Summary
Ovarian cancer (OC) is most often contained within the peritoneal cavity, making it an ideal disease for adenoviral-delivered gene therapies. In effort to develop a safe and effective gene therapy for OC, we created a replication deficient adenovirus bearing the herpes simplex thymidine kinase (HSV-tk) gene under direction of the tumor specific promoter human epididymis protein 4 (HE4). The purpose of this study was to investigate the ability of our adenoviral construct to transduce OC cells in vitro and mediate transgene expression of HSV-tk, thereby sensitizing OC to the pro-drug ganciclovir. Cisplatin-sensitive (CS) and -resistant (CR) A2780 OC cells, infected with virus for 6 hours at 100, 500, and 1000 multiplicity of infection followed by ganciclovir treatment every other day for 5 days, were assayed for cell viability. Adenoviral-mediated transgene expression increased with increasing amounts of virus and peaked at 48 hours after transduction in both A2780-CS and -CR. Unexpectedly, ganciclovir alone was slightly toxic to both A2780 cell lines (IC50 of 234.9 μg/mL and 257.2 μg/mL in A2780-CS and –CR, respectively). Transduction with ADV-HE4-HSV-tk followed by ganciclovir treatment increased (P<0.05) cell killing up to ten-fold, lowering the IC50 to 23.9 μg/mL and 32.6 μg/mL in A2780-CS and –CR, respectively, at 1000 multiplicity of infection. The results support the potential use of this approach as a gene therapy for OC, a disease that accounts for more deaths than any other cancer of the female reproductive system.
Keywords: ovarian cancer, gene therapy, adenovirus, herpes simplex virus thymidine kinase
I. Introduction
Ovarian cancer (OC) continues to be the deadliest of the gynecologic cancers. Despite continued research and technological advances, the standard treatment for OC has remained essentially unchanged, consisting of surgical de-bulking followed by treatment with platinum-based chemotherapies. Although most OC patients initially respond to current treatments, the large majority of patients experience fatal relapses as a result of chemo-resistance (Vaughan et al, 2011). Due to lack of symptoms and routine screening, the vast majority of OC cases are not diagnosed until they have already progressed to advanced stages resulting in a relative 5-year survival rate of less than 30% (Howlader N, 1975-2010).
Gene therapy, widely considered a promising treatment in the fight against cancer (Lam et al, 2013), specifically targets and kills cancer cells with minimal toxicity to normal cells. In particular, the herpes simplex virus thymidine kinase (HSV-tk) is a well-known suicide gene which has the ability to preferentially mono-phosphorylate the pro-drug ganciclovir (GCV) (Elion et al, 1977). GCV can then be further di- and triphosphorylated by host kinases allowing incorporation into elongating DNA chains resulting in chain termination and apoptosis (Freeman et al, 1993). Furthermore, GCV-triphosphate is often transferred to neighboring cells via gap junctions, a phenomenon known as the bystander effect, resulting in cell death when as little as 10% of the cell population is HSV-tk positive (Mesnil and Yamasaki, 2000). While the overall effectiveness of HSV-tk/GCV has been demonstrated (Dey and Evans, 2011; Fillat et al, 2003), limitations include inefficient gene delivery and lack of tumor specificity.
OC is most often contained within the abdominal cavity making it an ideal candidate for intraperitoneal delivery of recombinant viral vectors (Kim et al, 2012). Adenoviral vectors (ADV) have the ability to infect a broad range of dividing and non-dividing cells and are considered safe due to lack of chromosomal integration. ADV also offers a large cloning capacity (up to 8kb) and the ability to facilitate gene expression in as little as 12 hours after infection. The proficiency of ADV to infect cells is dependent upon the presence of coxsackievirus and adenovirus receptor (CAR) of which many tumor cells including OC cells express low levels (Kim et al, 2002). However, CAR-independent gene transfer can be mediated by the incorporation of an Arg-Gly-Asp (RGD) peptide on the fiber knob, which allows the virus to utilize an alternative receptor during the cell entry process resulting in more efficient transduction (Dmitriev et al, 1998). In order to combine efficient gene transfer, safety, and tumor specificity, we created an RGD-modified adenovirus bearing the HSV-tk gene driven by an OC-specific promoter.
The human epididymis protein 4 (HE4), normally expressed in the epididymis (Hellstrom et al, 2003; Kirchhoff, 1998), is over-expressed in 93% of serous and 100% of endometrioid epithelial ovarian cancer (Drapkin et al, 2005). HE4 has been shown to play an important role in OC cell adhesion and motility (Lu et al, 2012). Additionally, a specific region of the HE4 promoter (-530 bp from the ATG start site) was shown to be highly transcriptionally active in various OC cell lines with minimal activity in normal tissue (Berry et al, 2004). This region of the HE4 promoter has successfully been used to drive expression of a deadly toxin in mice bearing ovarian tumors resulting in decreased tumor burden and extended survival (Huang et al, 2009). HE4 has recently been identified as an early detection biomarker for OC (Moore et al, 2008). In conjunction with the typically used CA125, HE4 has aided in distinguishing between benign and malignant tumors, predicting OC progressiveness, and been shown to correctly diagnose the aggressive type II EOC at all stages and ages (Kalapotharakos et al, 2012; Kristjansdottir et al, 2013; Lin et al, 2012; Steffensen et al, 2012; Trudel et al, 2012). Based on these findings, the HE4 promoter may be a promising gene therapy approach tailored specifically toward patients with high serum levels of HE4. Toward this objective, the goal of the current study was to examine the ability of our ADV-HE4-HSV-tk construct to efficiently transduce OC and drive expression of HSV-tk, thereby creating a safe and tumor specific gene therapy.
II. Materials and Methods
A. Plasmid construction and sequence verification
The plasmids pDRIVE-hHE4ZeoR and pORF9-HSV1-tkShAmpR were purchased (InvivoGen, San Diego, CA) and double-digested with restriction enzymes NcoI/NheI, The HSV1-tk:Shble fragment (1587bp) was ligated into the pDRIVE-hHE4 backbone and double digestion confirmed correct size and orientation. PCR was performed using primers (set 1 forward - 5’CCTGATCCTGGGGGATT GTG and set 1 reverse - 5’GCAGTAGCGTG GGCATTTTC, and set 2 forward - 5’CGA GGAGCAGGACTGACC and set 2 reverse - 5’ATACCTGTCCGCCTTTCTCC) for verification of cloning. Samples were prepared and validated (100% match, correct orientation, no insertions/ deletions) by sequencing at the ISU Molecular Research Core Facility (Applied Biosystems 3130XL Capillary DNA Sequencer, Pocatello, ID).
B. Adenovirus construction and fluorescent imaging
A replication deficient (DE1/E3) and fiber modified (RGD) marker human adenovirus type 5 (ADV-CMV-GFP, 1×1010 PFU/mL) was used (Vector Biolabs, Philadelphia, PA) to test infection in OC cells. To create ADV-HE4-tk, pHE4-HSV1-tk:Shble (2506 bp) was excised using SpeI/SwaI, gel purified, then ligated into the DUAL-BASIC-EGFP Shuttle Vector (provided by Vector BioLabs) that had been linearized with BAMHI/XbaI. Production and purification of ADV-HE4-tk (3.5×1010 PFU/mL) was carried out by Vector BioLabs. Cells were seeded at 20,000cells/35mm2 dish, then infected the next day with 10 to 1000 MOI. After 24 hours, virus-infected media was replaced with fresh media and live images of cells expressing GFP were taken using a fluorescent confocal microscope (Fluoview, FV10i, Olympus) at 24, 48, and 72 hours after infection. Constant image parameters were maintained.
C. Cell culture, drug treatment, and cell toxicity assays
All cells were maintained in RPMI 1640 media (Corning Cellgro) containing 10% FBS and 2mM L-glutamine, 100U/mL penicillin and 100μg/mL Streptomycin (Corning Cellgro), at 37°C and 5% CO2. Cisplatin-sensitive and -resistant A2780 OC cells were described previously (Li et al, 2009). Ganciclovir (GCV; InvivoGen) was reconstituted to 10 mg/mL according to manufacture specifications for use in cell viability assays; cells were seeded at 1,000 cells/well in 100uL media on 96-well plates (polystyrene flat-bottom w/lid, tissue culture treated, Falcon) and infected the following day with 100, 500, or 1000 MOI for 6 hours, then virus media was removed and replaced with media containing GCV (from 10 to 200 μg/mL). Fresh GCV was added every other day. Toxicity assays using WTS-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetazolium, monosodium salt] (CCK-8 kit, Dojindo Laboratories, Santa Clara, CA) were performed on day 6. Absorbance was read at 450nm using a BioTek ELx800 microplate reader. Values were normalized to the average of untreated cells. All assays were performed independently in at least duplicate.
Prism software was used for graphing and statistical analysis (two-factor ANOVA analysis). All IC50 calculations were made by plotting log (inhibitor) vs. normalized response with a variable slope for P<0.05 (degrees freedom = 22).
D. Western blot analysis of HSV-tk protein expression
Cells were seeded at 250,000 cells/10 cm2 dish, allowed to incubate for a day, then infected the following day with ADV-HE4-tk at 100, 500, or 1000 MOI. After 48 hours, cells were pelleted for protein isolation and quantification (BioRad DC Protein Assay). Samples were diluted to the same concentration, mixed 5:1 using 5X SDS loading buffer, heated at 95°C for 5min, then 15 μL loaded and run on 12% bis-acrylamide gel using BioRad Miniprotein box. Samples were transferred to a nitrocellulose membrane, blocked (5% non-fat milk in TBS-T (.05% Tween) for 1 hour at room temperature, washed, and incubated with primary antibodies overnight at 4°C (HSV-1 Thymidine Kinase; cat: sc-28037, 1:200 dilution or GAPDH; cat: sc-25778, 1:500 dilution, Santa Cruz, Dallas, TX). Membranes were incubated (1 hour at RT) with secondary antibodies (HRP donkey anti-goat IgG, sc-2020, 1:2000 dilution, Santa Cruz or HRP goat anti-rabbit, #31460, 1:5000 dilution, Fisher), washed, incubated for 5 min. using BioRad Clarity Western ECL Substrate, and imaged (inverse digital images shown) side by side for chemiluminescence using a Gel Logic 2200 and Carestream Molecular Imaging Software.
III. Results and Discussion
In order to develop a potential gene therapy for OC, we utilized the HE4 promoter to drive expression of HSV-tk. An RGD-modified adenovirus was chosen as the mechanism of delivery in vitro. Experiments were carried out using the A2780 OC cell line, which was previously used in other studies that employed the HE4 promoter to drive high exogenous protein expression (Berry et al, 2004; Huang et al, 2009). Another advantage to using the A2780 cells was the availability of both cisplatin-sensitve (CS) and –resistant (CR) cell lines, which allowed us to test our construct in an aggressive drug-resistant model (Li et al, 2009). Once infectivity and viral-mediated HSV-tk expression were confirmed, cell viability assays were performed to evaluate the cell killing ability of ADV-HE4-HSV-tk upon treatment with GCV.
A. Adenoviral transduction
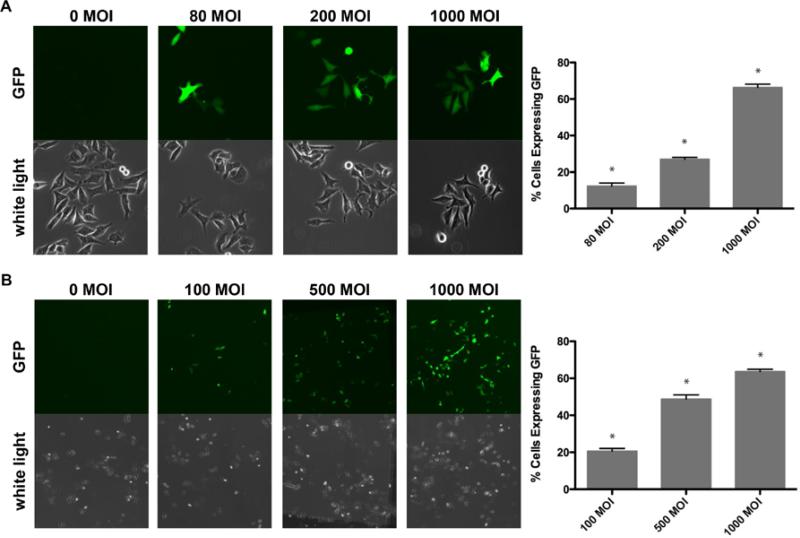
Initially, the marker ADV-CMV-GFP (Figure 1A) was tested in A2780-CS and A2780-CR cells, to demonstrate feasibility of the adenovirus to transduce OC cells and produce sufficient gene expression. GFP expression in cells transduced with ADVCMV-GFP was observed as early as 12 hours after infection at as little as 10 MOI, yet the highest (P<0.5) GFP expression was achieved using 1000 MOI (Figure 2A). The percent of cells expressing GFP increased (P<0.5) with increasing MOI in a titre-dependent manner (Figure 2A). No visible difference was detected in the ability of the ADV-CMV-GFP to infect and mediate GFP expression in A2780-CS versus -CR.
Figure 1. Construction of ADV-HE4-tk.
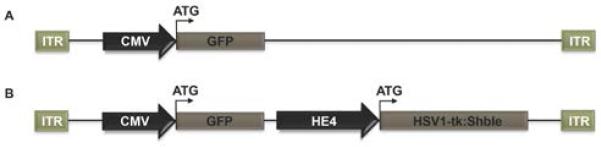
(A) A replication deficient (dE1/E3) and RGD fiber modified marker human adenovirus type 5 bearing the CMV promoter driven-GFP gene was initially used to test infectivity in A2780-CS and -CR ovarian cancer cells. (B) Once infectivity and GFP expression was established, ADV-HE4-tk was created by inserting the HE4 promoter-driven HSV1-tk:Shble gene.
Figure 2. Virus-mediated GFP expression with increasing MOI.
A2780-CS cells were seeded at (A) 30,000 cells/35mm2 dish, then infected the following day with marker ADV-CMV-GFP at various MOI (0, 80, 200, or 1000) or (B) seeded at 20,000 cells/35mm2 dish, then infected the following day with ADV-HE4-tk at various MOI (0, 100, 500, or 1000). After 24 hours, virus media was removed and replaced with fresh media, and pictures were taken of live cells using a fluorescent confocal microscope (A) 40x magnification (B) 10x magnification. (* indicates statistically different groups, P<.05)
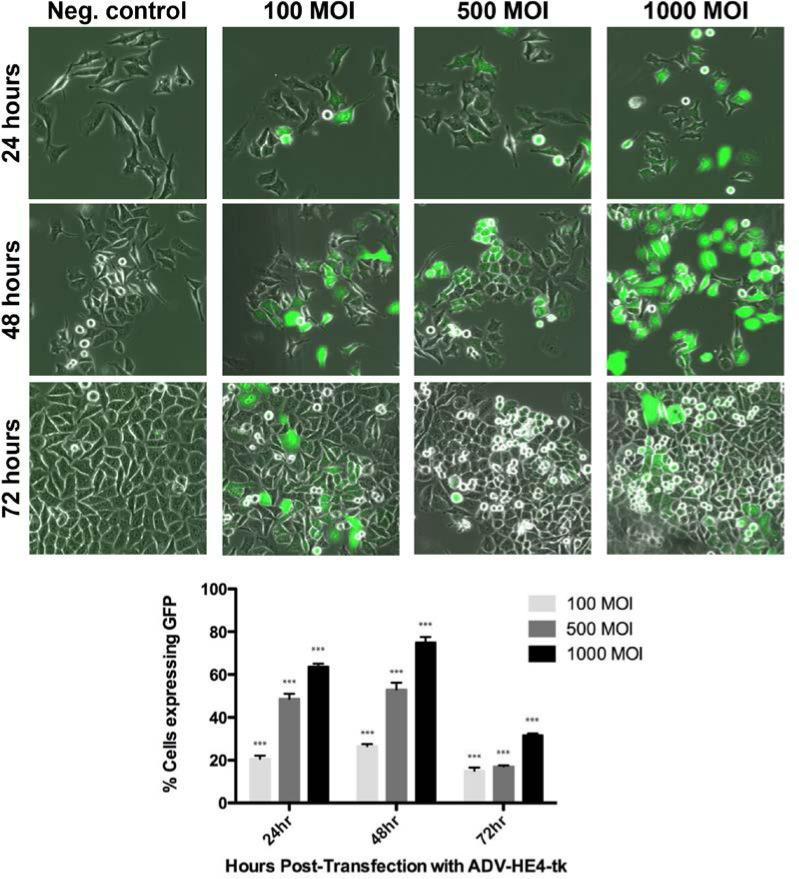
Once it was established that the marker adenovirus could successfully infect and mediate GFP expression, similar infectivity experiments were carried out using the ADVHE4-HSV-tk construct (Figure 1B), which also contained the GFP marker gene. Transduction with the ADV-HE4-HSV-tk construct yielded similar results in both cell lines, and the percentage of cells expressing GFP increased (P<0.05) with increasing MOI (Figure 2B). By examining the ADV-HE4-tk mediated expression of GFP over time and at increasing MOI, we were able to determine that the highest (P<0.05) GFP expression occurred using 1000 MOI at 48 hours after infection (Figure 3). Thus, the expression of HSV-tk under direction of the HE4 promoter should similarly peak around 48 hours. Western blot analysis for HSV-tk expression in both A2780 cell lines, performed 48 hours after infection with ADV-HE4-tk, confirmed that HE4 promoter driven HSV-tk expression was present and increased with increasing MOI (Figure 4).
Figure 3. GFP expression over time with increasing MOI using ADV-HE4-tk.
A2780-CS cells were seeded 20,000 cells/35mm2 dish, then infected the following day with ADV-HE4-tk. After 24 hours infection time, virus media was removed and replaced w/fresh media and pictures were taken of live cells 24, 48, and 72 hours after infection with 0, 100, 500, or 1000 MOI using a fluorescent confocal microscope, 30x magnification. (*** indicates statistically different groups, P<.01)
Figure 4. HSV-tk:Shble expression in ovarian cancer cells.
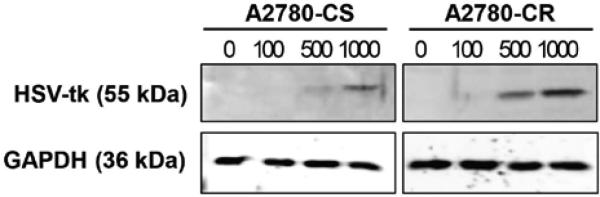
Western blot analysis was performed on protein isolated from A2780-CS and -CR cells 48 hours after transduction with ADV-HE4-tk with MOI ranging from 0 to 1000. Cells were seeded 250,000 cells per 10cm2 dish and allowed to recover for 24 hours. Virus-infected media was added at various MOI, incubated for 48 hours, and western blot analysis was performed.
B. Cell Viability Assays
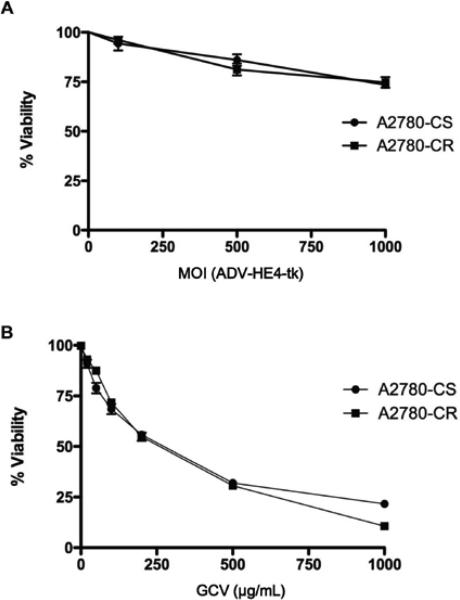
Toxicity assays were performed to assess the effect of ADV-HE4-tk on the A2780 cells without drug treatment. The percent viability of cells transduced using 100, 500, or 1000 MOI of ADV-HE4-tk decreased only slightly as the MOI increased (Figure 5A). The ADVHE4-tk incubation time (from 6 hours up to 24 hours incubation time) had no discernible effect on viability, and the virus was minimally toxic up to 1000 MOI.
Figure 5. ADV-HE4-tk viability and GCV sensitivity.
A2780-CS and –CR cells were seeded at 1,000 cells/well on 96-well plates. (A) Cells were infected on day 2 with ADV-HE4-tk at 100, 500, or 1000 MOI. After 6 hours incubation time, virus media was removed and replaced with fresh media. Media was replaced every other day. WTS-8 viability test was performed on day 6. (B) Cells were treated with increasing concentrations of GCV up to 1000 μg/mL starting 48 hours after seeding. Fresh drug was added every other day. WTS-8 viability tests were performed on day 6. Absorbance was read at 450 nm, and results show average viability normalized to untreated cells. Independent experiments were performed at least in duplicate (number of independent samples for each concentration/MOI = 24).
Surprisingly, treatment of both cell lines with GCV alone (Figure 5B) resulted in significant (P<0.05) cell death. Because GCV is recognized as a non-toxic pro-drug and converted into the monophosphate form only in the presence of herpes simplex viral thymidine kinases, we examined the A2780 cell lines for basal (endogenous) HSV-tk expression using whole transcriptome analysis, as described previously (Miller et al, 2013). The results revealed a low level of HSV1-tk and HSV2-tk in both the A2780-CS and –CR (supplemental figure 1). A search of the literature revealed only one paper that briefly (table 1 legend) mentioned sensitivity of A2780 cells to GCV alone (Selvakumaran et al, 2001). OC cell lines are not typically tested for Herpes Simplex Virus by the ATCC or the ECACC, yet our findings suggest epithelial derived cell lines may in fact be positive for the virus and should be tested for HSV-tk expression when performing studies using the HSV-tk/GCV system.
Table 1.
IC50 (μg of GCV/mL media) of ADV-HE4-tk transduced cells. A2780-CS and –CR cells were incubated with increasing amount of virus for 24 hours, then treated every other day with GCV followed by a WTS-8 cell viability test on day 6. Statistical summary of the IC50 values in both A2780-CS and -CR cells from figure 6 (P=.05).
| IC50 | 95% Confidence Interval | Fold Decrease | Log IC50 | Std. Error | Deg. Freedom | |
|---|---|---|---|---|---|---|
| A2780-CS | ||||||
| 0 MOI | 257.2 | 210.4 to 314.3 | 2.910 | 0.043 | 28 | |
| 100 MOI | 64.6 | 59.9 to 69.6 | 4.0 | 1.816 | 0.016 | 28 |
| 500 MOI | 41.0 | 37.6 to 44.7 | 6.3 | 1.613 | 0.018 | 28 |
| 1000 MOI | 32.6 | 28.9 to 36.7 | 7.9 | 1.513 | 0.026 | 28 |
| A2780-CR | ||||||
| 0 MOI | 234.9 | 208.3 to 265.0 | 2.371 | 0.026 | 28 | |
| 100 MOI | 87.4 | 79.5 to 96.0 | 2.7 | 1.942 | 0.020 | 28 |
| 500 MOI | 57.9 | 53.3 to 62.9 | 4.1 | 1.763 | 0.018 | 28 |
| 1000 MOI | 23.9 | 21.0 to 27.1 | 9.8 | 1.378 | 0.027 | 28 |
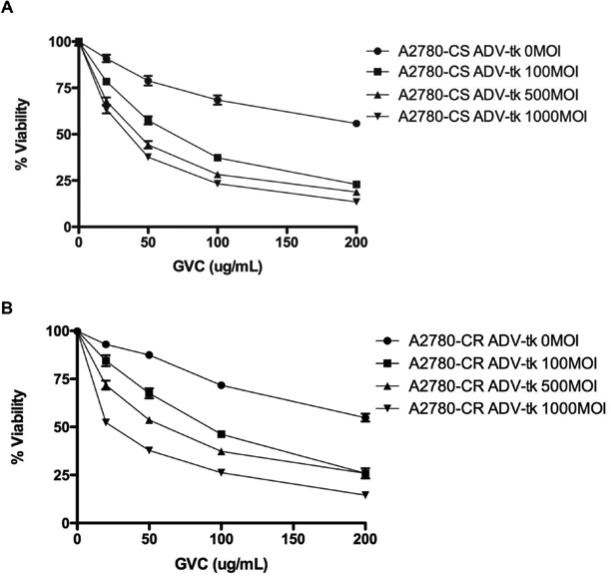
Despite results indicating OC sensitivity to GCV, the effect of GCV treatment in combination with ADV-HE4-HSV-tk was examined. We were able to further sensitize the A2780-CS as well as A2780-CR to GCV (Figure 6 & Table 1) resulting in decreased IC50 upon transduction with virus (Table 1). The IC50 in A2780-CS cells decreased (P<0.05) from 257 μg GCV/mL to 33 μg GCV/mL (7.9-fold decrease) upon transduction with 1000 MOI using ADV-HE4-tk, and a similar decrease was observed in the IC50 of A2780-CR cells from 234.9 μg GCV/mL to 23.9 μg GCV/mL (9.8-fold decrease). These results indicate that the HE4 promoter was transcriptionally active and able to drive HSV-tk expression.
Figure 6. ADV-HE4-tk sensitizes ovarian cancer cells to GCV.
(A) A2780-CS and (B) A2780-CR cells were seeded at 1,000 cells/well on 96-well plates, then infected the following day with ADV-HE4-tk at 100, 500, or 1000 MOI. After 6 hours incubation time, virus media cells were treated with GCV as indicated. Fresh drug was replaced every other day. WTS-8 viability tests were performed on day 6. Absorbance was read at 450 nm, and results show the average viability normalized to untreated cells. Independent experiments were performed at least in duplicate. (See Table 1 for statistical summary of the IC50 values).
It is concluded that even though low levels of endogenous HSV-tk were already present in this particular cell line, the HE4 promoter was accessible and sufficiently active to drive further expression of HSV-tk, facilitating increased cell death.
We treated both infected and uninfected A2780 cells with Zeocin. After 6 days, the majority of the uninfected cells had died while the cells infected with ADV-HE-tk (using 500 and 1000 MOI) showed increased ability to survive in the presence of up to 500 μg Zeocin/mL (data not shown). The resultant resistance to Zeocin upon transduction with ADV-HE4-tk strongly suggested that the HE4 promoter was able to drive HSV-tk:Sh ble expression, and that the increased sensitization of A2780 cells to GCV was viral-mediated via the HE4 promoter, and not solely the presence of endogenous HSV-tk.
IV. Conclusions
We have created an RGD fiber modified adenovirus bearing a potential gene therapy for OC. This therapy involves the use of the OC-specific promoter HE4 to drive expression of the suicide HSV-tk gene in cisplatin-sensitive and resistant A2780 cells, thus sensitizing the OC cells to GCV. We demonstrate that both cell lines are susceptible to adenoviral transduction with ADV-HE4-tk, by means of GFP expression as a marker, and exhibit cytotoxicity in response to HE4 promoter driven HSV-tk upon treatment with GCV.
The percent cell viability was titre-dependent, with increased cell killing corresponding to increased MOI. This adenoviral-delivered therapy is tailored specifically towards being able to treat OC patients identified with high levels of serum HE4, which is often an indicator of tumor aggressiveness and progression.
Future work involves examining the effectiveness of this therapy in other cell lines that have high genetic similarity to high-grade serious OC tumors (Domcke et al, 2013). Positive results would merit further testing in vivo by treating mice bearing ovarian tumors with intraperitoneal injections of ADV-HE4-tk. In an effort to achieve more sustained transgene expression and minimal inflammatory immune response or hepatic toxicity, long term studies may also involve the creation of a recombinant adeno-associated virus (Bui Nguyen et al, 2010; Lisowski et al, 2012) to deliver the HE4-tk gene.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the contributions of John Griffith for helping to facilitate research efforts, Dr. Nicholas Berry for helpful discussion, and BYU-Idaho for use of facilities and lab instrumentation.
The project described was supported by the INBRE Program, NIH Grant Nos. P20 RR016454 (National Center for Research Resources) and P20 GM103408 (National Institute of General Medical Sciences) and NCI- U54 CA113001. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
References
- Berry NB, Cho YM, Harrington MA, Williams SD, Foley J, Nephew KP. Transcriptional targeting in ovarian cancer cells using the human epididymis protein 4 promoter. Gynecol Oncol. 2004;923:896–904. doi: 10.1016/j.ygyno.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Bui Nguyen TM, Subramanian IV, Xiao X, Nguyen P, Ramakrishnan S. Adeno-associated virus-mediated delivery of kringle 5 of human plasminogen inhibits orthotopic growth of ovarian cancer. Gene Ther. 2010;175:606–615. doi: 10.1038/gt.2010.15. [DOI] [PubMed] [Google Scholar]
- Dey D, Evans GRD. Suicide Gene Therapy by Herpes Simplex Virus-1 Thymidine Kinase (HSV-TK) 2011.
- Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT. An Adenovirus Vector with Genetically Modified Fibers Demonstrates Expanded Tropism via Utilization of a Coxsackievirus and Adenovirus Receptor-Independent Cell Entry Mechanism. Journal of Virology. 1998;7212:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4 doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, Hecht JL. Human Epididymis Protein 4 (HE4) Is a Secreted Glycoprotein that Is Overexpressed by Serous and Endometrioid Ovarian Carcinomas. Cancer Research. 2005;656:2162–2169. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- Fillat C, Carrio M, Cascante A, Sangro B. Suicide gene therapy mediated by the Herpes Simplex virus thymidine kinase gene/Ganciclovir system: fifteen years of application. Curr Gene Ther. 2003;31:13–26. doi: 10.2174/1566523033347426. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL, Abraham GN. The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;5321:5274–5283. [PubMed] [Google Scholar]
- Hellstrom I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, Drescher C, Urban N, Hellstrom KE. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;6313:3695–3700. [PubMed] [Google Scholar]
- Howlader N NA, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975-2010. [Google Scholar]
- Huang YH, Zugates GT, Peng W, Holtz D, Dunton C, Green JJ, Hossain N, Chernick MR, Padera RF, Jr., Langer R, Anderson DG, Sawicki JA. Nanoparticle-delivered suicide gene therapy effectively reduces ovarian tumor burden in mice. Cancer Res. 2009;6915:6184–6191. doi: 10.1158/0008-5472.CAN-09-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalapotharakos G, Asciutto C, Henic E, Casslen B, Borgfeldt C. High preoperative blood levels of HE4 predicts poor prognosis in patients with ovarian cancer. J Ovarian Res. 2012;51:20. doi: 10.1186/1757-2215-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Dmitriev I, O'Malley JP, Wang M, Saddekni S, You Z, Preuss MA, Harris RD, Aurigemma R, Siegal GP, Zinn KR, Curiel DT, Alvarez RD. A Phase I Clinical Trial of Ad5.SSTR/TK.RGD, a Novel Infectivity-Enhanced Bicistronic Adenovirus, in Patients with Recurrent Gynecologic Cancer. Clinical Cancer Research. 2012;1812:3440–3451. doi: 10.1158/1078-0432.CCR-11-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Zinn KR, Barnett BG, Sumerel LA, Krasnykh V, Curiel DT, Douglas JT. The therapeutic efficacy of adenoviral vectors for cancer gene therapy is limited by a low level of primary adenovirus receptors on tumour cells. European Journal of Cancer. 2002;3814:1917–1926. doi: 10.1016/s0959-8049(02)00131-4. [DOI] [PubMed] [Google Scholar]
- Kirchhoff C. Molecular characterization of epididymal proteins. Rev Reprod. 1998;32:86–95. doi: 10.1530/ror.0.0030086. [DOI] [PubMed] [Google Scholar]
- Kristjansdottir B, Levan K, Partheen K, Sundfeldt K. Diagnostic performance of the biomarkers HE4 and CA125 in type I and type II epithelial ovarian cancer. Gynecologic Oncology. 2013;1311:52–58. doi: 10.1016/j.ygyno.2013.07.094. [DOI] [PubMed] [Google Scholar]
- Lam P, Khan G, Stripecke R, Hui KM, Kasahara N, Peng KW, Guinn BA. The innovative evolution of cancer gene and cellular therapies. Cancer Gene Ther. 2013;203:141–149. doi: 10.1038/cgt.2012.93. [DOI] [PubMed] [Google Scholar]
- Li M, Balch C, Montgomery J, Jeong M, Chung J, Yan P, Huang T, Kim S, Nephew K. Integrated analysis of DNA methylation and gene expression reveals specific signaling pathways associated with platinum resistance in ovarian cancer. BMC Medical Genomics. 2009;21:34. doi: 10.1186/1755-8794-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Qin J, Sangvatanakul V. Human epididymis protein 4 for differential diagnosis between benign gynecologic disease and ovarian cancer: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2012;712:00502–00507. doi: 10.1016/j.ejogrb.2012.10.036. [DOI] [PubMed] [Google Scholar]
- Lisowski L, Lau A, Wang Z, Zhang Y, Zhang F, Grompe M, Kay MA. Ribosomal DNA Integrating rAAV-rDNA Vectors Allow for Stable Transgene Expression. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;2010:1912–1923. doi: 10.1038/mt.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Sun X, Xiao R, Zhou L, Gao X, Guo L. Human epididymis protein 4 (HE4) plays a key role in ovarian cancer cell adhesion and motility. Biochem Biophys Res Commun. 2012;4192:274–280. doi: 10.1016/j.bbrc.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Mesnil M, Yamasaki H. Bystander Effect in Herpes Simplex Virus-Thymidine Kinase/Ganciclovir Cancer Gene Therapy: Role of Gap-junctional Intercellular Communication1. Cancer Research. 2000;6015:3989–3999. [PubMed] [Google Scholar]
- Miller DF, Yan PS, Buechlein A, Rodriguez BA, Yilmaz AS, Goel S, Lin H, Collins-Burow B, Rhodes LV, Braun C, Pradeep S, Rupaimoole R, Dalkilic M, Sood AK, Burow ME, et al. A new method for stranded whole transcriptome RNA-seq. Methods. 2013;632:126–134. doi: 10.1016/j.ymeth.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, Steinhoff M, Messerlian G, DiSilvestro P, Granai CO, Bast RC., Jr The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecologic Oncology. 2008;1082:402–408. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Selvakumaran M, Bao R, Crijns AP, Connolly DC, Weinstein JK, Hamilton TC. Ovarian epithelial cell lineage-specific gene expression using the promoter of a retrovirus-like element. Cancer Res. 2001;614:1291–1295. [PubMed] [Google Scholar]
- Steffensen KD, Waldstrom M, Brandslund I, Petzold M, Jakobsen A. The prognostic and predictive value of combined HE4 and CA-125 in ovarian cancer patients. Int J Gynecol Cancer. 2012;229:1474–1482. doi: 10.1097/IGC.0b013e3182681cfd. [DOI] [PubMed] [Google Scholar]
- Trudel D, Tetu B, Gregoire J, Plante M, Renaud MC, Bachvarov D, Douville P, Bairati I. Human epididymis protein 4 (HE4) and ovarian cancer prognosis. Gynecol Oncol. 2012;912:00736–00736. doi: 10.1016/j.ygyno.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Vaughan S, Coward JI, Bast RC, Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, Friedlander M, Gabra H, Kaye SB, Lord CJ, Lengyel E, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;1110:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.