. 2001a;158(3):377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 105.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J. Neurosci. 2001b;21(23):9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am. J. Psychiatry. 2004;161(2):242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- 107.Westover AN, McBride S, Haley RW. Stroke in young adults who abuse amphetamines or cocaine: a population-based study of hospitalized patients. Arch. Gen. Psychiatry. 2007;64(4):495–502. doi: 10.1001/archpsyc.64.4.495. [DOI] [PubMed] [Google Scholar]
- 108.Westover AN, Nakonezny PA, Haley RW. Acute myocardial infarction in young adults who abuse amphetamines. Drug Alcohol Depend. 2008;96(1–2):49–56. doi: 10.1016/j.drugalcdep.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Winslow BT, Voorhees KI, Pehl KA. Methamphetamine abuse. Am. Fam. Physician. 2007;76(8):1169–1174. [PubMed] [Google Scholar]
- 110.Wong W, Chaw JK, Kent CK, Klausner JD. Risk factors for early syphilis among gay and bisexual men seen in an STD clinic: San Francisco, 2002–2003. Sex. Transm. Dis. 2005;32:458–463. doi: 10.1097/01.olq.0000168280.34424.58. [DOI] [PubMed] [Google Scholar]
- 111.Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M. Psychiatric symptoms in methamphetamine users. Am. J. Addict. 2004;13(2):181–190. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]