Abstract
In bumble bees (Bombus spp.), where workers within the same colony exhibit up to a tenfold difference in mass, labor is divided by body size. Current adaptive explanations for this important life history feature are unsatisfactory. Within the colony, what is the function of the smaller workers? Here, we report on the differential robustness to starvation of small and large worker bumble bees (Bombus impatiens); when nectar is scarce, small workers remain alive significantly longer than larger workers. The presence of small workers, and size variation in general, might act as insurance against times of nectar shortage. These data may provide a novel, adaptive explanation, independent of division of labor, for size polymorphism within the worker caste.
Keywords: Polymorphism, Robustness, Social insects, Bumble bees, Bombus impatiens
Introduction
For organisms that are indisputable ecological successes, biologists have devoted decades to examining what traits might contribute fitness advantages. Have those traits evolved for specific purposes or were they successfully co-opted at a later time? How might characteristics confer a positive fitness consequence on the evolutionary lifespan of a genus? One of the most successful groups of organisms is the social Hymenoptera. Ants, bees, and wasps are ecologically dominant, species-rich, and contribute a very large proportion to the terrestrial biomass (Oster and Wilson, 1978; Wilson, 1987; Hölldobler and Wilson, 1990; Michener, 2000; Chapman and Bourke, 2001). In addition, social insects frequently display many interesting and unique life history traits whose adaptive function remains unclear.
For example, a bumble bee (Bombus spp.) worker may be up to tenfold greater in mass than her sister (Fig. 1) from the same colony (Cumber, 1949; Plowright and Jay, 1968; Alford, 1975; Goulson et al., 2002). This size polymorphism, symmetric about a single mean, is present throughout the colony cycle and independent of resource availability (Couvillon et al., submitted). Its presence is linked with division of labor within a bumble bee colony; the larger workers tend to forage, whereas smaller workers concentrate on intranidal tasks like nursing and incubating (Cumber, 1949; Goulson et al., 2002; Foster et al., 2004; Gardner et al., 2007; Jandt and Dornhaus, 2009). However, is effective division of labor the evolutionary reason for size polymorphism? There are good data to demonstrate that larger workers are better at foraging compared to smaller workers; larger foragers bring back more nectar per unit time (Goulson et al., 2002; Spaethe and Weidenmuller, 2002), fly at cooler temperatures (Free and Butler, 1959), probe deeper flowers (Peat et al., 2005), and may be less prone to predation (Goulson et al., 2002). However, if size polymorphism in bumble bees is an adaptation for division of labor, we would predict that smaller workers should also be better at their tasks. This has not yet been shown. In fact, there is even evidence to suggest that smaller workers are also less efficient at nursing compared to larger workers (Cnaani and Hefetz, 1994). Why then is size polymorphism such a consistent feature in Bombus?
Fig. 1.
Though bumble bee (Bombus impatiens) workers are highly related (r = 0.75), there may be a tenfold difference in mass between sisters of the same nest
Bumble bees evolved in temperate and alpine climates (Hines, 2008), which can experience rainy spells of many days or even weeks in which foraging is impossible. Bumble bee colonies are annual (Cumber, 1949) and do not store sufficient food to last for extended periods; thus, during such periods, a colony could potentially starve. However, if even a fraction of the workers remains alive, this could have positive fitness consequences, as they may raise remaining brood and also lay male eggs, ensuring a genetic contribution for the next generation.
Here we ask whether an individual worker’s physiology might preferentially predispose her to survive a nectar shortage. Specifically, we tested whether differential survival to starvation might be correlated with worker body size.
Methods
We obtained four queenright bumble bee colonies (B. impatiens, colonies 1–4) from a commercial breeder (Koppert Biological Systems, Romulus, MI, USA). At the start of the experiment, colonies typically had 15–30 workers plus queen with brood; each colony at its peak size had approximately 350 individual workers with natural body size variation (Cnaani et al., 2002) plus brood and honey stores (Fig. 2). Colonies were housed in wooden boxes (22 × 22 × 11 cm) with screened ventilation holes and a Plexiglas cover, which allowed us visible access to the entire nest. The colonies were connected by plastic tubing to a foraging chamber (58 × 36 × 40 cm), where sugar solution (“BeeHappy”, provided by Koppert) was presented in feeders ad libitum for 3 months. Defrosted pollen (1 rounded tsp, approximately 5 g) was delivered directly to the nest through a round opening in the Plexiglas cover daily. Colonies were kept at constant room temperature (25°C) and ambient humidity. The bumble bees behaved normally with the overhead lights (12:12 L:D) that were on during the day, as reported in previous studies (Couvillon and Dornhaus, 2009; Jandt and Dornhaus, 2009).
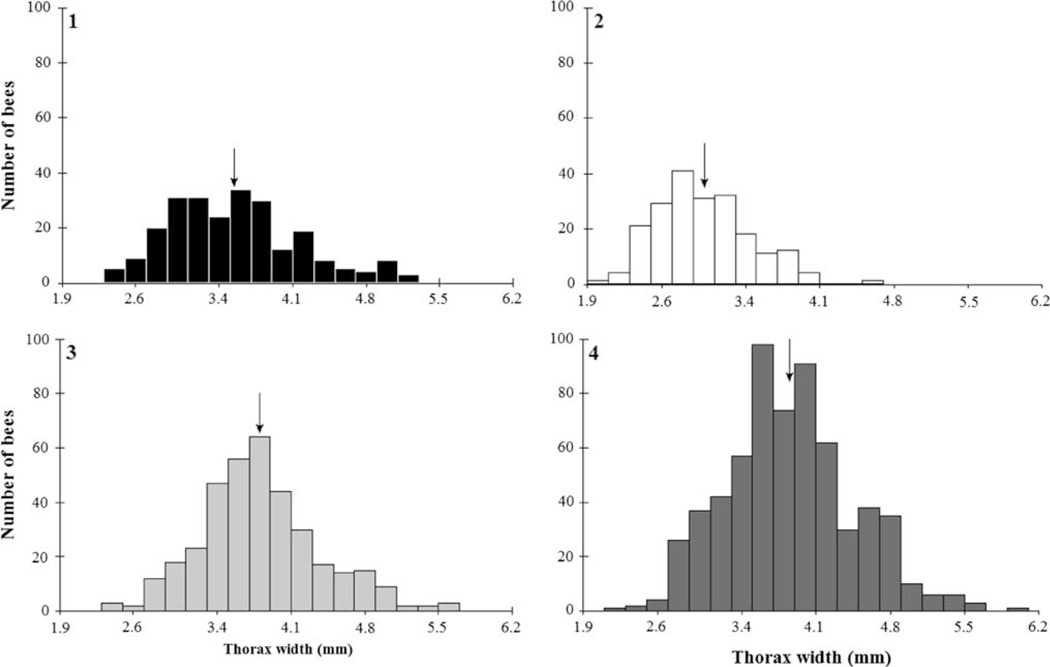
Fig. 2.
Mean (arrows) worker body size differed significantly among the colonies. Body size was not normally distributed (Kolmogorov–Smirnov, P < 0.05 for all colonies). Size distribution in all four colonies was positively skewed (Colony 1: n = 361, skew = 0.59, Colony 2: n = 623, skew = 0.5, Colony 3: n = 243, skew = 0.49, Colony 4: n = 205, skew = 0.30)
We did not control for the age of the workers; however, recent data demonstrate that all sizes of bees are produced throughout the entire colony cycle (Couvillon et al., submitted). Therefore, we do not expect large and small bees to differ in age, so any variation in lifespan is evenly distributed across the bees.
After 3 months, we discontinued feeding the colonies. For all colonies, the queen was still alive at the time of food discontinuation. Colonies survived for 3–6 days on stored honey; the honeypots were easy to see, as our colony nests are flat and spread out in the boxes. Once all visible honeypots were empty (of both honey and stored pollen, although pollen does not contain enough sugar to keep an adult alive), we collected dead bees every day at the same time (10:00 a.m.) and measured their body size using thorax width, which is a standard measurement of bumble bee size (Goulson et al., 2002). In total, we measured 1,432 dead bees from the four colonies.
Results
Body size was not normally distributed (Kolmogorov–Smirnov, P < 0.05 for all colonies), and the size distribution in all four colonies was positively skewed (Colony 1: skew = 0.59, Colony 2: skew = 0.5, Colony 3: skew = 0.49, Colony 4: skew = 0.30). Based on the Central Limit Theorem, our sample sizes were sufficient to justify the use of parametric statistics for our regression analysis (Sokal and Rohlf, 1995); however, we also analyzed using non-parametric statistics and obtained the same results.
The mean worker body size differed significantly among the colonies (Colony 1 (n = 361): 3.54 mm, Colony 2 (n = 623): 2.99 mm, Colony 3 (n = 243): 3.80 mm, Colony 4 (n = 205): 3.85 mm, One-way ANOVA, F = 125.00, P < 0.001; Fig. 2). This significance was maintained using non-parametric statistics (Kruskal–Wallis, H = 307.42, P < 0.001).
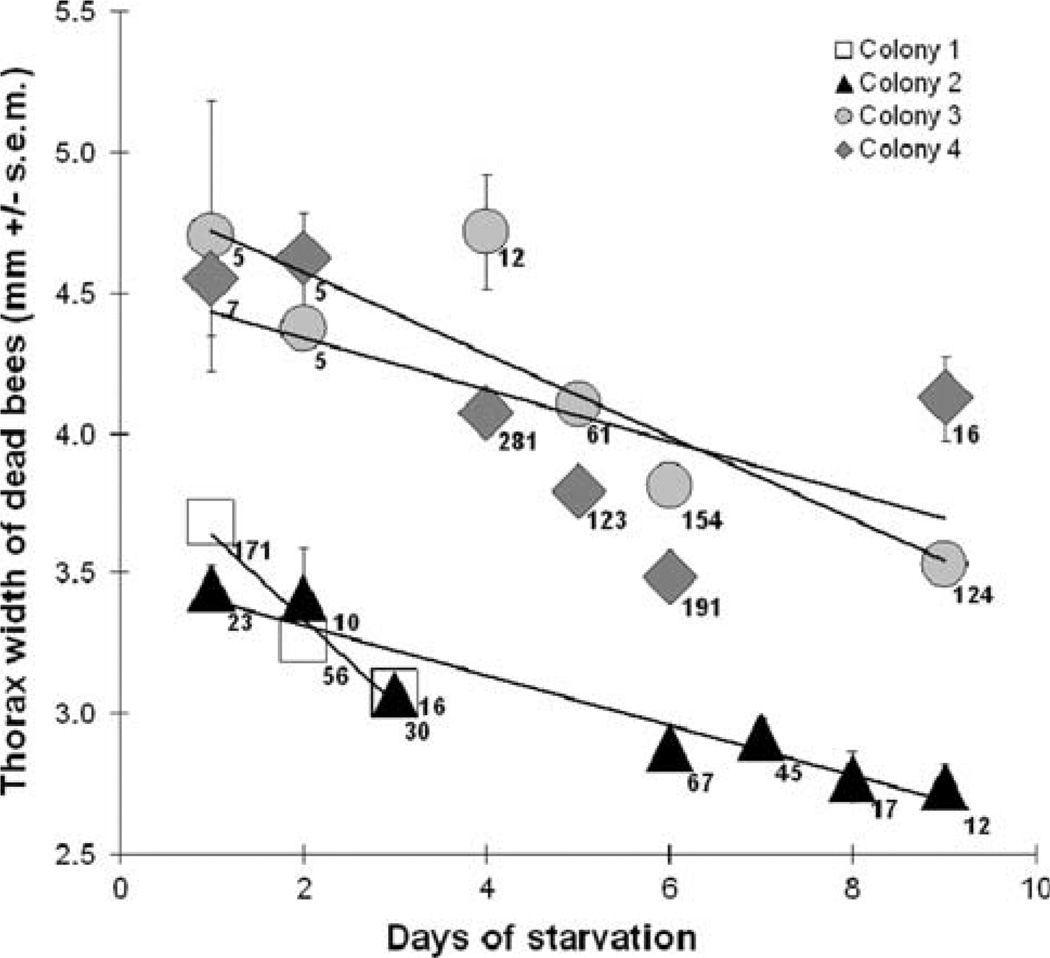
The average size of worker bees that died from starvation significantly decreased in all colonies the longer the colonies went without food, demonstrating that the larger bees died earlier. Thus, smaller workers are more robust to starvation compared to larger workers (Regression analyses—Colony 1: R2 = 0.12, F1,1 = 31.72, P < 0.001; Colony 2: R2 = 0.21, F1,1 = 53.01, P < 0.001; Colony 3: R2 = 0.19, F1,1 = 81.52, P < 0.001; Colony 4: R2 = 0.10, F1,1 = 71.84, P < 0.001) (Fig. 3). This significance was maintained using non-parametric statistics (Spearman’s Rank Correlation—Colony 1: −0.34, P < 0.001; Colony 2: −0.41, P < 0.001; Colony 3: −0.41, P < 0.001; Colony 4: −0.40, P < 0.001).
Fig. 3.
Smaller worker bees lived significantly longer than larger worker bees in all four colonies (Regression analysis, all P < 0.001; mean values ± SEM are shown). Different symbols represent different colonies, and the numbers beside each symbol are how many bees died and were measured on that day for that colony. “Days of starvation” is the time since honey stores were emptied
Discussion
We found that smaller worker bees were significantly more resilient to starvation than larger workers bees. This supports the hypothesis that individual worker physiology, specifically body size, might be correlated with ability to survive nectar dearth.
Why might smaller worker bumble bees better withstand starvation? One simple hypothesis would be that small workers need less food per individual per day to survive. Alternatively, smaller workers may store more nutrients in their body. If the latter is the case, starvation resistance may be a result of task specialization in the colony instead of body size per se. In particular, nurse bee physiology may better withstand starvation; in social hymenoptera, there is a general trend for nurses to carry a proportionally higher lipid content (Tschinkel, 1998; Markiewicz and O’Donnell, 2001; Toth and Robinson, 2005). Smaller bees tend to be nurses more often than larger bees (Jandt and Dornhaus, 2009). It was suggested that fatter nurses and, conversely, leaner foragers are adaptive because foraging typically occurs toward the end of a worker’s life, and keeping them lean is cheap as foragers experience higher mortality (i.e., a “disposable” caste) (Porter and Jorgensen, 1981). It is important to note, however, that the foragers in our study did not have very far to forage (<30 cm); therefore, it is unlikely that the bees disproportionately expended their stored energy, although this would be an interesting idea to test in both laboratory and field. Finally, bumble bee workers do not necessarily forage toward the end of their lives (Yerushalmi et al., 2006). Thus, whether or not bumble bee nurses also store more lipids or other nutrients, and how this relates to task specialization and age, remains unknown; we are now investigating this issue.
Mortality in our study may also have been affected by water loss in addition to starvation because we did not provide bees with separate water feeders; however, smaller bees, with a higher surface area:volume ratio, would be more susceptible to desiccation through the cuticle. In fact, there are much previous data to suggest that larger size in organisms reduces water loss (Chown and Gaston, 1999; Hoffmann and Harshman, 1999; Gilchrist et al., 2001; Telonis-Scott et al., 2006; Stillwell et al., 2007). Instead, smaller bees survived longer, which runs counter to previous work on starvation and desiccation resistance in other insects (Heinze et al., 2003; Stillwell et al., 2007) It might be interesting to model the optimal size distribution under the assumption of particular cost/benefit ratios in different conditions like starvation or desiccation, which would allow us to make predictions regarding the evolution of size distribution.
Of course, even if size polymorphism imparts fitness through increased survival to starvation, it may or may not also be an adaptation for division of labor (Couvillon and Dornhaus, 2009), as these hypotheses are not mutually exclusive. In division of labor, workers specialize, either permanently or temporarily, on a subset of tasks. This is thought to increase the efficiency (fitness) of a system as compared to one where all workers perform all tasks indiscriminately (Oster and Wilson, 1978; Beshers and Fewell, 2001; O’Donnell and Foster, 2001). Indeed, division of labor has been cited as a major reason for the incredible success of social insects (Wilson, 1985; Robinson, 1992). However, if this were the case in bumble bees, a difference in benefit/cost ratio of producing small and large workers for different tasks would have to be shown (Dornhaus, 2008). In addition, when thinking about adaptive explanations, it is important to note that efficiency, which describes superior performance under normal conditions, is not the only measure of biological fitness. Robustness is concerned with continued performance under non-ideal conditions (Bonabeau et al., 2000). When nectar is scarce, small workers remain alive significantly longer than larger workers. The presence of small workers, which might be less efficient at “normal” colony work of foraging and nursing, and size variation in general, might impart robustness, but not necessarily efficiency, to bumble bee colonies, allowing them to survive during times of high colony stress. Total colony fitness may be maximized by balancing the trade-off between the expensive, non-robust but very efficient large workers and the inexpensive, non-efficient but very robust small workers. As such, these data may provide a novel, adaptive explanation for size polymorphism that is independent of division of labor and open up a new area of investigation. Identifying what exactly in the physiology of smaller bees confers starvation robustness would be an interesting next step in studying the fitness benefits of size polymorphism.
Acknowledgments
We thank Jennifer Bonds for her help with data collection and Duncan Jackson and Dan Papaj for comments on the manuscript. This work was funded by a NIH PERT fellowship to MJC through the Center for Insect Science.
Contributor Information
M. J. Couvillon, Email: M.Couvillon@Sussex.ac.uk, Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721, USA; Laboratory of Apiculture and Social Insects, Department of Biology and Environmental Science, University of Sussex, Falmer, Brighton BN1 9QG, UK.
A. Dornhaus, Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721, USA
References
- Alford DV. Bumblebees. London: Davis-Poynter; 1975. [Google Scholar]
- Beshers SN, Fewell JH. Models of division of labor in social insects. Annu. Rev. Entomol. 2001;46:413–440. doi: 10.1146/annurev.ento.46.1.413. [DOI] [PubMed] [Google Scholar]
- Bonabeau E, Dorigo M, Theraulaz G. Inspiration for optimization from social insect behaviour. Nature. 2000;406:39–42. doi: 10.1038/35017500. [DOI] [PubMed] [Google Scholar]
- Chapman RE, Bourke AFG. The influence of sociality on the conservation biology of social insects. Ecol. Lett. 2001;4:650–662. [Google Scholar]
- Chown SL, Gaston KJ. Exploring links between physiology and ecology at macro-scales: the role of respiratory metabolism in insects. Biol. Rev. 1999;74:87–120. [Google Scholar]
- Cnaani J, Hefetz A. The effect of workers size frequency-distribution on colony development in Bombus terrestris. Insect. Soc. 1994;41:301–307. [Google Scholar]
- Cnaani J, Schmid-Hempel R, Schmidt JO. Colony development, larval development and worker reproduction in Bombus impatiens Cresson. Insect. Soc. 2002;49:164–170. [Google Scholar]
- Couvillon MJ, Dornhaus A. Location, location, location: larvae position inside the nest is correlated with adult body size in worker bumble bees (Bombus impatiens) Proc. R. Soc. B. 2009;276:2411–2418. doi: 10.1098/rspb.2009.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumber RA. The biology of humble-bees, with special reference to the production of the worker caste. Trans. R. Ent. Soc. Lond. 1949;100:1–45. [Google Scholar]
- Dornhaus A. Specialization does not predict individual efficiency in an ant. PLoS. 2008;6:e285. doi: 10.1371/journal.pbio.0060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RL, Brunskill A, Verdirame D, O’Donnell S. Reproductive physiology, dominance interactions, and division of labour among bumble bee workers. Physiol. Entomol. 2004;29:327–334. [Google Scholar]
- Free JB, Butler CG. Bumblebees. London: Collins; 1959. [Google Scholar]
- Gardner K, Foster R, O’Donnell S. Experimental analysis of worker division of labor in bumblebee nest thermoregulation (Bombus huntii, Hymenoptera: Apidae) Behav. Ecol. Sociobiol. 2007;61:783–792. [Google Scholar]
- Gilchrist GW, Huey RB, Serra L. Rapid evolution of wing size clines in Drosophila subobscura. Genetica. 2001;112:273–286. [PubMed] [Google Scholar]
- Goulson D, Peat J, Stout JC, Tucker J, Darvill B, Derwent LC, Hughes WOH. Can alloethism in workers of the bumblebee, Bombus terrestris, be explained in terms of foraging efficiency? Anim. Behav. 2002;64:123–130. [Google Scholar]
- Heinze J, Foitzik S, Fischer B, Wanke T, Kipyatkov VE. The significance of latitudinal variation in body size in a holarctic ant, Leptothorax acervorum. Ecography. 2003;26:349–355. [Google Scholar]
- Hines HM. Historical biogeography, divergence times, and diversification patterns of bumble bees (Hymenoptera: Apidae: Bombus) Syst. Biol. 2008;57:58–75. doi: 10.1080/10635150801898912. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Harshman LG. Desiccation and starvation resistance in Drosophila: patterns of variation at the species, population and intrapopulation levels. Heredity. 1999;83:637–643. doi: 10.1046/j.1365-2540.1999.00649.x. [DOI] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO. The Ants. Cambridge, Massachusetts: Harvard University Press; 1990. p. 732. [Google Scholar]
- Jandt J, Dornhaus A. Spatial organization and division of labor in the bumble bee, Bombus impatiens. Anim. Behav. 2009;77:641–651. [Google Scholar]
- Markiewicz D, O’Donnell S. Social dominance, task performance and nutrition: implications for reproduction in eusocial wasps. J. Comp. Physiol. A. 2001;187:327–333. doi: 10.1007/s003590100204. [DOI] [PubMed] [Google Scholar]
- Michener CD. The Bees of the World. Baltimore: Johns Hopkins University Press; 2000. [Google Scholar]
- O’Donnell S, Foster RL. Thresholds of response in nest thermoregulation by worker bumble bees, Bombus bifarius nearcticus (Hymenoptera: Apidae) Ethology. 2001;107:387–399. [Google Scholar]
- Oster GF, Wilson EO. Caste and Ecology in the Social Insects. Princeton, New Jersey: Princeton University Press; 1978. p. 352. [PubMed] [Google Scholar]
- Peat J, Tucker J, Goulson D. Does intraspecific size variation in bumblebees allow colonies to efficiently exploit different flowers? Ecol. Entomol. 2005;30:176–181. [Google Scholar]
- Plowright RC, Jay SC. Caste differentiation in bumblebees (Bombus Latr.: Hym.). 1. Determination of female size. Insect. Soc. 1968;15:171–192. [Google Scholar]
- Porter SD, Jorgensen CD. Foragers of the harvester ant, Pogonomyrmex owyheei: a disposable caste? Behav. Ecol. Sociobiol. 1981;9:247–256. [Google Scholar]
- Robinson GE. Regulation of division-of-labor in insect societies. Annu. Rev. Entomol. 1992;37:637–665. doi: 10.1146/annurev.en.37.010192.003225. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. New York: W.H. Freeman and Company; 1995. p. 850. [Google Scholar]
- Spaethe J, Weidenmuller A. Size variation and foraging rate in bumblebees (Bombus terrestris) Insect. Soc. 2002;49:142–146. [Google Scholar]
- Stillwell RAC, Morse GAE, Fox CA. Geographic variation in body size and sexual size dimorphism of a seedfeeding beetle. Amer. Nat. 2007;170:358–369. doi: 10.1086/520118. [DOI] [PubMed] [Google Scholar]
- Telonis-Scott M, Guthridge KM, Hoffmann AA. A new set of laboratory-selected Drosophila melanogaster lines for the analysis of desiccation resistance: response to selection, physiology and correlated responses. J. Exp. Biol. 2006;209:1837–1847. doi: 10.1242/jeb.02201. [DOI] [PubMed] [Google Scholar]
- Toth AL, Robinson GE. Worker nutrition and division of labour in honeybees. Anim. Behav. 2005;69:427–435. [Google Scholar]
- Tschinkel WR. Sociometry and sociogenesis of colonies of the harvester ant, Pogonomyrmex badius: worker characteristics in relation to colony size and season. Insect. Soc. 1998;45:385–410. [Google Scholar]
- Wilson EO. The sociogenesis of insect colonies. Science. 1985;228:1489–1495. doi: 10.1126/science.228.4707.1489. [DOI] [PubMed] [Google Scholar]
- Wilson EO. Causes of ecological success: the case of the ants. J. Anim. Ecol. 1987;56:1–9. [Google Scholar]
- Yerushalmi S, Bodenhaimer S, Bloch G. Developmentally determined attenuation in circadian rhythms links chronobiology to social organization in bees. J. Exp. Biol. 2006;209:1044–1051. doi: 10.1242/jeb.02125. [DOI] [PubMed] [Google Scholar]