Abstract
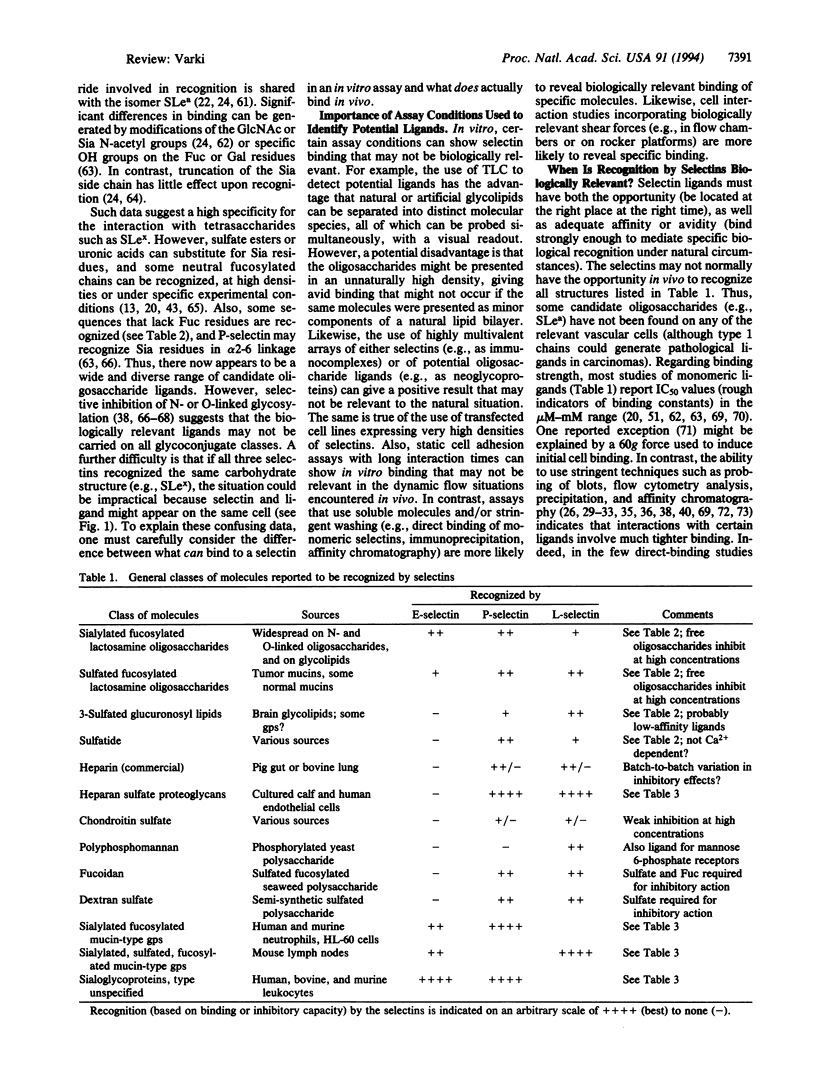
The selectins initiate many critical interactions among blood cells. The volume of information and diversity of opinions on the nature of the biologically relevant ligands for selectins is remarkable. This review analyzes the matter and suggests the hypothesis that at least some of the specificity may involve recognition of "clustered saccharide patches."
Full text
PDF

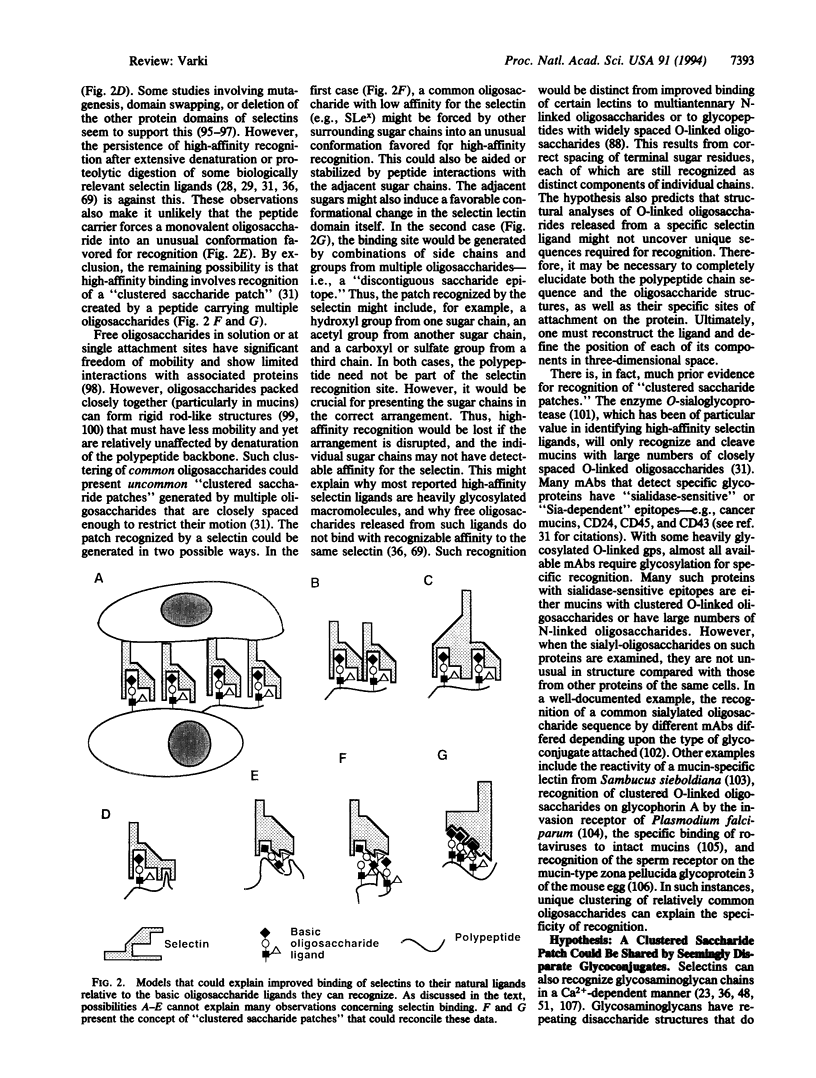

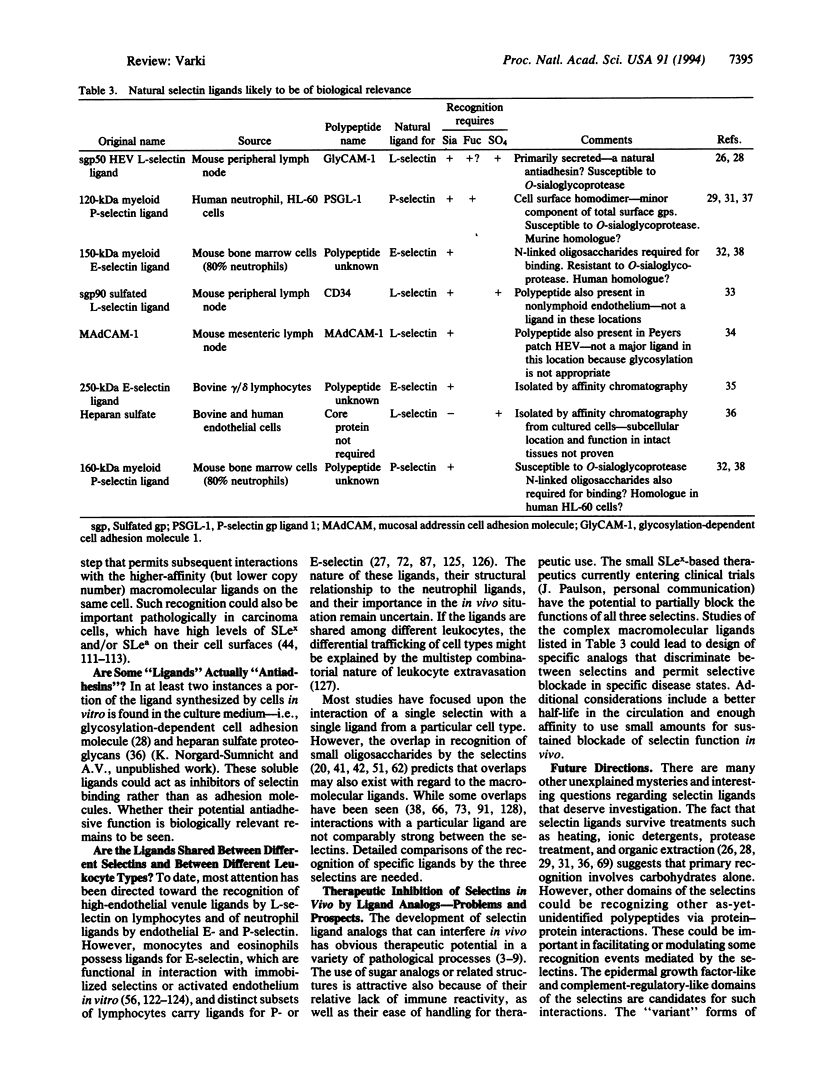


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Kolanus W., Walz G., Fredman P., Seed B. CD62/P-selectin recognition of myeloid and tumor cell sulfatides. Cell. 1991 Oct 4;67(1):35–44. doi: 10.1016/0092-8674(91)90570-o. [DOI] [PubMed] [Google Scholar]
- Asada M., Furukawa K., Kantor C., Gahmberg C. G., Kobata A. Structural study of the sugar chains of human leukocyte cell adhesion molecules CD11/CD18. Biochemistry. 1991 Feb 12;30(6):1561–1571. doi: 10.1021/bi00220a017. [DOI] [PubMed] [Google Scholar]
- Asako H., Kurose I., Wolf R., DeFrees S., Zheng Z. L., Phillips M. L., Paulson J. C., Granger D. N. Role of H1 receptors and P-selectin in histamine-induced leukocyte rolling and adhesion in postcapillary venules. J Clin Invest. 1994 Apr;93(4):1508–1515. doi: 10.1172/JCI117129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajorath J., Hollenbaugh D., King G., Harte W., Jr, Eustice D. C., Darveau R. P., Aruffo A. CD62/P-selectin binding sites for myeloid cells and sulfatides are overlapping. Biochemistry. 1994 Feb 15;33(6):1332–1339. doi: 10.1021/bi00172a007. [DOI] [PubMed] [Google Scholar]
- Baumheter S., Singer M. S., Henzel W., Hemmerich S., Renz M., Rosen S. D., Lasky L. A. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993 Oct 15;262(5132):436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Magnani J., Warnock R. A., Robinson M. K., Butcher E. C. Comparison of L-selectin and E-selectin ligand specificities: the L-selectin can bind the E-selectin ligands sialyl Le(x) and sialyl Le(a). Biochem Biophys Res Commun. 1992 Apr 30;184(2):1048–1055. doi: 10.1016/0006-291x(92)90697-j. [DOI] [PubMed] [Google Scholar]
- Berg E. L., McEvoy L. M., Berlin C., Bargatze R. F., Butcher E. C. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993 Dec 16;366(6456):695–698. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Robinson M. K., Mansson O., Butcher E. C., Magnani J. L. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991 Aug 15;266(23):14869–14872. [PubMed] [Google Scholar]
- Berg E. L., Robinson M. K., Warnock R. A., Butcher E. C. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991 Jul;114(2):343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg E. L., Yoshino T., Rott L. S., Robinson M. K., Warnock R. A., Kishimoto T. K., Picker L. J., Butcher E. C. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991 Dec 1;174(6):1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Nelson R. M. Selectins. J Clin Invest. 1993 Feb;91(2):379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady H. R., Spertini O., Jimenez W., Brenner B. M., Marsden P. A., Tedder T. F. Neutrophils, monocytes, and lymphocytes bind to cytokine-activated kidney glomerular endothelial cells through L-selectin (LAM-1) in vitro. J Immunol. 1992 Oct 1;149(7):2437–2444. [PubMed] [Google Scholar]
- Brandley B. K., Kiso M., Abbas S., Nikrad P., Srivasatava O., Foxall C., Oda Y., Hasegawa A. Structure-function studies on selectin carbohydrate ligands. Modifications to fucose, sialic acid and sulphate as a sialic acid replacement. Glycobiology. 1993 Dec;3(6):633–641. doi: 10.1093/glycob/3.6.633. [DOI] [PubMed] [Google Scholar]
- Buerke M., Weyrich A. S., Zheng Z., Gaeta F. C., Forrest M. J., Lefer A. M. Sialyl Lewisx-containing oligosaccharide attenuates myocardial reperfusion injury in cats. J Clin Invest. 1994 Mar;93(3):1140–1148. doi: 10.1172/JCI117066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Butenhof K. J., Gerken T. A. Structure and dynamics of mucin-like glycopeptides. Examination of peptide chain expansion and peptide-carbohydrate interactions by stochastic dynamics simulations. Biochemistry. 1993 Mar 16;32(10):2650–2663. doi: 10.1021/bi00061a025. [DOI] [PubMed] [Google Scholar]
- Carlos T., Kovach N., Schwartz B., Rosa M., Newman B., Wayner E., Benjamin C., Osborn L., Lobb R., Harlan J. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. Blood. 1991 May 15;77(10):2266–2271. [PubMed] [Google Scholar]
- Carver J. P., Michnick S. W., Imberty A., Cumming D. A. Oligosaccharide-protein interactions: a three-dimensional view. Ciba Found Symp. 1989;145:6–26. doi: 10.1002/9780470513828.ch2. [DOI] [PubMed] [Google Scholar]
- Corral L., Singer M. S., Macher B. A., Rosen S. D. Requirement for sialic acid on neutrophils in a GMP-140 (PADGEM) mediated adhesive interaction with activated platelets. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1349–1356. doi: 10.1016/0006-291x(90)91598-m. [DOI] [PubMed] [Google Scholar]
- Devine P. L., Harada H. Reactivity of mucin-specific lectin from Sambucus sieboldiana with simple sugars, normal mucins and tumor-associated mucins. Comparison with other lectins. Biol Chem Hoppe Seyler. 1991 Oct;372(10):935–942. doi: 10.1515/bchm3.1991.372.2.935. [DOI] [PubMed] [Google Scholar]
- Dowbenko D., Kikuta A., Fennie C., Gillett N., Lasky L. A. Glycosylation-dependent cell adhesion molecule 1 (GlyCAM 1) mucin is expressed by lactating mammary gland epithelial cells and is present in milk. J Clin Invest. 1993 Aug;92(2):952–960. doi: 10.1172/JCI116671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbe D. V., Watson S. R., Presta L. G., Wolitzky B. A., Foxall C., Brandley B. K., Lasky L. A. P- and E-selectin use common sites for carbohydrate ligand recognition and cell adhesion. J Cell Biol. 1993 Mar;120(5):1227–1235. doi: 10.1083/jcb.120.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbe D. V., Wolitzky B. A., Presta L. G., Norton C. R., Ramos R. J., Burns D. K., Rumberger J. M., Rao B. N., Foxall C., Brandley B. K. Identification of an E-selectin region critical for carbohydrate recognition and cell adhesion. J Cell Biol. 1992 Oct;119(1):215–227. doi: 10.1083/jcb.119.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzioni A., Frydman M., Pollack S., Avidor I., Phillips M. L., Paulson J. C., Gershoni-Baruch R. Brief report: recurrent severe infections caused by a novel leukocyte adhesion deficiency. N Engl J Med. 1992 Dec 17;327(25):1789–1792. doi: 10.1056/NEJM199212173272505. [DOI] [PubMed] [Google Scholar]
- Foxall C., Watson S. R., Dowbenko D., Fennie C., Lasky L. A., Kiso M., Hasegawa A., Asa D., Brandley B. K. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. J Cell Biol. 1992 May;117(4):895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz S. E., Hession C., Goff D., Griffiths B., Tizard R., Newman B., Chi-Rosso G., Lobb R. ELFT: a gene that directs the expression of an ELAM-1 ligand. Cell. 1990 Dec 21;63(6):1349–1356. doi: 10.1016/0092-8674(90)90430-m. [DOI] [PubMed] [Google Scholar]
- Goelz S., Kumar R., Potvin B., Sundaram S., Brickelmaier M., Stanley P. Differential expression of an E-selectin ligand (SLex) by two Chinese hamster ovary cell lines transfected with the same alpha (1,3)-fucosyltransferase gene (ELFT). J Biol Chem. 1994 Jan 14;269(2):1033–1040. [PubMed] [Google Scholar]
- Graves B. J., Crowther R. L., Chandran C., Rumberger J. M., Li S., Huang K. S., Presky D. H., Familletti P. C., Wolitzky B. A., Burns D. K. Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains. Nature. 1994 Feb 10;367(6463):532–538. doi: 10.1038/367532a0. [DOI] [PubMed] [Google Scholar]
- Green P. J., Tamatani T., Watanabe T., Miyasaka M., Hasegawa A., Kiso M., Yuen C. T., Stoll M. S., Feizi T. High affinity binding of the leucocyte adhesion molecule L-selectin to 3'-sulphated-Le(a) and -Le(x) oligosaccharides and the predominance of sulphate in this interaction demonstrated by binding studies with a series of lipid-linked oligosaccharides. Biochem Biophys Res Commun. 1992 Oct 15;188(1):244–251. doi: 10.1016/0006-291x(92)92376-9. [DOI] [PubMed] [Google Scholar]
- Handa K., Nudelman E. D., Stroud M. R., Shiozawa T., Hakomori S. Selectin GMP-140 (CD62; PADGEM) binds to sialosyl-Le(a) and sialosyl-Le(x), and sulfated glycans modulate this binding. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1223–1230. doi: 10.1016/0006-291x(91)92069-v. [DOI] [PubMed] [Google Scholar]
- Hollenbaugh D., Bajorath J., Stenkamp R., Aruffo A. Interaction of P-selectin (CD62) and its cellular ligand: analysis of critical residues. Biochemistry. 1993 Mar 30;32(12):2960–2966. doi: 10.1021/bi00063a006. [DOI] [PubMed] [Google Scholar]
- Huang K., Geoffroy J. S., Singer M. S., Rosen S. D. A lymphocyte homing receptor (L-selectin) mediates the in vitro attachment of lymphocytes to myelinated tracts of the central nervous system. J Clin Invest. 1991 Nov;88(5):1778–1783. doi: 10.1172/JCI115498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Lasky L. A., Rosen S. D. Further characterization of the interaction between L-selectin and its endothelial ligands. Glycobiology. 1992 Aug;2(4):373–381. doi: 10.1093/glycob/2.4.373. [DOI] [PubMed] [Google Scholar]
- Imai Y., Lasky L. A., Rosen S. D. Sulphation requirement for GlyCAM-1, an endothelial ligand for L-selectin. Nature. 1993 Feb 11;361(6412):555–557. doi: 10.1038/361555a0. [DOI] [PubMed] [Google Scholar]
- Imai Y., Singer M. S., Fennie C., Lasky L. A., Rosen S. D. Identification of a carbohydrate-based endothelial ligand for a lymphocyte homing receptor. J Cell Biol. 1991 Jun;113(5):1213–1221. doi: 10.1083/jcb.113.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990 Aug;15(8):291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Kansas G. S., Saunders K. B., Ley K., Zakrzewicz A., Gibson R. M., Furie B. C., Furie B., Tedder T. F. A role for the epidermal growth factor-like domain of P-selectin in ligand recognition and cell adhesion. J Cell Biol. 1994 Feb;124(4):609–618. doi: 10.1083/jcb.124.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. K., Warnock R. A., Jutila M. A., Butcher E. C., Lane C., Anderson D. C., Smith C. W. Antibodies against human neutrophil LECAM-1 (LAM-1/Leu-8/DREG-56 antigen) and endothelial cell ELAM-1 inhibit a common CD18-independent adhesion pathway in vitro. Blood. 1991 Aug 1;78(3):805–811. [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Kojima N., Handa K., Newman W., Hakomori S. Inhibition of selectin-dependent tumor cell adhesion to endothelial cells and platelets by blocking O-glycosylation of these cells. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1288–1295. doi: 10.1016/0006-291x(92)91872-n. [DOI] [PubMed] [Google Scholar]
- Kojima N., Handa K., Newman W., Hakomori S. Multi-recognition capability of E-selectin in a dynamic flow system, as evidenced by differential effects of sialidases and anti-carbohydrate antibodies on selectin-mediated cell adhesion at low vs. high wall shear stress: a preliminary note. Biochem Biophys Res Commun. 1992 Dec 30;189(3):1686–1694. doi: 10.1016/0006-291x(92)90272-m. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Kotovuori P., Tontti E., Pigott R., Shepherd M., Kiso M., Hasegawa A., Renkonen R., Nortamo P., Altieri D. C., Gahmberg C. G. The vascular E-selectin binds to the leukocyte integrins CD11/CD18. Glycobiology. 1993 Apr;3(2):131–136. doi: 10.1093/glycob/3.2.131. [DOI] [PubMed] [Google Scholar]
- Kuijpers T. W., Hoogerwerf M., van der Laan L. J., Nagel G., van der Schoot C. E., Grunert F., Roos D. CD66 nonspecific cross-reacting antigens are involved in neutrophil adherence to cytokine-activated endothelial cells. J Cell Biol. 1992 Jul;118(2):457–466. doi: 10.1083/jcb.118.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M., Ahern T. J., Stoll M. S., Shaffer M., Sako D., O'Brien J., Yuen C. T., Lawson A. M., Childs R. A., Barone K. M. Spectrum of sialylated and nonsialylated fuco-oligosaccharides bound by the endothelial-leukocyte adhesion molecule E-selectin. Dependence of the carbohydrate binding activity on E-selectin density. J Biol Chem. 1992 Jul 5;267(19):13661–13668. [PubMed] [Google Scholar]
- Larsen E., Palabrica T., Sajer S., Gilbert G. E., Wagner D. D., Furie B. C., Furie B. PADGEM-dependent adhesion of platelets to monocytes and neutrophils is mediated by a lineage-specific carbohydrate, LNF III (CD15). Cell. 1990 Nov 2;63(3):467–474. doi: 10.1016/0092-8674(90)90443-i. [DOI] [PubMed] [Google Scholar]
- Larsen G. R., Sako D., Ahern T. J., Shaffer M., Erban J., Sajer S. A., Gibson R. M., Wagner D. D., Furie B. C., Furie B. P-selectin and E-selectin. Distinct but overlapping leukocyte ligand specificities. J Biol Chem. 1992 Jun 5;267(16):11104–11110. [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Singer M. S., Dowbenko D., Imai Y., Henzel W. J., Grimley C., Fennie C., Gillett N., Watson S. R., Rosen S. D. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992 Jun 12;69(6):927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- Lee Y. C. Biochemistry of carbohydrate-protein interaction. FASEB J. 1992 Oct;6(13):3193–3200. doi: 10.1096/fasebj.6.13.1397841. [DOI] [PubMed] [Google Scholar]
- Leeuwenberg J. F., Jeunhomme T. M., Buurman W. A. Role of ELAM-1 in adhesion of monocytes to activated human endothelial cells. Scand J Immunol. 1992 Mar;35(3):335–341. doi: 10.1111/j.1365-3083.1992.tb02866.x. [DOI] [PubMed] [Google Scholar]
- Leeuwenberg J. F., Tan A., Jeunhomme T. M., Ploegh H. L., Buurman W. A. The ligand recognized by ELAM-1 on HL60 cells is not carried by N-linked oligosaccharides. Eur J Immunol. 1991 Dec;21(12):3057–3059. doi: 10.1002/eji.1830211225. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Weyrich A. S., Buerke M. Role of selectins, a new family of adhesion molecules, in ischaemia-reperfusion injury. Cardiovasc Res. 1994 Mar;28(3):289–294. doi: 10.1093/cvr/28.3.289. [DOI] [PubMed] [Google Scholar]
- Lenter M., Levinovitz A., Isenmann S., Vestweber D. Monospecific and common glycoprotein ligands for E- and P-selectin on myeloid cells. J Cell Biol. 1994 Apr;125(2):471–481. doi: 10.1083/jcb.125.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinovitz A., Mühlhoff J., Isenmann S., Vestweber D. Identification of a glycoprotein ligand for E-selectin on mouse myeloid cells. J Cell Biol. 1993 Apr;121(2):449–459. doi: 10.1083/jcb.121.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K., Gaehtgens P., Fennie C., Singer M. S., Lasky L. A., Rosen S. D. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991 Jun 15;77(12):2553–2555. [PubMed] [Google Scholar]
- Li S. H., Burns D. K., Rumberger J. M., Presky D. H., Wilkinson V. L., Anostario M., Jr, Wolitzky B. A., Norton C. R., Familletti P. C., Kim K. J. Consensus repeat domains of E-selectin enhance ligand binding. J Biol Chem. 1994 Feb 11;269(6):4431–4437. [PubMed] [Google Scholar]
- Lowe J. B., Kukowska-Latallo J. F., Nair R. P., Larsen R. D., Marks R. M., Macher B. A., Kelly R. J., Ernst L. K. Molecular cloning of a human fucosyltransferase gene that determines expression of the Lewis x and VIM-2 epitopes but not ELAM-1-dependent cell adhesion. J Biol Chem. 1991 Sep 15;266(26):17467–17477. [PubMed] [Google Scholar]
- Lowe J. B., Stoolman L. M., Nair R. P., Larsen R. D., Berhend T. L., Marks R. M. ELAM-1--dependent cell adhesion to vascular endothelium determined by a transfected human fucosyltransferase cDNA. Cell. 1990 Nov 2;63(3):475–484. doi: 10.1016/0092-8674(90)90444-j. [DOI] [PubMed] [Google Scholar]
- Majuri M. L., Mattila P., Renkonen R. Recombinant E-selectin-protein mediates tumor cell adhesion via sialyl-Le(a) and sialyl-Le(x). Biochem Biophys Res Commun. 1992 Feb 14;182(3):1376–1382. doi: 10.1016/0006-291x(92)91885-t. [DOI] [PubMed] [Google Scholar]
- McEver R. P. Selectins. Curr Opin Immunol. 1994 Feb;6(1):75–84. doi: 10.1016/0952-7915(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Mebius R. E., Watson S. R. L- and E-selectin can recognize the same naturally occurring ligands on high endothelial venules. J Immunol. 1993 Sep 15;151(6):3252–3260. [PubMed] [Google Scholar]
- Moore K. L., Stults N. L., Diaz S., Smith D. F., Cummings R. D., Varki A., McEver R. P. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992 Jul;118(2):445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. L., Thompson L. F. P-selectin (CD62) binds to subpopulations of human memory T lymphocytes and natural killer cells. Biochem Biophys Res Commun. 1992 Jul 15;186(1):173–181. doi: 10.1016/s0006-291x(05)80790-9. [DOI] [PubMed] [Google Scholar]
- Moore K. L., Varki A., McEver R. P. GMP-140 binds to a glycoprotein receptor on human neutrophils: evidence for a lectin-like interaction. J Cell Biol. 1991 Feb;112(3):491–499. doi: 10.1083/jcb.112.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Lowe J. B., Larsen R. D., Paulson J., Zheng Z. L., DeFrees S., Maemura K., Fukuda M., Ward P. A. Protective effects of sialylated oligosaccharides in immune complex-induced acute lung injury. J Exp Med. 1993 Aug 1;178(2):623–631. doi: 10.1084/jem.178.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Paulson J. C., De Frees S., Zheng Z. L., Lowe J. B., Ward P. A. Protective effects of oligosaccharides in P-selectin-dependent lung injury. Nature. 1993 Jul 8;364(6433):149–151. doi: 10.1038/364149a0. [DOI] [PubMed] [Google Scholar]
- Nakamori S., Kameyama M., Imaoka S., Furukawa H., Ishikawa O., Sasaki Y., Kabuto T., Iwanaga T., Matsushita Y., Irimura T. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 1993 Aug 1;53(15):3632–3637. [PubMed] [Google Scholar]
- Needham L. K., Schnaar R. L. The HNK-1 reactive sulfoglucuronyl glycolipids are ligands for L-selectin and P-selectin but not E-selectin. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1359–1363. doi: 10.1073/pnas.90.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. M., Cecconi O., Roberts W. G., Aruffo A., Linhardt R. J., Bevilacqua M. P. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993 Dec 1;82(11):3253–3258. [PubMed] [Google Scholar]
- Nelson R. M., Dolich S., Aruffo A., Cecconi O., Bevilacqua M. P. Higher-affinity oligosaccharide ligands for E-selectin. J Clin Invest. 1993 Mar;91(3):1157–1166. doi: 10.1172/JCI116275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard-Sumnicht K. E., Varki N. M., Varki A. Calcium-dependent heparin-like ligands for L-selectin in nonlymphoid endothelial cells. Science. 1993 Jul 23;261(5120):480–483. doi: 10.1126/science.7687382. [DOI] [PubMed] [Google Scholar]
- Norgard K. E., Han H., Powell L., Kriegler M., Varki A., Varki N. M. Enhanced interaction of L-selectin with the high endothelial venule ligand via selectively oxidized sialic acids. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1068–1072. doi: 10.1073/pnas.90.3.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard K. E., Moore K. L., Diaz S., Stults N. L., Ushiyama S., McEver R. P., Cummings R. D., Varki A. Characterization of a specific ligand for P-selectin on myeloid cells. A minor glycoprotein with sialylated O-linked oligosaccharides. J Biol Chem. 1993 Jun 15;268(17):12764–12774. [PubMed] [Google Scholar]
- Ohmori K., Takada A., Ohwaki I., Takahashi N., Furukawa Y., Maeda M., Kiso M., Hasegawa A., Kannagi M., Kannagi R. A distinct type of sialyl Lewis X antigen defined by a novel monoclonal antibody is selectively expressed on helper memory T cells. Blood. 1993 Nov 1;82(9):2797–2805. [PubMed] [Google Scholar]
- Orlandi P. A., Klotz F. W., Haynes J. D. A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac(alpha 2-3)Gal- sequences of glycophorin A. J Cell Biol. 1992 Feb;116(4):901–909. doi: 10.1083/jcb.116.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Butcher E. C. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Warnock R. A., Burns A. R., Doerschuk C. M., Berg E. L., Butcher E. C. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991 Sep 6;66(5):921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Pinola M., Renkonen R., Majuri M. L., Tiisala S., Saksela E. Characterization of the E-selectin ligand on NK cells. J Immunol. 1994 Apr 1;152(7):3586–3594. [PubMed] [Google Scholar]
- Polley M. J., Phillips M. L., Wayner E., Nudelman E., Singhal A. K., Hakomori S., Paulson J. C. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6224–6228. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. D., Ginsburg V. Sulfated glycolipids and cell adhesion. Arch Biochem Biophys. 1988 Dec;267(2):405–415. doi: 10.1016/0003-9861(88)90046-x. [DOI] [PubMed] [Google Scholar]
- Rosen S. D. Cell surface lectins in the immune system. Semin Immunol. 1993 Aug;5(4):237–247. doi: 10.1006/smim.1993.1028. [DOI] [PubMed] [Google Scholar]
- Rosen S. D., Singer M. S., Yednock T. A., Stoolman L. M. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science. 1985 May 24;228(4702):1005–1007. doi: 10.1126/science.4001928. [DOI] [PubMed] [Google Scholar]
- Saito S., Levery S. B., Salyan M. E., Goldberg R. I., Hakomori S. Common tetrasaccharide epitope NeuAc alpha 2-->3Gal beta 1-->3(Neu-Ac alpha 2-->6)GalNAc, presented by different carrier glycosylceramides or O-linked peptides, is recognized by different antibodies and ligands having distinct specificities. J Biol Chem. 1994 Feb 25;269(8):5644–5652. [PubMed] [Google Scholar]
- Sako D., Chang X. J., Barone K. M., Vachino G., White H. M., Shaw G., Veldman G. M., Bean K. M., Ahern T. J., Furie B. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993 Dec 17;75(6):1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- Sawada R., Lowe J. B., Fukuda M. E-selectin-dependent adhesion efficiency of colonic carcinoma cells is increased by genetic manipulation of their cell surface lysosomal membrane glycoprotein-1 expression levels. J Biol Chem. 1993 Jun 15;268(17):12675–12681. [PubMed] [Google Scholar]
- Shimizu Y., Shaw S., Graber N., Gopal T. V., Horgan K. J., Van Seventer G. A., Newman W. Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature. 1991 Feb 28;349(6312):799–802. doi: 10.1038/349799a0. [DOI] [PubMed] [Google Scholar]
- Skinner M. P., Fournier D. J., Andrews R. K., Gorman J. J., Chesterman C. N., Berndt M. C. Characterization of human platelet GMP-140 as a heparin-binding protein. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1373–1379. doi: 10.1016/0006-291x(89)91821-4. [DOI] [PubMed] [Google Scholar]
- Skinner M. P., Lucas C. M., Burns G. F., Chesterman C. N., Berndt M. C. GMP-140 binding to neutrophils is inhibited by sulfated glycans. J Biol Chem. 1991 Mar 25;266(9):5371–5374. [PubMed] [Google Scholar]
- Smeets E. F., de Vries T., Leeuwenberg J. F., van den Eijnden D. H., Buurman W. A., Neefjes J. J. Phosphorylation of surface E-selectin and the effect of soluble ligand (sialyl Lewisx) on the half-life of E-selectin. Eur J Immunol. 1993 Jan;23(1):147–151. doi: 10.1002/eji.1830230124. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Kishimoto T. K., Abbassi O., Hughes B., Rothlein R., McIntire L. V., Butcher E., Anderson D. C., Abbass O. Chemotactic factors regulate lectin adhesion molecule 1 (LECAM-1)-dependent neutrophil adhesion to cytokine-stimulated endothelial cells in vitro. J Clin Invest. 1991 Feb;87(2):609–618. doi: 10.1172/JCI115037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertini O., Luscinskas F. W., Gimbrone M. A., Jr, Tedder T. F. Monocyte attachment to activated human vascular endothelium in vitro is mediated by leukocyte adhesion molecule-1 (L-selectin) under nonstatic conditions. J Exp Med. 1992 Jun 1;175(6):1789–1792. doi: 10.1084/jem.175.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertini O., Luscinskas F. W., Kansas G. S., Munro J. M., Griffin J. D., Gimbrone M. A., Jr, Tedder T. F. Leukocyte adhesion molecule-1 (LAM-1, L-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesion. J Immunol. 1991 Oct 15;147(8):2565–2573. [PubMed] [Google Scholar]
- Steininger C. N., Eddy C. A., Leimgruber R. M., Mellors A., Welply J. K. The glycoprotease of Pasteurella haemolytica A1 eliminates binding of myeloid cells to P-selectin but not to E-selectin. Biochem Biophys Res Commun. 1992 Oct 30;188(2):760–766. doi: 10.1016/0006-291x(92)91121-6. [DOI] [PubMed] [Google Scholar]
- Stone J. P., Wagner D. D. P-selectin mediates adhesion of platelets to neuroblastoma and small cell lung cancer. J Clin Invest. 1993 Aug;92(2):804–813. doi: 10.1172/JCI116654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolman L. M. Adhesion molecules controlling lymphocyte migration. Cell. 1989 Mar 24;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Stoolman L. M., Yednock T. A., Rosen S. D. Homing receptors on human and rodent lymphocytes--evidence for a conserved carbohydrate-binding specificity. Blood. 1987 Dec;70(6):1842–1850. [PubMed] [Google Scholar]
- Streeter P. R., Rouse B. T., Butcher E. C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988 Nov;107(5):1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D. R., Abdullah K. M., Cyopick P., Mellors A. Cleavage of the cell-surface O-sialoglycoproteins CD34, CD43, CD44, and CD45 by a novel glycoprotease from Pasteurella haemolytica. J Immunol. 1992 Mar 1;148(5):1458–1464. [PubMed] [Google Scholar]
- Suzuki Y., Toda Y., Tamatani T., Watanabe T., Suzuki T., Nakao T., Murase K., Kiso M., Hasegawa A., Tadano-Aritomi K. Sulfated glycolipids are ligands for a lymphocyte homing receptor, L-selectin (LECAM-1), Binding epitope in sulfated sugar chain. Biochem Biophys Res Commun. 1993 Jan 29;190(2):426–434. doi: 10.1006/bbrc.1993.1065. [DOI] [PubMed] [Google Scholar]
- Takada A., Ohmori K., Yoneda T., Tsuyuoka K., Hasegawa A., Kiso M., Kannagi R. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993 Jan 15;53(2):354–361. [PubMed] [Google Scholar]
- Tamatani T., Kuida K., Watanabe T., Koike S., Miyasaka M. Molecular mechanisms underlying lymphocyte recirculation. III. Characterization of the LECAM-1 (L-selectin)-dependent adhesion pathway in rats. J Immunol. 1993 Mar 1;150(5):1735–1745. [PubMed] [Google Scholar]
- Thorpe S. J., Feizi T. Species differences in the expression of carbohydrate differentiation antigens on mammalian blood cells revealed by immunofluorescence with monoclonal antibodies. Biosci Rep. 1984 Aug;4(8):673–685. doi: 10.1007/BF01121021. [DOI] [PubMed] [Google Scholar]
- Tiemeyer M., Swiedler S. J., Ishihara M., Moreland M., Schweingruber H., Hirtzer P., Brandley B. K. Carbohydrate ligands for endothelial-leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1138–1142. doi: 10.1073/pnas.88.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True D. D., Singer M. S., Lasky L. A., Rosen S. D. Requirement for sialic acid on the endothelial ligand of a lymphocyte homing receptor. J Cell Biol. 1990 Dec;111(6 Pt 1):2757–2764. doi: 10.1083/jcb.111.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D., James P., Rao N., Foxall C., Abbas S., Dasgupta F., Nashed M., Hasegawa A., Kiso M., Asa D. Structural requirements for the carbohydrate ligand of E-selectin. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10372–10376. doi: 10.1073/pnas.88.22.10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiyama S., Laue T. M., Moore K. L., Erickson H. P., McEver R. P. Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J Biol Chem. 1993 Jul 15;268(20):15229–15237. [PubMed] [Google Scholar]
- Walcheck B., Watts G., Jutila M. A. Bovine gamma/delta T cells bind E-selectin via a novel glycoprotein receptor: first characterization of a lymphocyte/E-selectin interaction in an animal model. J Exp Med. 1993 Sep 1;178(3):853–863. doi: 10.1084/jem.178.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Zona pellucida glycoproteins. Annu Rev Biochem. 1988;57:415–442. doi: 10.1146/annurev.bi.57.070188.002215. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Imai Y., Fennie C., Geoffrey J., Singer M., Rosen S. D., Lasky L. A. The complement binding-like domains of the murine homing receptor facilitate lectin activity. J Cell Biol. 1991 Oct;115(1):235–243. doi: 10.1083/jcb.115.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. R., Imai Y., Fennie C., Geoffroy J. S., Rosen S. D., Lasky L. A. A homing receptor-IgG chimera as a probe for adhesive ligands of lymph node high endothelial venules. J Cell Biol. 1990 Jun;110(6):2221–2229. doi: 10.1083/jcb.110.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P. F., Rand T. H., Goelz S. E., Chi-Rosso G., Lobb R. R. Human eosinophil adherence to vascular endothelium mediated by binding to vascular cell adhesion molecule 1 and endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7430–7433. doi: 10.1073/pnas.88.16.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby R. E. Rotaviruses preferentially bind O-linked sialylglycoconjugates and sialomucins. Glycobiology. 1993 Oct;3(5):437–445. doi: 10.1093/glycob/3.5.437. [DOI] [PubMed] [Google Scholar]
- Wong C. S., Gamble J. R., Skinner M. P., Lucas C. M., Berndt M. C., Vadas M. A. Adhesion protein GMP140 inhibits superoxide anion release by human neutrophils. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2397–2401. doi: 10.1073/pnas.88.6.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N. W., Jalkanen S., Streeter P. R., Butcher E. C. Evolutionary conservation of tissue-specific lymphocyte-endothelial cell recognition mechanisms involved in lymphocyte homing. J Cell Biol. 1988 Nov;107(5):1845–1851. doi: 10.1083/jcb.107.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yago K., Zenita K., Ginya H., Sawada M., Ohmori K., Okuma M., Kannagi R., Lowe J. B. Expression of alpha-(1,3)-fucosyltransferases which synthesize sialyl Le(x) and sialyl Le(a), the carbohydrate ligands for E- and P-selectins,in human malignant cell lines. Cancer Res. 1993 Nov 15;53(22):5559–5565. [PubMed] [Google Scholar]
- Yednock T. A., Rosen S. D. Lymphocyte homing. Adv Immunol. 1989;44:313–378. doi: 10.1016/s0065-2776(08)60645-8. [DOI] [PubMed] [Google Scholar]
- Yuen C. T., Bezouska K., O'Brien J., Stoll M., Lemoine R., Lubineau A., Kiso M., Hasegawa A., Bockovich N. J., Nicolaou K. C. Sulfated blood group Lewis(a). A superior oligosaccharide ligand for human E-selectin. J Biol Chem. 1994 Jan 21;269(3):1595–1598. [PubMed] [Google Scholar]
- Yuen C. T., Lawson A. M., Chai W., Larkin M., Stoll M. S., Stuart A. C., Sullivan F. X., Ahern T. J., Feizi T. Novel sulfated ligands for the cell adhesion molecule E-selectin revealed by the neoglycolipid technology among O-linked oligosaccharides on an ovarian cystadenoma glycoprotein. Biochemistry. 1992 Sep 29;31(38):9126–9131. doi: 10.1021/bi00153a003. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Moore K. L., Smith D. F., Varki A., McEver R. P., Cummings R. D. The selectin GMP-140 binds to sialylated, fucosylated lactosaminoglycans on both myeloid and nonmyeloid cells. J Cell Biol. 1991 Oct;115(2):557–564. doi: 10.1083/jcb.115.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G. Transport and sorting of membrane lipids. Curr Opin Cell Biol. 1993 Aug;5(4):661–673. doi: 10.1016/0955-0674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- von Andrian U. H., Berger E. M., Ramezani L., Chambers J. D., Ochs H. D., Harlan J. M., Paulson J. C., Etzioni A., Arfors K. E. In vivo behavior of neutrophils from two patients with distinct inherited leukocyte adhesion deficiency syndromes. J Clin Invest. 1993 Jun;91(6):2893–2897. doi: 10.1172/JCI116535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., Berg E. L., Michie S. A., Brown D. A., Karolak D., Ramezani L., Berger E. M., Arfors K. E., Butcher E. C. L-selectin mediates neutrophil rolling in inflamed venules through sialyl LewisX-dependent and -independent recognition pathways. Blood. 1993 Jul 1;82(1):182–191. [PubMed] [Google Scholar]
- von Asmuth E. J., Smeets E. F., Ginsel L. A., Onderwater J. J., Leeuwenberg J. F., Buurman W. A. Evidence for endocytosis of E-selectin in human endothelial cells. Eur J Immunol. 1992 Oct;22(10):2519–2526. doi: 10.1002/eji.1830221009. [DOI] [PubMed] [Google Scholar]