Abstract
Remarkable progress has been made highlighting the importance of cap-dependent mRNA translation in cancer progression. 4E-BP1 is a translation initiation repressor by sequestering the mRNA cap-binding protein eIF4E and consequently inhibiting the translation of certain key oncogenic mRNAs encoding proteins for cell proliferation, survival, angiogenesis and malignancy. In most tumors, however, the repressive function of 4E-BP1 is compromised by reduction of its expression or phosphorylation mediated by oncogenic signaling pathways. We recently unveiled that 4E-BP1-regulated cap-dependent translation integrates oncogenic effects of the AKT and ERK signaling pathways on tumor growth and metastatic progression. Mechanistically, we demonstrate that AKT and ERK pathways selectively upregulate survivin expression at the level of translation by convergent activation of the mTORC1/4E-BP1/eIF4E signaling axis. In addition, loss of 4E-BP1 function induces epithelial–mesenchymal transition and increases metastatic capability of cancer cells by translational activation of Snail. Continuous translation of survivin and Snail is important for colorectal cancer progression to metastasis. Herein we discuss our findings concerning deregulation of translation in cancer progression and metastasis and highlight 4E-BP1 as a potential biomarker and therapeutic target.
Keywords: 4E-BP1, AKT, ERK, mTOR, translational regulation, Snail, Survivin, EMT, tumorigenesis, metastasis
Introduction
Regulation of gene expression occurs at multiple levels from epigenetic modification to mRNA translation. Although transcriptional regulation is a well-established mechanism that regulates protein expression, a growing body of evidence indicates that gene transcription and steady-state levels of mRNA expression are poor predictors of protein levels [1–5]. It has become clear that translational regulation is also actively involved in cancer development by specifically altering the levels of a subset of mRNAs into proteins while the most of transcripts remain unchanged [6–8]. In this research highlight, we provide an update of our recent efforts on the biology and clinical relevance of translational regulation in cancer progression and therapeutics.
Cap-dependent translation and its regulation in cancer
The majority of newly synthesized eukaryotic mRNAs is modified by the addition of a 7-methylguanosine cap structure at their 5′ end, and translation of those capped mRNAs relies on a protein complex termed eIF4F. The formation of eIF4F complex begins at binding of translation initiation factor eIF4E to 5′-capped mRNAs in the nucleus, followed by the recruitment of scaffolding protein eIF4G and RNA helicase eIF4A in the cytoplasm [6]. The eIF4F complex initiates and regulates ribosome recruitment and subsequent protein synthesis. The assembly of eIF4F complex is thought to be a rate limiting step for translation initiation and is largely dependent on eIF4E availability. Emerging evidence indicates that translation of certain key oncogenic mRNAs bearing long and highly structured 5′-untranslated regions is strongly dependent on the eIF4E [6, 7]; these mRNAs include those encoding proteins involved in cell cycle progression and cell survival, such as D-cyclins, VEGF, c-Myc, Bcl-2 and Mcl1. Consequently, these oncogenic mRNAs are selectively regulated by eIF4E availability and are sensitive to alteration in the levels of eIF4E [4, 7]. Over expression of eIF4E is frequently observed in a variety of human cancers [7, 9]. In experimental models including the transgenic mouse model, ectopic eIF4E expression can induce cellular transformation and tumor genesis, and increase the incidence of multiple cancers [10, 11].
The level of free eIF4E can be regulated by its binding proteins, 4E-BPs, mainly 4E-BP1 and 4E-BP2 [4, 6]. These proteins repress the formation of eIF4F complex by binding to eIF4E surface antagonistically with eIF4G using the same binding motif [4, 6]. To date, mutation or deletion of 4E-BP genes has not yet been identified in human cancers [12]. However, 4E-BP1 is frequently hyperphosphorylated in cancer cells by the oncogenic signals such as the PI3K/AKT and RAS/RAF/MEK/ERK signaling pathways [7], which causes 4E-BP1 to disassociate from eIF4E and thus inactivates the competitive function of 4E-BP1, and increases the level of free eIF4E [7]. The mTOR kinase complex 1 (mTORC1) is an important regulator of cap-dependent translation as it phosphorylates 4E-BP1 on T37 and T46, and these phosphorylations promote subsequent phosphorylations on S65 and T70 [13]. Hyperphosphorylation of 4E-BP1 or reduction of 4E-BP1 expression occurs in several cancer types and is associated with malignant progression and poor prognosis [7, 14–16].
Cross-talk between AKT and ERK pathways
Activation of the PI3K/AKT and RAS/RAF/MEK/ERK pathways is a common feature in cancers. Mutations in genes that encode components of these two pathways occur at high frequency in cancer. In a majority of human cancers, the PI3K/AKT pathway is frequently activated by the activating mutations of PI3K p110α (PIK3CA) and the loss or inactivating mutation of PTEN, whereas hyper activation of MEK/ERK signaling driven by mutant RAS and BRAF is also a common oncogenic event in a variety of cancers [17, 18]. Moreover, the AKT and ERK pathways are concurrently activated by separate mutations in many human tumors. For instance, KRAS and PIK3CA mutation; BRAF and PIK3CA mutation; and BRAF and PTEN mutation occur simultaneously in colorectal carcinoma, thyroid carcinoma and melanoma, respectively [19–23]. Deregulated AKT and ERK pathways are proven to be actively involved in maintaining malignant properties in tumor cells and promoting cancer progression and metastasis [24, 25]. Thus, a number of small molecule inhibitors targeting components of these two pathways have been aggressively developed for the treatment of cancers [17, 18, 26, 27].
Preclinical studies and clinical trials with selective PI3K and AKT inhibitors have shown that tumors with PIK3CA mutations are likely to be dependent on the PI3K/AKT pathway and are sensitive to inhibition of that pathway [28–30]. We found that in PIK3CA mutant tumors, the AKT dependence of 4E-BP1 phosphorylation is closely correlated with tumor growth [28, 31]. On the other hand, the BRAF inhibitor vemurafenib or the MEK inhibitor trametinib produces high response rates in mutant BRAF V600E-driven melanoma [32, 33]. However, tumor cells with PIK3CA or PTEN mutations are not all sensitive to the inhibitors of PI3K or AKT [31, 34]. Similarly, mutant KRAS or BRAF tumors are not always dependent on ERK signaling and sensitive to the BRAF and MEK inhibitors [31, 35, 36]. We demonstrated that coexistent KRAS mutation renders PIK3CA mutant tumors independent of PI3K/AKT signaling, whereas PIK3CA mutation uncouples tumor growth from MEK/ERK and mutant KRAS signaling [31, 36]. In tumors with mutational activation of both PI3K/AKT and MEK/ERK pathways, inhibition of either pathway alone has minor or negligible effects on cell survival and tumor growth [31]. However, combined inhibition of both pathways effectively induces apoptosis and suppresses tumor growth [31]. These data suggest that AKT and ERK pathways may activate a common set of downstream targets that integrate their function in tumors, thus reducing ‘oncogenic addiction’ on AKT or ERK signaling pathway and causing resistance to inhibition of either pathway alone.
4E-BP1 is a key effector of the oncogenic action of AKT and ERK signaling pathways in tumorigenesis
We discovered that redundant phosphorylation of 4E-BP1 with concomitant activation of cap-dependent translation mediated by the AKT and ERK pathways is associated with the resistance to targeted inhibition of either pathway alone in tumors with coexistent pathway activation [31]. In the experimental model of colorectal cancer (CRC) with coexistent KRAS and PIK3CA mutations, 4E-BP1 phosphorylation is unresponsive or less affected by inhibition of either AKT or ERK pathway alone. However, combined inhibition of both pathways effectively inhibits 4E-BP1 phosphorylation, which in turn activates 4E-BP1 binding to the eIF4E-mRNA cap complex and thus represses eIF4E-initiated cap-dependent translation, with an associated synergistic induction of apoptosis and suppression of tumor growth [31]. Moreover, using a non-phoshorylated mutant 4E-BP1 allele with four known phosphorylation sites (T37, T46, S65, T70) substituted with alanine (4E-BP1-4A), which causes constitutive binding to eIF4E and inhibition of cap-dependent translation, we were able to show that this active 4E-BP1 mutant exerts similar inhibitory effects on CRC tumor growth as does the combined inhibition of AKT and ERK pathways. Others studies also show that the active 4E-BP1 can block tumorigenesis in PTEN mutant breast cancer, AKT-driven lymphoma and KRAS mutant non-small cell lung cancer [37–39]. In contrast, knockdown of 4E-BP1 expression or overexpression of eIF4E profoundly attenuates dependence of colon tumors on activated AKT and ERK signaling for translation and survival [31]. In addition, we further demonstrated that phosphorylation of 4E-BP1 is critical, compared with the phosphorylation of 4E-BP2 or other translation regulators including S6K and S6 ribosomal protein, to the oncogenic action of AKT and ERK in colon tumors. In sum, our data suggest that 4E-BP1 functions as a key integrator of the effects of AKT and ERK activation on cap-dependent translation and that convergent phosphorylation of 4E-BP1 by activated AKT and ERK pathways plays a critical role in maintaining the transformed phenotype in tumors with activation of both pathways.
4E-BP1-mediated survivin translation is critical for cancer metastatic progression by AKT and ERK signaling pathways
Metastasis is a complicated process that requires numerous proteins cooperatively to impart tumor invasion, mediate angiogenesis, suppress apoptosis and cause proliferation at a secondary site for tumor genesis [40]. In contrast to the frequent mutations observed in the classic oncogenes and tumor suppressor genes, many of the genes that drive primary tumor progression to metastasis are inappropriately expressed. It is believed that the initial step for metastasis, acquisition of migratory and invasive capability, is rate-limiting. Our recent studies show that phosphorylated 4E-BP1-mediated activation of cap-dependent translation by cooperative AKT and ERK signaling is also crucial for promotion of cancer cell motility and metastasis [41]. We found that in CRC cells with concurrent mutational activation of AKT and ERK pathways, combined inhibition of both pathways is required for effective inhibition of cell migration and invasion, while inhibition of either pathway alone shows little effects. Genetic disruption of eIF4F complex formation by eIF4E knockdown or expression of the non-phoshorylated 4E-BP1 mutant 4E-BP1-4A profoundly repressed CRC cell migration and invasion as well as lung and liver metastases in the mouse model of CRC. Conversely, activation of cap-dependent translation by eIF4E overexpression or 4E-BP1 knockdown greatly reduced dependence on AKT and ERK signaling for cell migration and invasion. Mechanistically, we identified that AKT and ERK signaling cooperate to selectively upregulate survivin protein expression at the level of translation mediated by their common downstream of the mTORC1/4E-BP1/eIF4E signaling axis. We found that continuous translation of survivin by AKT and ERK signaling is critical for colon cancer progression to metastasis [41]. These findings highlight 4E-BP1 and eIF4E-initiated cap-dependent translation as a key effector or downstream process of the oncogenic activation of the AKT and ERK pathways responsible for metastatic progression of cancer.
4E-BP1 loss induces EMT and increases metastatic capability of cancer cells by translational activation of Snail
Epithelial–mesenchymal transition (EMT) is proposed to be a crucial mechanism regulating cell migratory and invasive capabilities in metastatic progression [42]. Our most recent work demonstrated for the first time that loss of 4E-BP1 function by silencing its expression induces EMT followed by promotion of cell migratory and invasive capabilities as well as metastasis in colon and breast cancer models [43]. Mechanistically, we determined that loss of 4E-BP1 selectively increases the translation of Snail mRNA, an EMT inducer, with concomitant decreased expression of the epithelial marker E-cadherin. Inhibition of cap-dependent translation by the non-phoshorylated and dominant active mutant 4E-BP1-4A, by mTORC1 inhibition or by directly targeting the eIF4F translation initiation complex using a selective eIF4E/eIF4G interaction inhibitor, 4EGI-1 [44], profoundly attenuated Snail expression and cell motility, whereas knockdown of 4E-BP1 or over expression of Snail significantly reversed the inhibitory effects [43]. Thus, our findings uncover a novel role of 4E-BP1 in the regulation of EMT and metastatic progression of cancer through translational control of Snail expression and activity, and suggest that targeted inhibition of cap-dependent translation may be a promising approach for blocking key oncoproteins including the commonly considered “undruggable” oncoprotein Snail.
mTORC1 largely mediates the oncogenic effects of AKT and ERK signaling via 4E-BP1 phosphorylation
The mTOR kinase forms two distinct functional complexes, mTORC1 and mTORC2, with respective substrates. It is well-known that mTORC1 phosphorylates 4E-BP1, which in turn, activates cap-dependent translation, while mTORC2 mediates the phosphorylation of AKT on S473 [45]. mTORC1 has been shown to be activated by both AKT and ERK signaling via phosphorylation of TSC2 [46, 47]. It is thus reasonable to speculate that mTORC1 is a translation regulatory node integrating the effects of AKT and ERK signaling pathways in CRC with coexistent pathway activation. In these CRC cells, we found that genetic or pharmacologic inhibition of mTORC1 via knockdown of its obligatory component raptor or using the mTOR kinase inhibitors greatly represses 4E-BP1 phosphorylation and survivin translation with the similar inhibitory effects induced by combined inhibition of AKT and ERK [41]. Moreover, we and others, established that raptor knockdown can profoundly suppress cell invasion and metastasis of CRC [41, 48]. Thus, our data strongly suggest that the oncogenic action of AKT and ERK signaling in translational control of CRC invasion and metastasis is largely mediated in the mTORC1-dependent manner. A recent study also shows that mTORC1 plays an important role in translational regulation of a subset of gene expression responsible for metastatic progression of prostate cancer [49]. Thus, targeting mTORC1 is a potential therapeutic strategy for suppression of cancer invasion and metastasis. However, it is important to note that mTOR inhibitors, including the allosteric inhibitors of mTORC1 (rapamycin and its rapalogs) and the second generation of mTOR kinase inhibitors that inhibit both mTORC1 and mTORC2, are known to activate receptor tyrosine kinases and their downstream PI3K/AKT and ERK signaling in tumors via the loss of a negative feedback mechanism [50–53]. This feedback activation of AKT and ERK signaling may attenuate therapeutic effects of mTOR inhibitors. Furthermore, incomplete inhibition of 4E-BP1 phosphorylation or mTOR-independent phosphorylation of 4E-BP1 is believed to be an additional mechanism that causes tumor cell resistance to mTOR inhibitors [54–57]. We have found that combined inhibition of mTORC1 and AKT activities induces more profound inhibition of 4E-BP1 phosphorylation, and a robust inhibition of cell migration and invasion in CRC cells [41]. Other studies also demonstrate that mTORC1 inhibition in combination with the dual PI3K/mTOR or AKT inhibitors exhibit more marked inhibitory effects on phosphorylation of 4E-BP1 and cap-dependent translation [58], and increase therapeutic efficacy in cancer [58–60]. These findings suggest that phosphorylation status of 4E-BP1 is an important biomarker associated with the antitumor effect of mTOR inhibitors.
Conclusions and Perspectives
Our studies provide novel insights into the biology and clinical relevance of translational regulation in CRC metastatic progression and therapy. Our findings reveal 4E-BP1 as a key translation regulatory switch that integrates the effects of the mutational activation of the PI3K/AKT and RAS/RAF/MEK/ERK signaling pathways on tumorigenesis and metastasis. This integration allows cancer cells the flexibility to rely on either pathway to maintain their transformed phenotypes regardless of which single pathway is inhibited (Figure 1). A number of small molecule inhibitors of PI3K, AKT, RAF, MEK and mTOR kinases have been tested in the clinic for the treatment of cancer. Our work suggests that genotyping of patients’ tumors for mutations in components of PI3K/KAT and RAS/RAF/MEK/ERK pathways is important for optimizing clinical care because tumors with mutations in both pathways may have an unfavorable response to inhibition of one pathway alone. This hypothesis needs to be evaluated in current clinical trials. Furthermore, dephosphorylation of 4E-BP1 could serve as an important biomarker for predicting response to cancer therapy using the AKT and ERK pathway inhibitors in the clinic.
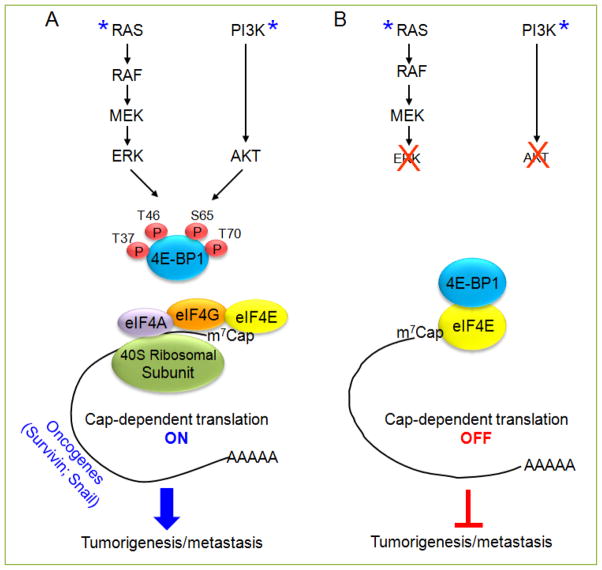
Figure 1. A schematic model for the role of 4E-BP1 as a key translation regulatory switch that integrates effects of the PI3K/AKT and RAS/RAF/MEK/ERK signaling pathways on tumorigenesis and metastasis.
(A) In colorectal cancer cells with coexistent KRAS and PIK3CA mutations (as indicated with asterisk), activated AKT and ERK pathways cooperate to maintain tumor growth and promote metastatic progression by convergent phosphorylation of the translational repressor 4E-BP1 followed by a selective increase in eIF4E-initiated cap-dependent translation of certain oncogenic mRNAs such as survivin and Snail. (B) Combined inhibition of AKT and ERK pathways is required for effective inhibition of 4E-BP1 phosphorylation, which in turn induces 4E-BP1 binding to the eIF4E-mRNA cap complex and thus represses translation of oncogenic mRNAs, with an associated suppression of tumorigenesis and metastasis.
Our findings further suggest that targeting the convergence of oncogenic AKT and ERK signals on eIF4F translation initiation complex may be a useful therapeutic alternative to combinations of both pathway inhibitors. Given the importance of 4E-BP1-regulated cap-dependent translation as a key downstream node that integrates multiple oncogenic signaling pathways for tumor growth and metastasis [28, 31, 41], compounds that mimic the biochemical function of 4E-BP1 by disruption of eIF4E-eIF4G interaction or target other translation initiation components may be effective for cancer therapeutics. Indeed, several translation initiation inhibitors, including eIF4E antisense-oligonucleotides and silvestrol that inhibits the RNA helicase eIF4A, have recently produced encouraging anti-tumor effects with limited toxicity in mouse; some of these inhibitors have been tested in clinical studies [61–64]. Considering that accumulated adverse side effects produced by a combination of therapies that inhibits multiple canonical signaling pathways, and that the mTOR inhibition-induced feedback activation of upstream receptor tyrosine kinases and AKT and ERK signaling that may reduce anti-tumor effects of mTOR inhibitors, targeting 4E-BP1-regulated translation that can block upstream oncogenic signals on the expression of multiple, important oncoproteins may provide a potentially viable therapeutic strategy against the metastatic progression of cancer with less toxicity.
Acknowledgments
This work was supported by grants from NCI R01CA175105, NIH/NCATSUL1RR033173-KL2RR0033171 and ACS IRG85-001-22 to Q-B She.
References
- 1.Ideker T, Thorsson V, Ranish JA, Christmas R, Buhler J, Eng JK, Bumgarner R, et al. Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science. 2001;292:929–934. doi: 10.1126/science.292.5518.929. [DOI] [PubMed] [Google Scholar]
- 2.de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5:1512–1526. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maier T, Guell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583:3966–3973. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 4.Livingstone M, Atas E, Meller A, Sonenberg N. Mechanisms governing the control of mRNA translation. Phys Biol. 2010;7:021001. doi: 10.1088/1478-3975/7/2/021001. [DOI] [PubMed] [Google Scholar]
- 5.Vogel C, de Abreu RS, Ko D, Le SY, Shapiro BA, Burns SC, et al. Sequence signatures and mRNA concentration can explain two-thirds of protein abundance variation in a human cell line. Molecular systems biology. 2010;6:400. doi: 10.1038/msb.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nature Rev Cancer. 2010;10:254–266. doi: 10.1038/nrc2824. [DOI] [PubMed] [Google Scholar]
- 8.Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nature Rev Mol Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–3199. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- 10.Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature. 1990;345:544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- 11.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 12.Martineau Y, Azar R, Bousquet C, Pyronnet S. Anti-oncogenic potential of the eIF4E-binding proteins. Oncogene. 2013;32:671–677. doi: 10.1038/onc.2012.116. [DOI] [PubMed] [Google Scholar]
- 13.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, et al. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armengol G, Rojo F, Castellvi J, Iglesias C, Cuatrecasas M, Pons B, et al. 4E-binding protein 1: a key molecular “funnel factor” in human cancer with clinical implications. Cancer Res. 2007;67:7551–7555. doi: 10.1158/0008-5472.CAN-07-0881. [DOI] [PubMed] [Google Scholar]
- 15.Martin ME, Perez MI, Redondo C, Alvarez MI, Salinas M, Fando JL. 4E binding protein 1 expression is inversely correlated to the progression of gastrointestinal cancers. Int J Biochem Cell Biol. 2000;32:633–642. doi: 10.1016/s1357-2725(00)00007-8. [DOI] [PubMed] [Google Scholar]
- 16.Kim YY, Von Weymarn L, Larsson O, Fan D, Underwood JM, Peterson MS, et al. Eukaryotic initiation factor 4E binding protein family of proteins: sentinels at a translational control checkpoint in lung tumor defense. Cancer Res. 2009;69:8455–8462. doi: 10.1158/0008-5472.CAN-09-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vakiani E, Solit DB. KRAS and BRAF: drug targets and predictive biomarkers. J Pathol. 2011;223:219–229. doi: 10.1002/path.2796. [DOI] [PubMed] [Google Scholar]
- 19.Simi L, Pratesi N, Vignoli M, Sestini R, Cianchi F, Valanzano R, Nobili S, et al. High-resolution melting analysis for rapid detection of KRAS, BRAF, and PIK3CA gene mutations in colorectal cancer. Am J Clin Pathol. 2008;130:247–253. doi: 10.1309/LWDY1AXHXUULNVHQ. [DOI] [PubMed] [Google Scholar]
- 20.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou P, Liu D, Shan Y, Hu S, Studeman K, Condouris S, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007;13:1161–1170. doi: 10.1158/1078-0432.CCR-06-1125. [DOI] [PubMed] [Google Scholar]
- 22.Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol. 2004;122:337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janku F, Lee JJ, Tsimberidou AM, Hong DS, Naing A, Falchook GS, et al. PIK3CA mutations frequently coexist with RAS and BRAF mutations in patients with advanced cancers. PloS One. 2011;6:e22769. doi: 10.1371/journal.pone.0022769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 25.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nature Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 27.Haluska F, Pemberton T, Ibrahim N, Kalinsky K. The RTK/RAS/BRAF/PI3K pathways in melanoma: biology, small molecule inhibitors, and potential applications. Semin Oncol. 2007;34:546–554. doi: 10.1053/j.seminoncol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 28.She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PloS One. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Tanaka H, Yoshida M, Tanimura H, Fujii T, Sakata K, Tachibana Y, et al. The selective class I PI3K inhibitor CH5132799 targets human cancers harboring oncogenic PIK3CA mutations. Clin Cancer Res. 2011;17:3272–3281. doi: 10.1158/1078-0432.CCR-10-2882. [DOI] [PubMed] [Google Scholar]
- 30.Janku F, Wheler JJ, Naing A, Falchook GS, Hong DS, Stepanek VM, et al. PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early-phase clinical trials. Cancer Res. 2013;73:276–284. doi: 10.1158/0008-5472.CAN-12-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer cell. 2010;18:39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 34.Yu K, Toral-Barza L, Shi C, Zhang WG, Zask A. Response and determinants of cancer cell susceptibility to PI3K inhibitors: combined targeting of PI3K and Mek1 as an effective anticancer strategy. Cancer Biol Ther. 2008;7:307–315. doi: 10.4161/cbt.7.2.5334. [DOI] [PubMed] [Google Scholar]
- 35.Wee S, Jagani Z, Xiang KX, Loo A, Dorsch M, Yao YM, et al. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res. 2009;69:4286–4293. doi: 10.1158/0008-5472.CAN-08-4765. [DOI] [PubMed] [Google Scholar]
- 36.Halilovic E, She QB, Ye Q, Pagliarini R, Sellers WR, Solit DB, et al. PIK3CA Mutation Uncouples Tumor Growth and Cyclin D1 Regulation from MEK/ERK and Mutant KRAS Signaling. Cancer Res. 2010;70:6804–6814. doi: 10.1158/0008-5472.CAN-10-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avdulov S, Li S, Michalek V, Burrichter D, Peterson M, Perlman DM, et al. Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer cell. 2004;5:553–563. doi: 10.1016/j.ccr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer cell. 2010;17:249–261. doi: 10.1016/j.ccr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobson BA, Alter MD, Kratzke MG, Frizelle SP, Zhang Y, Peterson MS, et al. Repression of cap-dependent translation attenuates the transformed phenotype in non-small cell lung cancer both in vitro and in vivo. Cancer Res. 2006;66:4256–4262. doi: 10.1158/0008-5472.CAN-05-2879. [DOI] [PubMed] [Google Scholar]
- 40.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 41.Ye Q, Cai W, Zheng Y, Evers BM, She QB. ERK and AKT signaling cooperate to translationally regulate survivin expression for metastatic progression of colorectal cancer. Oncogene. 2014;33:1828–1839. doi: 10.1038/onc.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Cai W, Ye Q, She QB. Loss of 4E-BP1 function induces EMT and promotes cancer cell migration and invasion via cap-dependent translational activation of snail. Oncotarget. 2014;5:6015–6027. doi: 10.18632/oncotarget.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moerke NJ, Aktas H, Chen H, Cantel S, Reibarkh MY, Fahmy A, et al. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell. 2007;128:257–267. doi: 10.1016/j.cell.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 45.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 47.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 48.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–3256. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 51.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011;1:248–259. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ducker GS, Atreya CE, Simko JP, Hom YK, Matli MR, Benes CH, et al. Incomplete inhibition of phosphorylation of 4E-BP1 as a mechanism of primary resistance to ATP-competitive mTOR inhibitors. Oncogene. 2014;33:1590–1600. doi: 10.1038/onc.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Zheng XF. mTOR-independent 4E-BP1 phosphorylation is associated with cancer resistance to mTOR kinase inhibitors. Cell Cycle. 2012;11:594–603. doi: 10.4161/cc.11.3.19096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazzoletti M, Bortolin F, Brunelli L, Pastorelli R, Di Giandomenico S, Erba E, et al. Combination of PI3K/mTOR inhibitors: antitumor activity and molecular correlates. Cancer Res. 2011;71:4573–4584. doi: 10.1158/0008-5472.CAN-10-4322. [DOI] [PubMed] [Google Scholar]
- 59.Thomas HE, Mercer CA, Carnevalli LS, Park J, Andersen JB, Conner EA, et al. mTOR inhibitors synergize on regression, reversal of gene expression, and autophagy in hepatocellular carcinoma. Sci Transl Med. 2012;4:139ra184. doi: 10.1126/scitranslmed.3003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Floc’h N, Kinkade CW, Kobayashi T, Aytes A, Lefebvre C, Mitrofanova A, et al. Dual Targeting of the Akt/mTOR Signaling Pathway Inhibits Castration-Resistant Prostate Cancer in a Genetically Engineered Mouse Model. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graff JR, Konicek BW, Vincent TM, Lynch RL, Monteith D, Weir SN, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest. 2007;117:2638–2648. doi: 10.1172/JCI32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cencic R, Carrier M, Galicia-Vazquez G, Bordeleau ME, Sukarieh R, Bourdeau A, et al. Antitumor activity and mechanism of action of the cyclopenta[b]benzofuran, silvestrol. PloS One. 2009;4:e5223. doi: 10.1371/journal.pone.0005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cencic R, Hall DR, Robert F, Du Y, Min J, Li L, et al. Reversing chemoresistance by small molecule inhibition of the translation initiation complex eIF4F. Proc Natl Acad Sci U S A. 2011;108:1046–1051. doi: 10.1073/pnas.1011477108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blagden SP, Willis AE. The biological and therapeutic relevance of mRNA translation in cancer. Nature Rev Clin Oncol. 2011;8:280–291. doi: 10.1038/nrclinonc.2011.16. [DOI] [PubMed] [Google Scholar]