Abstract
Antiestrogen therapy is commonly used to treat estrogen receptor (ER)+ breast cancers but acquired and de novo resistance limits their overall curative potential. An endoplasmic reticulum stress pathway, the unfolded protein response, and autophagy are both implicated in the development of antiestrogen therapy resistance in estrogen receptor-α (ER) positive breast cancer. Thus, we recently investigated how ERα can regulate autophagy and the unfolded protein response (Cook et al., FASEBJ, 2014). We showed that inhibiting ERα signaling stimulates autophagosome formation and flux. Moreover, we showed that ERα knockdown inhibited the unfolded protein response (UPR) signaling components. Here we support and extend this recent report showing additional data on ERα localization and provide a schematic of the overall signaling implicated by our results. Differential activation of UPR and autophagy highlight the pivotal role of ERα in regulating pro-survival signaling in breast cancer through UPR and autophagy. Furthermore, these data suggest new approaches to successful targeting ERα and preventing the regulation of key pro-survival signaling that confers resistance to endocrine therapies.
Introduction
About 232,000 new cases of breast cancer are diagnosed annually within the USA, and approximately 70% of these tumors express the estrogen receptor (ER)-α [1]. Due to the high prevalence of ER+ breast cancer, an ERα targeted therapy such as tamoxifen (TAM), faslodex (fulvestrant, ICI), or aromatase inhibitors like letrozole are often used to treat this breast cancer subtype [2]. However, resistance to these therapies often develops, limiting their respective abilities to cure all ER+ breast cancers [3]. Understanding how antiestrogen resistance occurs, and the signaling pathways involved in resistance, remain critical goals in breast cancer research. Clarifying the biology of resistance may lead to improvements in how we treat the disease and reduce breast cancer mortality. Our group has shown how the unfolded protein response (UPR, an endoplasmic reticulum stress pathway) and autophagy play an integral role in the development and maintenance of antiestrogen resistance in ER+ breast cancer [4–9]. More recently, we defined a central role for ERα in this integrated signaling [2]. Here we provide additional support and discussion of these findings.
Autophagy is a process of “self-eating” whereby old or dysfunctional organelles and cellular material are labeled for degradation, engulfed by a double membrane, and digested by lysosomal hydrolases [5]. UPR is activated by the accumulation of unfolded or misfolded proteins in the endoplasmic reticulum [4]. UPR activation results in an inhibition of protein translation and promotes both the transcription of protein chaperones and antioxidant signaling [4, 10, 11]. While both autophagy and UPR can be either pro-survival or pro-death, for endocrine therapies both UPR and autophagy promote the development of therapy resistance and breast cancer cell survival [4].
Our recent publication showed that inhibition of ERα expression, through RNAi, resensitized antiestrogen resistant cells and potentiated antiestrogen-mediated cell death in endocrine sensitive breast cancer cells [2]. This observation, consistent with a previous report [12], lead to a perplexing conundrum: how does reducing ERα (the molecular target for ICI) increase antiestrogen therapy responsiveness in ER+ breast cancer cells? We showed that ERα knockdown resulted in changes in other secondary activities of ERα (such as UPR or autophagy signaling) that may explain the observed effects [2]. We used various molecular techniques including electron microscopy, confocal microscopy, flow cytometry, gene knockdown/over expression, western blot hybridization, and mathematical modeling to explore our hypothesis. We determined that ERα ablation inhibited UPR signaling, thereby preventing UPR-mediated antioxidant response, resulting in elevated reactive oxygen species formation and cell death in response to antiestrogen treatment[2]. The data included in this report supplement our previous study and focus on ERα localization and the potential effect of changes in this localization on ERα-mediated UPR activation.
Material and Methods
Materials
ICI 182,780 (Tocris Bioscience, Ellisville, MO); Improved Minimal Essential Medium (IMEM; Gibco Invitrogen BRL, Carlsbad, CA); and bovine calf charcoal stripped serum (CCS) (Equitech-Bio Inc, Kerrville, TX). Mouse IgG negative control antibody (Dako, Glostrup, Denmark) and ERα (Vector Laboratories) were used for IHC studies. ERα (Vector Laboratories), goat anti-mouse Alexa Fluor ® 568 secondary antibody (Invitrogen), and DAPI were used for confocal microscopy.
Cell Culture
MCF7/LCC1 (LCC1) and MCF7/LCC9 (LCC9) breast carcinoma cells, previously derived in this laboratory [13, 14], were grown in phenol-red free IMEM media containing 5% charcoal-treated calf serum (CCS). Cells were grown at 37°C in a humidified, 5% CO2:95% air atmosphere.
Confocal Microscopy
LCC1/LCC9 cells were treated with 0.1% v/v ethanol vehicle or 500 nM ICI for 24 h. Cells were permeabilized and incubated with an ERα antibody. ERα localization was observed by confocal microscopy. Confocal microscopy was performed using an Olympus IX-70 confocal microscope (LCCC Imaging Shared Resources).
Orthotopic xenografts in athymic mice
Five week old ovariectomized athymic nude mice (Harlan Laboratories, Fredrick, MD) were injected orthotopically into the mammary fat pads with a suspension of 1 × 106 LCC1 or LCC9 cells in Matrigel. Where appropriate, mice were supplemented with s.c. implantation of a 17β-estradiol pellet (0.72 mg, 60-day release; Innovative Research of America, Sarasota, FL). Mice were sacrificed after 9 weeks, and tumors removed at necropsy, fixed in neutral buffered formalin, and processed using routine histological methods.
Immunohistochemistry (IHC)
Tumors were fixed in 10% formalin for 24 h prior to embedding in paraffin. Embedded tumors were cut into 5 μm thick sections and stained with hemotoxylin and eosin for histopathologic analysis. Immunostaining was performed with an antibody to ERα (1:100, LCCC Histopathology Core Shared Resources), or a non-specific antibody (negative control) using the streptavidin-biotin method. Stained sections were visualized and photographed.
Results
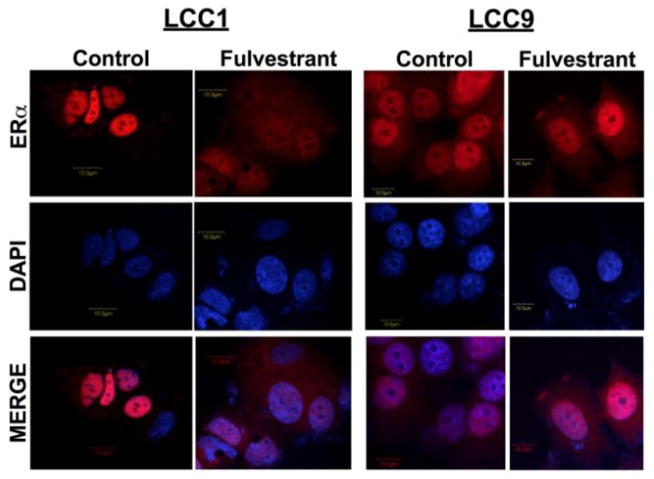
Localization of ERα was confirmed by confocal microscopy. LCC1 and LCC9 cells were treated with vehicle or 500 nM ICI for 24 hours, stained for ERα, and counterstained with DAPI for nuclear localization (Figure 1). In LCC1 cells, ERα is predominantly localized in the nucleus under basal growth conditions, while treatment with 500nM ICI increased the cytosolic distribution of ERα. Localization of ERα differs in LCC9 cells. In the antiestrogen resistant breast cancer cells, ERα is located in both the cytosol and nucleus under basal growth conditions and 500 nM ICI treatment has no overall effect on ERα localization.
Figure 1. Localization of ERα in LCC1 and LCC9 cells treated with fulvestrant.
LCC1 (A) and LCC9 (B) breast cancer cells were treated with 500 nM ICI for 24 hours. Confocal microscopy indicates localization of the ERα (red) and nucleus (blue). These data give background on ER localization and indicate possible mechanism of ICI stimulating UPR signaling which was shown in Cook et al. 2014 [2].
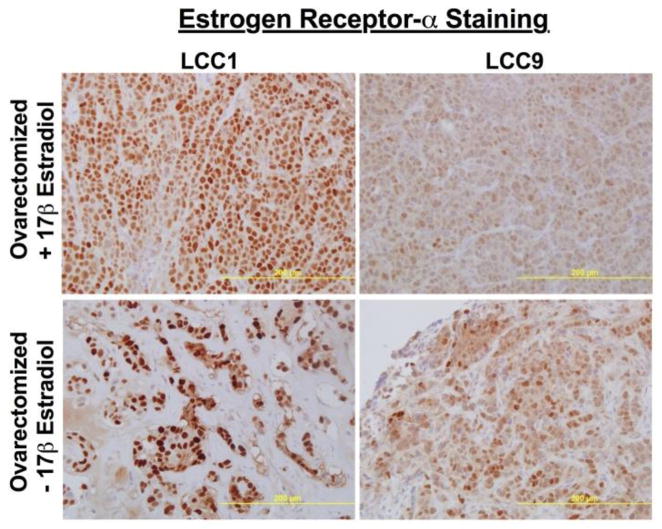
LCC1 and LCC9 xenografts were grown in ovariectomized female mice with or without an implanted 60 daytime-release 17β-estradiol pellet (E2) to determine the effect of estrogen on ERα levels and localization in vivo (Figure 2). Tumor sections were stained with ERα and counterstained with hemotoxylin. ERα was mainly expressed in the nucleus of LCC1 tumors, while a reduced but more dispersed ERα localization was observed in LCC9 tumors. Grown in the absence of estrogen supplementation, LCC1 and LCC9 tumors exhibited a dramatic increase in ERα expression, with a similar localization pattern to that observed in their respective estrogen treated tumors. An upregualtion of receptors in the absence of ligand is a common pharmacological response. The high number of receptor molecules can enable the cells to respond to very low concentrations of ligand; significant receptor upregulation can create a “spare receptor” phenotype.
Figure 2. Localization of ERα in LCC1 and LCC9 xenografts grown in the presence or absence of 17-β estradiol.
Immunohistochemical analysis of LCC1 and LCC9 tumor sections exposed or deprived of estrogen show differences in ERα levels and localization. These data give background on ER localization in vivo, suggesting a possible mechanism for ICI-mediated UPR signaling which was shown in Cook et al. 2014 [2].
Discussion
Understanding the development of therapeutic resistance remains a critical question in breast cancer biology. Knowledge of how resistance develops and the molecular signaling pathways that confer/maintain this phenotype could greatly impact the design of future clinical trials and the treatment of breast cancer. Preventing the development of endocrine resistance, and/or resensitizing resistant tumors to endocrine therapies, would reduce breast cancer mortality.
We showed that autophagy and UPR are two vital molecular signaling pathways involved in antiestrogen therapy resistance [2]. We determined that ERα regulates these survival pathways through two different mechanisms; inhibition of ERα signaling promotes pro-survival autophagy, while the aggregation of ERα likely stimulates UPR signaling [2]. Knockdown of ERα prevented pro-survival UPR signaling [2]. In a study investigating the combination of ICI and proteasome inhibitors in MCF-7 cells, ICI was suggested to induce UPR signaling through aggregation of ERα molecules in the cytoplasm, thereby enhancing proteasomal inhibitor-mediated cell death [15]. We show the localization of ERα using confocal microscopy in LCC1 and LCC9 (Figure 1) in basal growth conditions and in response to ICI. ERα is localized in the nucleus in LCC1 cells. Antiestrogen treatment reduces overall ERα levels and increases the cytoplasmic distribution of ERα, consistent with previous reports on fulvestrant activity on ERα localization [15]. In contrast, ERα is distributed in both the nucleus and the cytoplasm independent of ICI treatment in the antiestrogen resistant LCC9 cells. Thus, the increased levels of UPR signaling in antiestrogen resistant cell lines may be partly due to the need to remove aggregated cytoplasmic ERα proteins.
LCC1 and LCC9 xenografts show a similar pattern of ERα localization when grown in the presence or absence of 17β-estradiol (Figure 2), with a potent and stable induction of ERα in estrogen deprived growth conditions. Estrogen deprivation also results in a more diffuse ERα localization. Growing LCC1 and LCC9 xenografts in a very low estrogen environment likely increases cellular stress, leading to increased UPR signaling and stimulation of autophagy. Increased autophagy likely helps supplement cellular metabolism when ER+ breast cancer cells are deprived of adequate E2. Increased autophagy and UPR is also apparent in vitro with the transition from estrogen dependent to estrogen independent and from antiestrogen sensitive to antiestrogen resistant [5, 7, 8, 16].
Working closely with our collaborators, we recently modeled the switch between estrogen receptor and growth factor signaling in ER+ breast cancer [17, 18]. The novel mathematical models detail ERα activation of growth factor signaling, which potentiates estrogen-independent growth and can promote endocrine resistance. The studies further describe how understanding survival-signaling switches can be beneficial in the design of potential clinical trials to overcome endocrine resistance in ER+ breast cancers. We propose that precise timing of cycling therapies may result in increased sensitization to drugs and prevent, delay, or reduce resistance [17, 18]. We show evidence supporting this idea here in Figure 2. ER+ breast tumors grown without estrogen (in ovariectomized mice) have elevated ERα expression when compared with tumors grown in the presence of estrogen. Based upon the mathematical model, cycling aromastase inhibitors with SERM therapies may result in increased ERα expression that would result in a better response to tamoxifen and limiting the development of resistance.
Conclusion
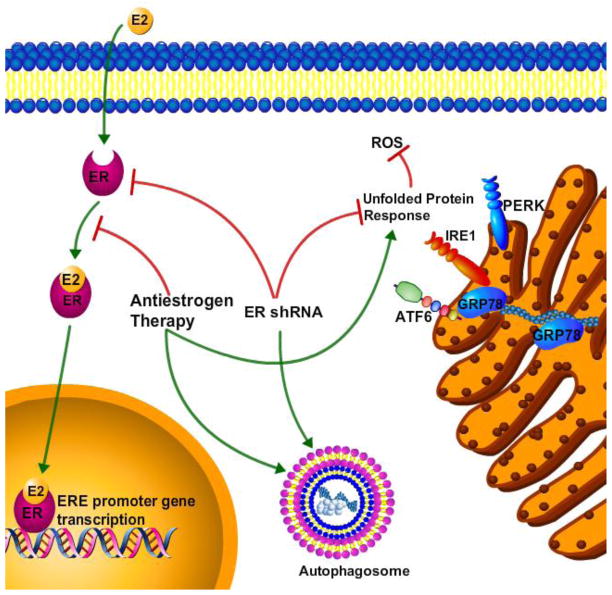
The report here supports and extends our recent study of the role of ERα in regulating UPR and autophagy [2]. We further highlighted the pro-survival activities of antiestrogen-mediated UPR and autophagy that may promote endocrine-based therapeutic resistance. We showed that antiestrogen drugs induce autophagic signaling through inhibition of ERα signaling, while ICI promotes UPR signaling through aggregation of cytoplasmic ERα [2]. ERα down regulation inhibited UPR signaling and resulted in pro-death ROS generation, stimulating antiestrogen-induced cell death in both endocrine therapy sensitive and resistant breast cancer cells[2]. An overview of the proposed signaling is represented in Figure 3. Our previous reports have linked UPR and autophagy signaling, indicating that GRP78 is critical to antiestrogen-mediated autophagy induction [7, 19]. Moreover, another recent study suggests that inhibiting autophagy successfully restored tamoxifen sensitivity to resistant ER+ breast tumors [20]. These observations suggest that combining UPR and autophagy inhibitors with antiestrogen drug regimens may benefit ER+ breast cancer patients by preventing or reducing the occurrence of drug resistance.
Figure 3.
Overview of the signaling schematic of ant estrogen therapy and ERα in ER+ breast cancer cells, summarizing the data shown in Cook et al., 2014 [2].
Acknowledgments
Katherine Cook is supported by a DOD Breast Cancer Research Program Postdoctoral Fellowship (BC112023). This research was also supported in part by awards from the US Department of Health and Human Services (R01-CA131465 and U54-CA149147) to Robert Clarke.
Footnotes
Conflict of interests
Authors declare that they have no conflict of interests.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Cook KL, Clarke PA, Parmar J, Hu R, Schwartz-Roberts JL, Abu-Asab M, et al. Knockdown of estrogen receptor-alpha induces autophagy and inhibits antiestrogen-mediated unfolded protein response activation, promoting ROS-induced breast cancer cell death. FASEB J. 2014 doi: 10.1096/fj.13-247353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riggins RB, Bouton AH, Liu MC, Clarke R. Antiestrogens, aromatase inhibitors, and apoptosis in breast cancer. Vitam Horm. 2005;71:201–237. doi: 10.1016/S0083-6729(05)71007-4. [DOI] [PubMed] [Google Scholar]
- 4.Clarke R, Cook KL, Hu R, Facey CO, Tavassoly I, Schwartz JL, et al. Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate. Cancer Res. 2012;72:1321–1331. doi: 10.1158/0008-5472.CAN-11-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook KL, Shajahan AN, Clarke R. Autophagy and endocrine resistance in breast cancer. Expert Rev Anticancer Ther. 2011;11:1283–1294. doi: 10.1586/era.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford AC, Riggins RB, Shajahan AN, Zwart A, Clarke R. Co-inhibition of BCL-W and BCL2 restores antiestrogen sensitivity through BECN1 and promotes an autophagy-associated necrosis. PLoS One. 2010;5:e8604. doi: 10.1371/journal.pone.0008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook KL, Shajahan AN, Warri A, Jin L, Hilakivi-Clarke LA, Clarke R. Glucose-regulated protein 78 controls cross-talk between apoptosis and autophagy to determine antiestrogen responsiveness. Cancer Res. 2012;72:3337–3349. doi: 10.1158/0008-5472.CAN-12-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez BP, Riggins RB, Shajahan AN, Klimach U, Wang A, Crawford AC, et al. Human X-box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. FASEB J. 2007;21:4013–4027. doi: 10.1096/fj.06-7990com. [DOI] [PubMed] [Google Scholar]
- 9.Gu Z, Lee RY, Skaar TC, Bouker KB, Welch JN, Lu J, et al. Association of interferon regulatory factor-1, nucleophosmin, nuclear factor-kappaB, and cyclic AMP response element binding with acquired resistance to Faslodex (ICI 182,780) Cancer Res. 2002;62:3428–3437. [PubMed] [Google Scholar]
- 10.Chen CS, Tseng YT, Hsu YY, Lo YC. Nrf2-Keap1 Antioxidant Defense and Cell Survival Signaling Are Upregulated by 17beta-Estradiol in Homocysteine-Treated Dopaminergic SH-SY5Y Cells. Neuroendocrinology. 2012 doi: 10.1159/000342692. [DOI] [PubMed] [Google Scholar]
- 11.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuske B, Naughton C, Moore K, Macleod KG, Miller WR, Clarke R, et al. Endocrine therapy resistance can be associated with high estrogen receptor alpha (ERalpha) expression and reduced ERalpha phosphorylation in breast cancer models. Endocr Relat Cancer. 2006;13:1121–1133. doi: 10.1677/erc.1.01257. [DOI] [PubMed] [Google Scholar]
- 13.Brunner N, Boulay V, Fojo A, Freter CE, Lippman ME, Clarke R. Acquisition of hormone-independent growth in MCF-7 cells is accompanied by increased expression of estrogen-regulated genes but without detectable DNA amplifications. Cancer Res. 1993;53:283–290. [PubMed] [Google Scholar]
- 14.Brunner N, Boysen B, Jirus S, Skaar TC, Holst-Hansen C, Lippman J, et al. MCF7/LCC9: an antiestrogen-resistant MCF-7 variant in which acquired resistance to the steroidal antiestrogen ICI 182,780 confers an early cross-resistance to the nonsteroidal antiestrogen tamoxifen. Cancer Res. 1997;57:3486–3493. [PubMed] [Google Scholar]
- 15.Ishii Y, Papa L, Bahadur U, Yue Z, Aguirre-Ghiso J, Shioda T, et al. Bortezomib enhances the efficacy of fulvestrant by amplifying the aggregation of the estrogen receptor, which leads to a proapoptotic unfolded protein response. Clin Cancer Res. 2011;17:2292–2300. doi: 10.1158/1078-0432.CCR-10-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samaddar JS, Gaddy VT, Duplantier J, Thandavan SP, Shah M, Smith MJ, et al. A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol Cancer Ther. 2008;7:2977–2987. doi: 10.1158/1535-7163.MCT-08-0447. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Baumann WT, Clarke R, Tyson JJ. Modeling the estrogen receptor to growth factor receptor signaling switch in human breast cancer cells. FEBS Lett. 2013;587:3327–3334. doi: 10.1016/j.febslet.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyson JJ, Baumann WT, Chen C, Verdugo A, Tavassoly I, Wang Y, et al. Dynamic modelling of oestrogen signalling and cell fate in breast cancer cells. Nat Rev Cancer. 2011;11:523–532. doi: 10.1038/nrc3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook KL, Clarke R. Heat shock 70 kDa protein 5/glucose-regulated protein 78 “AMP”ing up autophagy. Autophagy. 2012;8:1827–1829. doi: 10.4161/auto.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook KL, Warri A, Soto-Pantoja DR, Clarke PA, Cruz MI, Zwart A, et al. Hydroxychloroquine Inhibits Autophagy to Potentiate Antiestrogen Responsiveness in ER+ Breast Cancer. Clin Cancer Res. 2014;20:3222–3232. doi: 10.1158/1078-0432.CCR-13-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]